Abstract
The functional recovery of the immune system in HIV-infected persons receiving HAART and the role of adjuvant immune therapy are still matters of intensive investigation. We analysed the effects of HAART combined with cytokines in 22 naive asymptomatic individuals, randomized to receive HAART (n = 6), HAART plus a low dose (1000 000 U/daily) of rIL-2 (n = 8), and HAART plus rIL-2 after previous administration of granulocyte colony-stimulating factor (n = 8). After 3 months of therapy, increased CD4+ T cell counts and diminished viral loads were observed in all patients, independently of cytokine addition. A decreased expression of CD95 (Apo 1/Fas) was evident in all groups when compared with values before therapy. The percentages of peripheral blood mononuclear cells (PBMC) expressing CD95 after therapy decreased by 15%, 22% and 18% in the three treatment groups, respectively (P < 0·05). Analysis of PBMC subsets demonstrated that CD95 expression was significantly reduced on CD45RA+CD62L+ naive T cells (25·3%, 22·4%, and 18·6%, respectively; P < 0·05) in each group, after therapy. Accordingly, all patients showed a reduced rate of in vitro spontaneous apoptosis (P < 0·05). Another effect induced by HAART was a significant increase in IL-2Rα expression on total PBMC (P < 0·05), independently of cytokine addition. Altogether, our results suggest that very low dose administration of rIL-2 (1000 000 U/daily) may be not enough to induce a significant improvement in the immune system as regards HAART alone. The employment of higher doses of recombinant cytokines and/or different administration protocols in clinical trials might however contribute to ameliorate the immune reconstitution in patients undergoing HAART.
Keywords: HAART, CD95, IL-2, apoptosis, naive cells
INTRODUCTION
The introduction of HAART has been successful in significantly reducing plasma viral load and increasing CD4 counts [1–3]. Specifically, the restoration of CD4+ T cell reactivity against common opportunistic pathogens, such as cytomegalovirus, Mycobacterium tuberculosis, and Candida albicans, is achievable in HIV-infected individuals receiving HAART [2–4]. Shortly after the initiation of HAART however the restoration of specific immunity against microbial pathogens may contribute to the reactivation of subclinical infections already present prior to treatment [5]. Interestingly, the CD4+ T cell recovery observed after anti-retroviral therapy seems to be strictly dependent on the amplitude and duration of viral load reduction [6,7], as demonstrated by high levels of HIV replication and CD4+ T cell loss when therapy is suspended or failed. A remodelling of the peripheral T cell compartment (both CD4+ and CD8+ T cells) is observed as a consequence of a significant increase in the memory population shortly after initiation of HAART, and a successive slow increase of naive cells [8]. Elevated numbers of memory cells following therapy may also be consequent to a significant decline in the apoptosis rate in this subset [9]. Moreover, the overall rate of lymphocyte apoptosis in the peripheral blood of HIV-infected persons observed at various stages of disease [10–13] is significantly reduced by anti-retroviral therapy [9,14]. The high degree of tissue apoptosis present in the lymphoid compartments [13,15] however has been shown to be reduced only upon 12 weeks of HAART [16].
Association of HAART with cytokines (especially rIL-2) has been recently introduced to improve immune system recovery in HIV-infected individuals. Patients receiving subcutaneous low doses of rIL-2 showed sustained rises of CD4+ T cells compared with patients receiving high doses, and no toxic effects or increased plasma HIV levels were reported [17–19]. Moreover, both chemokine production and chemokine receptor expression on peripheral blood mononuclear cells (PBMC) from HIV-infected individuals receiving HAART and low doses of rIL-2 were not significantly modified when compared with patients receiving HAART alone [20].
The aim of our study was to evaluate the effect of anti-retroviral therapy and the influence of low-dose cytokine administration on the immune reconstitution of HIV-infected individuals. Particularly, we investigated the expression of the CD95 (Apo 1/Fas) death receptor and the CD25 activation marker (IL-2 receptor α-chain) on different subsets of lymphocytes.
PATIENTS AND METHODS
Patients
The study involved 22 patients (12 males and 10 females) with a mean age of 36 years, selected as follows: asymptomatic HIV disease (A, CDC 1993), CD4 T cell absolute number in the range 400–600/μl, HIV-RNA >5000 copies/ml. HIV+ patients treated with anti-retroviral drugs, immunomodulators (interferons), corticosteroids or affected by hepatitis C virus (HCV), hepatitis B virus (HBV) and autoimmune diseases were excluded. The transmission of HIV was by homosexual contact in 11 individuals, heterosexual in the other patients. The patients were enrolled in three randomized groups. Six patients (group 1) were treated with HAART (Indinavir 2400 mg/day; Stavudine 60–80 mg/day; Lamivudine 300 mg/day), eight patients (group 2) were treated with HAART and rIL-2 (Aldesleukin; Chiron Corp., Emeryville, CA; 1000 000 U/day, subcutaneously, 5 days/week at alternative weeks), eight patients (group 3) received granulocyte colony-stimulating factor (G-CSF; Filgrastim, 5 μg/kg per day, for 5 consecutive days) to stimulate haematopoietic progenitor cell mobilization before starting HAART and rIL-2 [21]. All patients were treated with HAART for 1 month before receiving differentiated therapies (HAART; HAART +rIL-2; (G-CSF) HAART +rIL-2) for an additional 12/24 weeks. No patients interrupted the therapeutic protocol during the period of observation reported in the study. All patients provided informed written consent according to the Ethical Committee.
Blood collection and PBMC preparation
PBMC collected from HIV-1-infected patients prior to therapy (t = 0) and after 12/28 weeks of anti-retroviral therapy were isolated by Ficoll–Hypaque gradient (Lymphoprep; Nycomed, Oslo, Norway) and analysed.
Antibodies
MoAbs were purchased from Becton Dickinson (San Jose, CA): isotype-matched control fluoresceinated MoAbs, APC-conjugated and PerCP-conjugated anti-CD3, FITC-conjugated and PerCP-conjugated anti-CD4, PE-labelled anti-CD8, FITC-conjugated anti-CD45RA, FITC-conjugated anti-CD45RO, PE-conjugated anti-CD62L, APC-conjugated anti-CD95, FITC-conjugated anti-CD14, PerCP-conjugated anti-CD19, CyC-conjugated anti-CD16, PE-labelled anti-CD25, PE-labelled anti-CD122. All antibodies were used at saturating concentrations according to the manufacturer's instructions.
Immunophenotyping of PBMC
The analysis of PBMC by multiparameter flow cytometry was performed at baseline and 12 weeks after therapy. Briefly, 5 × 105 PBMC were incubated with MoAbs for 15 min on ice and, after two washes in PBS + 1% bovine serum albumin (BSA), resuspended in ice-cold 0·5% paraformaldehyde/PBS. A total of 2 × 104 PBMC for each sample were acquired using a FACSCalibur Flow Cytometer (Becton Dickinson) equipped with a 15-mW 488-nm argon–ion laser and a 635-nm red diode laser. Cell Quest software was used for analysis. Appropriate isotype-matched negative controls were run in parallel.
Plasma viraemia
HIV RNA was extracted from plasma and quantified by reverse transcriptase-polymerase chain reaction (RT-PCR) procedure (HIV-1 Amplicor Monitor; Roche Molecular Systems, Branchburg, NJ) according to the manufacturer's instructions. Plasma levels of HIV-1 RNA were expressed as HIV-RNA copies/ml with a threshold level of 400 copies/ml.
Spontaneous apoptosis
Detection of spontaneous apoptosis was performed before (t = 0) and after 24 weeks therapy as previously described [22]. PBMC isolated by Ficoll–Isopaque gradient were incubated in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (FCS), l-glutamine and antibiotics at 37°C for 72 h in 24-well plates (4 × 105 cells/ml) in duplicates. At day 0 and day 3 of culture, cells were stained with 0·5 μg/ml ethidium bromide and acquired with a cytometer (Cytoron Absolute; Ortho Diagnostic Systems, Raritan, NJ) to evaluate the absolute number of viable cells (cells not stained with ethidium) and compared with healthy donors. Mortality rate in percent (%) was calculated as follows: 100 – (absolute number of viable cells at day 3)/(absolute number of viable cells at day 0) × 100. Apoptotic cells showed a smaller size (mean forward scatter 62) and an increased granularity (mean side scatter 33) compared with viable cells (mean forward scatter 100, and mean side scatter 19), and appeared negative, ‘dim’ or ‘bright’ after staining with ethidium, according to the cell death phase.
Statistical analysis
All data were analysed blind. Data are given as arithmetic mean percentages and s.e.m. Mean differences between groups were evaluated for statistical significance by using a two-tailed Student's t-test and non-parametric Wilcoxon test for paired data; mean differences between groups were evaluated by Kruskall–Wallis test. P <0·05 was assumed to indicate statistical significance.
RESULTS
Response to anti-viral therapy and levels of spontaneous apoptosis
This study involved 22 adults with HIV-1 infection, naive for anti-retroviral therapy. After 12 weeks of therapy, the CD4+ T cell counts significantly increased in all patients, whereas CD8+ T cell counts remained stable. In all patients, HIV-1 RNA plasma levels decreased below the detection limit. In general, no significant differences were observed between the three groups in terms of CD4+ and CD8+ T cell counts or HIV-RNA reduction. The clinical characterization of patients is shown in Table 1.
Table 1.
Effects of differentiated anti-retroviral therapies in HIV-1-infected individuals
| HAART | HAART +IL-2 | (G-CSF) HAART +IL-2 | ||||
|---|---|---|---|---|---|---|
| t = 0 | t = 12 weeks | t = 0 | t = 12 weeks | t = 0 | t = 12 weeks | |
| CD4+ T cells | 453 + 66 | 726 + 228 | 497 + 56 | 931 + 226 | 546 + 64 | 848 + 229 |
| CD8+ T cells | 1017 + 418 | 996 + 216 | 1553 + 563 | 1424 + 608 | 1211 + 476 | 1138 + 41 |
| CD16+ B cells | 224 + 129 | 230 + 120 | 115 + 79 | 113 + 51 | 112 + 105 | 223 + 92 |
| CD19+ B cells | 161 + 42 | 207 + 91 | 146 + 56 | 251 + 182 | 181 + 90 | 295 + 307 |
| Percent in vitro | 37 + 6 | 27 + 6* | 44 + 10 | 26 + 4* | 43 + 7·5 | 25 + 3* |
| spontaneous apoptosis | ||||||
| RNA copies/ml (log) | 4·4 + 0·3 | 2·6 + 0 | 4·7 + 0·78 | 2·6 + 0·1 | 4·9 + 0·7 | 2·6 + 0·1 |
Values refer to absolute cell counts/μl and relative standard deviations. In vitro spontaneous apoptosis was detected after 24 weeks of therapy.
The level of spontaneous apoptosis of PBMC was analysed at t = 0 and after 24 weeks of therapy (t = 24 weeks). Collected PBMC were cultured for 3 days and cell viability was assessed at days 0 and 3 of culture by ethidium bromide staining as previously described [22]. All HIV+ patients showed an increased rate of spontaneous apoptosis when compared with normal uninfected individuals (P < 0·05). Although after 24 weeks of treatment the level of spontaneous apoptosis decreased in all groups, a more pronounced, but statistically not significant, effect was detected in patients receiving rIL-2 (Table 1).
Memory and naive T cell recovery
T cells expressing both CD45RA and CD62L markers were considered as naive cells, whereas CD45RA+CD62L− T cells and CD45RO+ T cells were identified as memory lymphocytes [14,23]. In accordance with previous studies [8], we observed a considerable increase in both CD4+ and CD8+ memory T cell numbers (data not shown) and a slight tendency to increase the numbers of naive T cells already present after 12 weeks of anti-retroviral therapy. Although many patients showed a similar behaviour in naive T cell recovery after anti-retroviral treatment, the increase in naive cell numbers was different and specific for any patient (Fig. 1a,b,c). To characterize PBMC subsets with a naive phenotype, single-cell analysis of peripheral blood lymphocytes at t = 0 and t = 12 weeks of therapy was performed by flow cytometry. We observed that the increased percentage of naive T cells among total PBMC was mainly due to CD4+ lymphocytes with a naive phenotype (Fig. 1d,e,f) and to a lower extent to CD8+ T cells. Moreover, naive natural killer (NK) cells did not show any considerable difference before or after therapy in any group (data not shown).
Fig. 1.

Frequencies of naive cells in HIV-infected individuals undergoing anti-retroviral therapy. Detection of peripheral blood mononuclear cells (PBMC) with a naive phenotype (identified as expression of CD45RA+ and CD62L+ receptors) was performed by single-cell analysis using a 4-fluorescence cytometric protocol at t = 0 (before therapy; □) and at t = 12 weeks (after 12 weeks of therapy; ▪). The percentage of naive cells analysed in each patient is shown in (a) (group 1), (b) (group 2), and (c) (group 3). In parallel, CD4+ T cells with naive phenotype were also analysed and relative percentages are shown in (d) (group 1), (e) (group 2), and (f) (group 3).
Analysis of CD95-expressing cells
Analysis of the expression of the CD95 death receptor was performed on PBMC before and after 12 weeks of therapy by single-cell analysis. All HIV-infected individuals, independently of therapy, showed a significant (P < 0·05) decrease of CD95 expression on total PBMC when compared with values before therapy (Fig. 2a). The percentages of CD95 cell decrease, following therapy, were 15%, 22% and 18%, respectively, in each group. Single-cell analysis of CD95 expression on cell subsets was performed by flow cytometry at t = 0 and t = 12 weeks. CD95 expression in the CD4+, CD8+ and NK lymphocytes for each group of patients is shown in Fig. 2b–d. The CD4+ subset showed a significant increase (P < 0·05) of the mean percentage of CD95-expressing cells in groups 2 and 3 after therapy (Fig. 2b). In contrast, no significant differences were observed in CD8+ lymphocytes and NK cells before or after treatment in any patient (Fig. 2c,d).
Fig. 2.
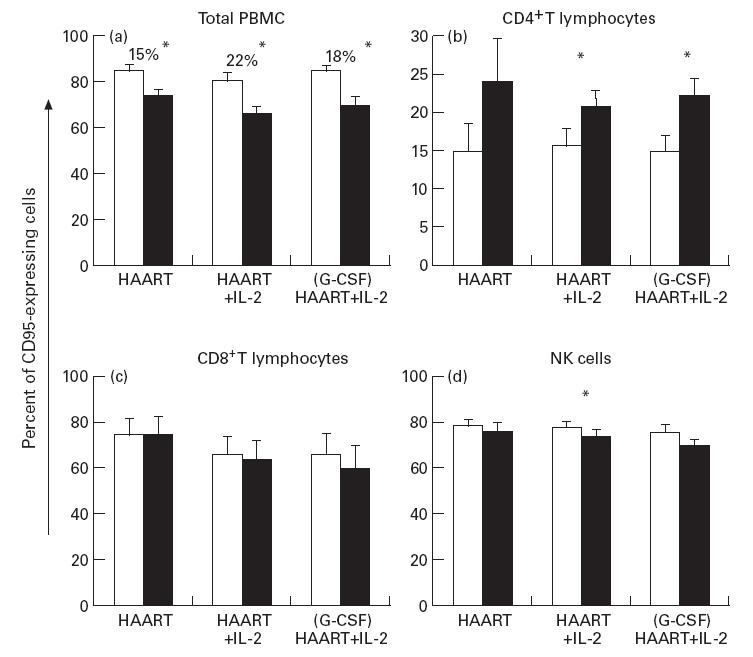
Analysis of CD95 expression on peripheral blood mononuclear cells (PBMC) obtained from HIV-infected individuals undergoing therapy. The expression of the CD95 receptor on total PBMC was investigated by single-cell analysis at t = 0 (□) and at t = 12 weeks (▪). (a) Changes in the percentage of total PBMC expressing the CD95 receptor in each group, before and after differentiated therapies. Percentages of CD95+ cell decrease, relative to each group following therapy, are shown. Results of phenotypical single-cell analysis of CD95+ PBMC subsets are shown in (b) (percentage of CD4+CD95+ T cells), (c) (percentage of CD8+CD95+ T cells), and (d) (percentage of natural killer (NK) CD16+CD95+ cells). Data represent the arithmetic mean percentage; error bars indicate s.e.m. P values are indicated: *P < 0·05.
The analysis of CD95 expression was then carried out on the naive subset of total PBMC by using a 4-fluorescence flow cytometer. The expression of CD95 on naive PBMC was analysed by gating cells co-expressing CD45RA and CD62L markers. In each group of patients undergoing therapy, a significant (P < 0·05) decrease in CD95 expression was observed on naive total PBMC, without any effect of cytokines after 12 weeks of therapy (Fig. 3a). The percentage of CD95 cell decrease, following therapy, was 25·3% in group 1, 22·4% in group 2 and 18·6% in group 3. Figure 3b–e shows representative flow cytometry images of one high responding patient of group 2. Prior to therapy, 81% of naive cells were CD95+ (Fig. 3b,d), but after 12 weeks of therapy only 28·7% of naive cells were CD95+ (Fig. 3c,e). In this case the percentage of CD95+ cell decrease was up to 64·8%. The CD95 reduced expression following HAART may be a consequence of an increased proportion of CD95− naive cells.
Fig. 3.

Decrease of CD95 death receptor expression on naive subset of total peripheral blood mononuclear cells (PBMC) after differentiated therapy in HIV-infected individuals. (a) Changes in the percentage of naive (CD45RA+CD62L+) cells expressing the CD95 receptor, before (□) and after 12 weeks of therapy (▪) in each group of patients. Mean percentages of decrease, relative to each group following therapy, are reported in the text. Data represent the arithmetic mean percentages; error bars indicate s.e.m. P values are indicated: *P < 0·05. (b–e) Representative flow cytometric images of a patient undergoing (G-CSF) HAART +IL-2, before (b,d) and after (c,e) therapy. Naive cells, shown in (b,c) by gating CD45RA+CD62L+ cells (R2), were then analysed for the expression of CD95 at t = 0 (d) and t = 12 weeks (e). Percentages of CD95+ naive cells were 81% at starting therapy (d) and 28·7% after 3 months (e).
Analysis of IL-2 receptor expression
The effect of rIL-2 administration on the expression of IL-2 receptors (IL-2Rα and IL-2Rβ) was analysed by flow cytometry on total PBMC after 3 months of HAART, and compared with values prior to treatment (Fig. 4a). A significant increase (P < 0·05) in α IL-2R (CD25) chain receptor expression on total PBMC was observed in all patients (the increase in CD25+ cell numbers from t = 0 to t = 12 weeks was 77·7% in the control group, 77·5% in group 2, and 105·5% in group 3; Fig. 4a). Analysis of CD25 expression was also performed on the different subsets of PBMC by single-cell analysis. Patients receiving HAART did not show any changes in the mean percentages of different CD25+ cell subsets after therapy (Fig. 4b). Patients undergoing HAART combined with a low dose of rIL-2 demonstrated a significant expansion in CD25+ cell numbers in all subsets when analysed before and after therapy (Fig. 4c,d; P < 0·05), with the exception of CD4+ lymphocytes. It is worth noting that cytotoxic cells (CD8+ and CD16+ cells) and B lymphocytes represent the major subsets expressing higher levels of CD25 after therapy in groups 2 and 3, even though the statistical analysis (Wilcoxon test) of these data did not show any difference between the studied groups, thus suggesting that this dose of rIL-2 was not sufficient to enhance the expression of CD25 on PBMC subsets.
Fig. 4.

rIL-2 administration during HAART affects the expression of CD25 (IL-2Rα) in HIV-infected individuals undergoing therapy. (a) Changes in the percentage of CD25-expressing total peripheral blood mononuclear cells (PBMC) in all groups, investigated by single-cell analysis at t = 0 (□) and at t = 12 weeks (▪). Mean percentages of CD25+ cell increase, relative to each group following therapy, are reported in the text. Analysis of different subsets of PBMC expressing CD25 was carried out by flow cytometry on the three groups of patients, and percentages reported in (b,c,d). A significant rIL-2 treatment-induced increase in the percentages of CD3+, CD8+, CD16+ and CD19+ lymphocytes was observed after therapy in groups 2 and 3, when compared with baseline values at starting treatment. Data represent the arithmetic mean percentage; error bars indicate s.e.m. P values are indicated: *P < 0·05.
Analysis of CD122 (IL-2Rβ) expression on total PBMC displayed no changes in CD122 expression (Fig. 5). Some patients exhibited a significant increase in CD122 expression after HAART on CD16+ NK or CD8+ or CD3+ subsets, but without significant differences within the three groups studied and between the groups, before or after therapy (Fig. 5).
Fig. 5.

rIL-2 administration during HAART does not affect the expression of CD122 (IL-2Rβ) in HIV-infected individuals undergoing therapy. (a) Mean percentages of CD122-expressing total peripheral blood mononuclear cells (PBMC) in all groups, investigated by single-cell analysis at t = 0 (□) and at t = 12 weeks (▪). Mean percentages of CD122+ cells did not change significantly after therapy in all groups. Different subsets of PBMC expressing CD122 were then analysed and are reported in (b,c,d). No significant treatment-induced changes in the percentage of CD122+ lymphocytes were observed after therapy, when compared with baseline values at starting treatment. Data represent the arithmetic mean percentage; error bars indicate s.e.m. P values are indicated: *P < 0·05.
DISCUSSION
HAART induces a dramatic decrease in plasma viral load and a parallel increase in peripheral T cell numbers, through the reappearance of both memory and naive T cell subsets [8]. The increase in T cell numbers is a consequence of T cell redistribution from lymph nodes to the peripheral blood [24]. Such a phenomenon is accompanied by a rapid increase in circulating memory T cells [8] and paralleled by a continuous slow re-population with newly produced naive T cells [24]. This subset may derive from the pool of naive T cells present in the periphery [25] and/or from de novo thymic generation [26]. The reappearance of naive cells is considered to be a predictive marker of immune reconstitution [2]. Moreover, the CD4+ T cell repertoire, disrupted in HIV-infected individuals, may be partially restored following anti-retroviral therapy, and it is a critical event for a prolonged immune recovery [27]. This study confirms that anti-retroviral therapy contributes to immune reconstitution by remodelling the compartment of naive T lymphocytes. A slow increase in naive T cell counts was observed when HIV+ individuals received HAART. In contrast to other reports [28] however, no effect of cytokines on naive cell distribution was observed. This may be due to different schedules of rIL-2 administration during HAART.
A decrease in susceptibility to undergo apoptosis was previously described in lymphocytes from patients receiving anti-retroviral therapy [9]. In keeping with this, we detected a decreased PBMC susceptibility to undergo in vitro spontaneous apoptosis and a parallel reduction in CD95 expression, as previously reported [16,29].
Naive CD4+ T cells are relatively resistant to viral-induced cytolysis, and HIV replication preferentially occurs in memory cells [30,31]. Moreover, naive T lymphocytes expressing CD95 (Fas) are highly resistant to Fas-mediated apoptosis [16]. Although HIV infection and replication, as well as T cell activation, may render these cells susceptible to apoptosis, it is worth noting that the intrinsic resistance to apoptosis in the naive T cell compartment is maintained following HIV infection and is not modified following HAART [9]. We showed that the increase in the percentage of naive T cells occurs in parallel with the reduction of spontaneous apoptosis in circulating lymphocytes after HAART. An important feature of naive cells was the decline of CD95 death receptor expression following HAART. This result suggests that the rise in numbers of apoptosis-resistant lymphocytes with a naive phenotype may represent a relevant effect of anti-retroviral therapy.
Defective production of IL-2 and a failure in IL-2Rα expression were reported during HIV infection [32], accompanied by increased levels of tumour necrosis factor-alpha (TNF-α) [33] and IL-6 [34,35]. In HIV-infected individuals, triple combination therapy was unable to increase the endogenous production of IL-2 [28] but the pattern of IL-2R expression has been described to approach normal levels [36]. Nevertheless, PBMC subsets from these patients displayed in vitro a clear IL-2 reactivity, indicating an enhanced response of the cytotoxic compartment, induced by HAART [36]. This study analysed IL-2R expression in patients receiving HAART combined with a low dose of rIL-2. Specifically, we observed a general increase in the expression of CD25 (IL-2Rα) in total PBMC after 12 weeks of HAART, independently of cytokine addition. It is worth noting that CD8+ T cells, CD16+ NK cells and CD19+ B cells showed a larger, even though statistically not significant, increase in CD25 expression when HAART was combined with rIL-2. These observations may suggest that administration of low doses of rIL-2 (1000 000 U/daily) is not adequate to enhance significantly the basal level of activation of effector cells induced by HAART; alternatively, the short course of therapy (3 months) could explain the partial effects observed on immune reconstitution after HAART. In light of this, we believe that the combined therapy of HAART and rIL-2 might be a useful approach to improve the immune reconstitution, and that higher doses of the cytokine are needed. Induction of higher levels of IL-2R expression on helper and cytotoxic T cells could improve the anti-viral activity, contributing to the suppression of virus spread and elimination of HIV-infected cells [37]. During primary HIV-1 infection, a pool of latently infected resting CD4+ T cells is established in lymph nodes and in the peripheral blood of infected individuals [38]. Although plasma viraemia decreases below the level of detectability in commonly used assays [39,40], a detectable reservoir of latently infected resting CD4+ T cells persists during HAART, thus representing the major obstacle to eradication of HIV in infected individuals [41]. Activation of these cells following immune restoration in the presence of a normal network of cytokines may result in protective immunity and clearance of infection, by elimination of the proviral reservoir cells through activation-induced cell death [42]. According to this hypothesis, we observed that the frequencies of latently infected cells were significantly lower in our patients treated with HAART combined with low doses of rIL-2 (F. Dianzani et al., Antiviral Res, in press). Moreover, it has been demonstrated that the combination of cytokines IL-2, IL-6 and TNF-α can reactivate HIV replication in vitro in latently infected CD4+ T cells obtained both from untreated patients and from patients responding to HAART [43–45]. The subsequent release of virus however may result in the death of these CD4+ T cells, confirming that cytokine treatment may contribute to purge the pool of latently infected cells.
Anti-retroviral therapy combined with rIL-2 has been indicated to enhance the immune reconstitution mediated by HAART. In our experience, the administration of low doses of rIL-2 may circumvent the side-effects induced by high doses [19], but it seems unable to promote an adequate improvement of the immune recovery. Although G-CSF was shown to mobilize totipotent stem cells [46], we observed that our schedule of cytokine administration plus rIL-2 was unable to modify the immunological and virological parameters, compared with HAART alone.
In conclusion, our data may contribute to define clinical trials aimed at improving the immune response to microbial pathogens in HIV-infected individuals with higher rIL-2 dose and/or different administration protocols.
Acknowledgments
We would like to express our gratitude to the staff of the IV Day Hospital of the Institute for Infectious Diseases ‘L. Spallanzani’: M. Lupi, G. Cianca, G. Preziosi, L. Bolognesi, and D. Menna. This study was partially supported by the I.S.S. project on AIDS, partially by Current and Finalized Research Project of Italian Ministry of Health, and in the frame of EU Copernicus Project. A.A. was supported by an I.S.S. AIDS fellowship. We thank Miss Simona Bach for stimulating discussions. The I.R.H.A.N. (Immune Reconstitution HIV Antiretroviral Naive) group is composed of: G. D'Offizi, V. Galati, P. Narciso, F. Aiuti, F. Pandolfi, M. Marziali, M. L. Bernardi, M. Pierdominici, F. Dianzani, G. Antonelli, and O. Turriziani.
REFERENCES
- 1.Collier AC, Coombs RW, Schoenfeld DA, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine and zalcitabine. N Engl J Med. 1996;334:1011–7. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 2.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 3.Mathez D, Bagnarelli P, Gorin I, et al. Reduction in viral load and increases in T lymphocyte numbers in treatment-naive patients with advanced HIV-1 infection treated with ritonavir, zidovudine, zalcitabine triple therapy. Antiviral Ther. 1997;2:175–83. [PubMed] [Google Scholar]
- 4.Pontesilli O, Kerkhof-Garde S, Pakker NG, et al. Antigen-specific T-lymphocyte proliferative responses during highly active antiretroviral therapy (HAART) of HIV-1 infection. Immunol Letters. 1999;66:213–7. doi: 10.1016/s0165-2478(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 5.Foudraine NA, Hovenkamp E, Notermans DW, et al. Immunopathology as a result of highly active antiretroviral therapy in HIV-1-infected patients. AIDS. 1999;13:177–84. doi: 10.1097/00002030-199902040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Li TS, Tubiana R, Katlama C, et al. Long-lasting recovery in CD4 T cell function and viral load reduction after highly active antiretroviral therapy in advanced HIV disease. Lancet. 1998;351:1682–6. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 7.Bisset LR, Cone RW, Huber W, et al. Highly active antiretroviral therapy during early HIV infection reverses T-cell activation and maturation abnormalities. Swiss HIV Cohort study. AIDS. 1998;12:2115–23. doi: 10.1097/00002030-199816000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Roeder M. Getting to the HAART of T cell dynamics. Nat Med. 1998;4:145–6. doi: 10.1038/nm0298-145. [DOI] [PubMed] [Google Scholar]
- 9.Gougeon ML, Lecoeur H, Sasaki Y. Apoptosis and CD95 system in HIV disease: impact of highly active anti-retroviral therapy. Immunol Letters. 1999;66:97–103. doi: 10.1016/s0165-2478(98)00167-9. [DOI] [PubMed] [Google Scholar]
- 10.Groux H, Torpier G, Monté D, et al. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–40. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyaard L, Otto SA, Jonker RR, et al. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–9. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 12.Oyaizu N, McCloskey TW, Coronesi M, et al. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood. 1993;82:3392–400. [PubMed] [Google Scholar]
- 13.Gougeon ML, Lecour H, Dulioust A, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 14.Bohler T, Walcher J, Holzl-Wenig G, et al. Early effects of antiretroviral combination therapy on activation, apoptosis and regeneration of T cells in HIV-1-infected children and adolescents. AIDS. 1999;13:779–89. doi: 10.1097/00002030-199905070-00006. [DOI] [PubMed] [Google Scholar]
- 15.Amendola A, Gougeon ML, Poccia F, et al. Induction of ‘tissue’ transglutaminase indicates high rate of apoptosis in peripheral CD4+ and accessory cells of lymph nodes of HIV-infected persons. PNAS USA. 1996;93:11057–62. doi: 10.1073/pnas.93.20.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badley AD, Dockrell DH, Algeciras A, et al. In vitro analysis of Fas/FasL interactions in HIV-infected patients. J Clin Invest. 1998;102:79–87. doi: 10.1172/JCI2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. PNAS USA. 1996;93:10405–10. doi: 10.1073/pnas.93.19.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey RT, Chaitt DG, Piscitelli SC, et al. Subcutaneous administration of interleukin-2 in human immunodeficiency virus type 1-infected persons. J Infect Dis. 1997;175:781–9. doi: 10.1086/513971. [DOI] [PubMed] [Google Scholar]
- 19.Davey RT, Chaitt DG, Albert JM, et al. A randomized trial of high-versus low-dose subcutaneous interleukin-2 outpatient therapy for early human immunodeficiency virus type 1 infection. J Infect Dis. 1999;179:849–58. doi: 10.1086/314678. [DOI] [PubMed] [Google Scholar]
- 20.Blanco J, Cabrera C, Jou A, et al. Chemokine and chemokine receptor expression after combined anti-HIV-1 interleukin-2 therapy. AIDS. 1999;13:547–55. doi: 10.1097/00002030-199904010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Slobod KS, Bennett TA, Freiden PJ, et al. Mobilization of CD34+ progenitor cells by granulocyte colony-stimulating factor in human immunodeficiency virus type 1-infected adults. Blood. 1996;88:3329–35. [PubMed] [Google Scholar]
- 22.Pandolfi F, Pierdominici M, Oliva A, et al. Apoptosis-related mortality in vitro of mononuclear cells from patients with HIV infection correlates with disease severity and progression. J Acquir Immune Def Syndr Hum Retrovir. 1995;9:450–8. [PubMed] [Google Scholar]
- 23.Kepler TB, Borrero M, Rugerio B, et al. Interdependence of N nucleotide addition and recombination site choice in V(D)J rearrangement. J Immunol. 1996;157:4451–7. [PubMed] [Google Scholar]
- 24.Pakker NG, Notermans DW, DeBoer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 25.Haynes B, Hale LP, Weinhold KJ, et al. Analysis of the adult thymus in reconstitution of peripheral T lymphocytes in HIV-1 infection. J Clin Invest. 1999;103:453–60. doi: 10.1172/JCI5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 27.Gorochov G, Neuman A, Kereveur A, et al. Perturbation of CD4+ and CD8+ T cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nature Med. 1998;4:215–21. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 28.Zanussi S, Simonelli C, Bortolin MT, et al. Immunological changes in peripheral blood and in lymphoid tissue after treatment of HIV-infected subjects with highly active anti-retroviral therapy (HAART) or HAART+IL-2. Clin Exp Immunol. 1999;116:486–92. doi: 10.1046/j.1365-2249.1999.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sousa AE, Chaves AF, Doroana M, et al. Early reduction of the over-expression of CD40, OX40 and Fas on T cells in HIV-1 infection during triple anti-retroviral therapy: possible implications for lymphocyte traffic and functional recovery. Clin Exp Immunol. 1999;116:307–15. doi: 10.1046/j.1365-2249.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods TC, Roberts BD, Butera ST, et al. Loss of inducible virus in CD45RA naive cells after human immunodeficiency virus-1 entry accounts for preferential viral replication in CD45RO memory cells. Blood. 1997;89:1635–41. [PubMed] [Google Scholar]
- 31.Roeder M, Raju PA, Mitra DK, et al. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J Clin Invest. 1997;99:1555–64. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poli G, Fauci A. Role of cytokines in the pathogenesis of human immunodeficiency virus infection. In: Aggarval BB, Puri RK, editors. Human cytokines: their role in disease and therapy. Cambridge, MA: Blackwell Science; 1995. pp. 421–49. [Google Scholar]
- 33.Aukrust P, Muller F, Lien E, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 34.Gurram M, Chirmule N, Wang XP, et al. Increased spontaneous secretion of interleukin-6 and tumor necrosis factor alpha by peripheral blood lymphocytes of human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1994;13:496–501. doi: 10.1097/00006454-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Levine AM, Tulpule A, Espina B, et al. Low dose methotrexate, bleomicin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone with zalcitabine in patients with acquired immunodeficiency syndrome-related lymphoma. Effect on human immunodeficiency virus and serum interleukin-6 levels over time. Cancer. 1996;78:517–26. doi: 10.1002/(SICI)1097-0142(19960801)78:3<517::AID-CNCR20>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.David D, Bani L, Moreau JL, et al. Regulatory dysfunction of the interleukin-2 receptor during HIV infection and the impact of triple combination therapy. PNAS USA. 1998;95:11348–53. doi: 10.1073/pnas.95.19.11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chun TW, Engel D, Mizell SB, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–5. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 38.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 39.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nature Med. 1997;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 40.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 41.Chun TW, Engel D, Berrey MM, et al. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. PNAS USA. 1998;95:8869–73. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chun TW, Engel D, Mizell S, et al. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho DD, Neumann AU, Perelson AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 44.Wei X, Ghosh SK, Taylor ME, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–22. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 45.Perelson AS, Neumann AU, Markowitz M, et al. HIV dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–6. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 46.Welte K, Gabrilove J, Bronchud MH, et al. Filgrastim (R-metHuG-CSF): the first 10 years. Blood. 1988;6:1907–29. [PubMed] [Google Scholar]


