Abstract
The human immune system undergoes continuous remodelling with the advancement of age. Since age-associated functional alterations in the immune system could be caused by a possible change in helper T cell regulation in elderly subjects, we comparatively studied the function of CD4+ T cells in peripheral blood obtained from both young and old healthy volunteers. Upon cell activation by phorbol myristate acetate and ionomycin, the proportion of CD4+ T cells containing interferon-gamma (IFN-γ) was found to be greater in the old subjects. Utilizing a co-culture system, which activated CD4+ T cells via the TCR/CD3 complex and CD28, we found that CD4+ T cells from the old subjects secreted more IFN-γ and IL-2, but less IL-4, than those from the young subjects. Upon cell activation by co-culture, CD4+ T cells from the old subjects expressed more CD26, CD40L, and LFA-1, but less CD30, than those from the young. These results together suggest that the microenvironment in which CD4+ T cells develop in older people may cause production of more cells committed to Th1 than that in younger subjects.
Keywords: ageing, helper T cell, cell activation, lymphokine, CD40L, costimulation, cell adhesion molecules, immunosenescence
INTRODUCTION
Progressive alterations in immunological activities with ageing appear to be responsible for an impaired resistance to infection and a decline in surveillance over neoplastic lesions in the elderly population. For instance, an increasing proportion of all forms of reactivation of tuberculosis, which is an intracellular infection that is eliminated by cell-mediated immune response (CMI) driven by Th1 lymphokines, occurs in patients over the age of 60 years [1,2]. Moreover, other evidence suggests that elderly people may have defects in delayed type immune response, such as a failure to be sensitized by delayed type antigens and a reduction in response to dinitrochlorobenzene or dinitrofluorobenzene [3,4]. As a result, the decline of CMI is considered to be a major reason for the reactivation of tuberculosis or decrease of DTH often seen in older patients. However, the cellular mechanisms responsible for the decline of CMI in the elderly are not well understood.
Since the function of macrophages is also known to decline along with ageing [5] and macrophages from old mice have a suppressive effect on T cells from young mice [6,7], the age-associated defects of CMI could be caused by a hypofunction of macrophages, which are the effector cells at the final stage of CMI.
In order to dissect alterations of the immune system involved with ageing, we studied the functions of CD4+ T cells, which regulate immune response by producing an array of lymphokines upon cell activation. In particular, we investigated such lymphokines produced by CD4+ T cells as IL-2, IL-4, and interferon-gamma (IFN-γ), which collectively regulate the clonal expansion of antigen-specific T cells and determine differentiation of Th1 and Th2 subsets [8–10]. Specifically, T helper type 1 cells, which secrete IL-2, IFN-γ, and lymphotoxin, have a major role in cellular immunity with intracellular pathogens, virus infections, and cancers, whereas Th2 cells, which secrete IL-4, IL-5 and IL-10, play a predominant role in humoral immunity against extracellular parasites [11], along with some forms of allergies and autoimmune diseases. Although cytokines such as IFN-γ, IL-12 and IL-4, have been shown to play major roles in regulating the Th cell repertoire [12], the strength of signals mediated by TCR [13,14] and such costimulatory molecules as CD30 [15,16], CD28, CD40L, and LFA-1 have also been reported to play an auxiliary role in Th cell differentiation. Previous reports have compared the function of lymphocytes obtained from SENIEUR old subjects [17] with that of young controls and indicated either a decrease of IFN-γ[18,19], no change [20], or an increase when normalized by the number of CD3+ cells [21]. These conclusions were based on experiments which utilized whole blood mononuclear cell cultures and did not measure IL-4 simultaneously.
The purpose of the current study was to evaluate the function of CD4+ T cells from young and old populations for their ability to secrete cytokines and express cell adhesion molecules, as an attempt to explain age-associated alterations of immune function.
SUBJECTS AND METHODS
Study and control groups
Young and old individuals were selected for this study according to the following criteria. Peripheral blood was obtained from 33 healthy subjects: 17 young adults of mean age 19 years (range 18–22 years) and 16 elderly adults with mean age of 61 years (range 55–65 years) who donated blood at the Red Cross Blood Centre (Kyoto, Japan).
Exclusion criteria were (i) clinical information; (ii) laboratory data; and (iii) pharmacological interference [17]. All individuals underwent medical interview and physical examination. Individuals were accepted only if they had not suffered from an inflammatory disease from an infection during the previous 6 weeks. Persons vaccinated in the previous 6 weeks were not accepted. In addition, it was required that subjects had had no surgical intervention, pregnancy, myocardial infarction or serious illness during the previous 6 months. Individuals with malignancies, hepatitis in the past or at present were not accepted. Individuals who had used medications known to influence the immune system in the previous 6 months were also excluded. Each blood sample underwent routine laboratory tests, including those described previously [17]. Blood samples were excluded if they had abnormal test values in references to the Blood Centre's normal range determined for each age group. Ethical approval for this study was granted by the Review Board of Kyoto University Hospital.
T cells and CD4+ T cell preparation
T cells were selected from the peripheral blood of healthy donors by E-rosette followed by a Ficoll–Conray method as described elsewhere. CD4+ T cells were further purified by positive selection using a MACS beads cell separation system (Milteny Biotec, Sunnyvale, CA). Purity was usually >90%, which was estimated by flow cytometric analysis using FITC-conjugated anti-CD4 (Nu-TS/C; Nichirei, Tokyo, Japan) and PE-conjugated anti-CD3 (Nu-TS/C; Nichirei).
Detection of intracellular cytokine synthesis
Purified CD4+ T cells were incubated in the media containing phorbol myristate acetate (PMA; 50 ng/ml), ionomycin (100 ng/ml) and monencin (20 μm) (Sigma Chemical Co., St. Louis, MO) for 6 h. Then CD4+ T cells were collected and intracellular staining of IFN-γ and IL-4 was performed. Flow cytometric determination of intracellular IL-4 and IFN-γ production was performed according to the method described previously [16]. Briefly, CD4+ T cells were washed with PBS, supplemented with 0·5% bovine serum albumin (BSA; Gibco, Grand Island, NY). After washing, cells were fixed with 4% formaldehyde for 15 min at room temperature, washed twice with PBS containing 0·5% BSA and then permeabilized with PBS containing 0·5% BSA and 0·5% saponin. Cells were incubated with PE-conjugated anti-IL-4 or with FITC-conjugated anti-IFN-γ MoAb. After staining, cells were washed with PBS containing 0·5% BSA and 0·5% saponin and next washed with PBS. Lastly, cells were stained with biotin-labelled anti-CD4 MoAb, followed by RED670-conjugated streptavidin. After washing with PBS, the samples were finally analysed on a flow cytometer (EPICS XL; Coulter Electronics, Hialeah, FL), and percentage values of cells expressing a given cytokine were recorded.
LCD32 cells expressing CD80 molecule and cell culture conditions
L cells co-expressing CD32 alone (LCD32) or together with CD80 (LCD32/CD80) were generated as described previously [22]. LCD32 cells and LCD32/CD80 cells were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal calf serum (FCS; Bio Whittaker, Walkersville, MD), 50 μm 2-mercaptoethanol, 5% NCTC109 (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin. HAT and G418 were added to the medium for the LCD32 and LCD32/CD80 cells.
Co-culture system
LCD32 and LCD32/CD80 cells were used in the co-culture experiments after irradiation (70 Gy). For flow cytometric analysis and cytokine analysis, 2·5 × 105 T cells and 5 × 104 irradiated L cells were co-cultured in 1 ml of media comprising RPMI 1640 supplemented with 10% FCS, NaHCO3 2·0 g/l, 100 U/ml penicillin and 100 μg/ml streptomycin. Anti-CD3 antibody (0·01 μg/ml) (OKT3, ATCC CRL-8001) was added to the medium.
Cytokine assay
The supernatants were collected after 36 h of co-culture and assayed for the production of IL-2, IL-4, and IFN-γ. IL-2 was measured using the CTLL-2 cell line (ATCC; TIB 214) as described elsewhere [23]. IL-4 was measured by proliferation assay using the CT-h4S cell line which expresses the human IL-4 receptor (kindly provided by Dr W. Paul, Laboratory of Immunology/NIAID/NIH) [24]. The detection limit for the IL-4 in this assay was 1 pg/ml. IFN-γ was quantified by ELISAs using mouse anti-human IFN-γ (Genzyme, Cambridge, MA) as capture MoAb, biotinylated mouse anti-human IFN-γ (PharMingen, San Diego, CA) and alkaliphosphatase-labelled mouse antibiotin (Zymed, San Francisco, CA) as detection antibodies in 12-well assay plates (Nunc Nunc-Immuno Plate MaxiSorp, Napierville, IL). The detection limit for the IFN-γ assay was 1 ng/ml.
Flow cytometric analysis
T cells (5 × 105) were stained with optimally diluted PE-conjugated mouse anti-human CD4 (Dako, Glostrup, Denmark), PE-conjugated mouse anti-human CD2 (Nu-TER; Nichirei), FITC-conjugated anti-CD26 (Dako), FITC-conjugated anti-CD30 (Dako), biotin-conjugated mouse anti-CD40L (Ancell Corp., Bayport, MN), PE-conjugated anti-human CD45RO (UCHL1; Nichirei), PE-conjugated CD45RA (Nichirei) and FITC-conjugated anti-CD11a (LFA-1) (Caltag Labs, San Francisco, CA). For detection of CD40L, the samples were further incubated with avidin-conjugated FITC (PharMingen) and FITC-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates, Inc., Birmingham, AL). Finally, propidium iodide (PI) was added to each cell suspension so that gates could be set to exclude dead cells. Surface immunofluorescence was assessed using a flow cytometer (EPICS XL; Coulter Electronics).
Statistical analysis
Statistical analysis was done using Student's t-test.
RESULTS
Intracellular cytokines in CD4+ T cells of young and old subjects
In order to examine the presence of Th precursor cells in the peripheral blood of young and old subjects, we assessed intracellular lymphokine production by CD4 T cells. Upon stimulation with PMA plus ionomycin, CD4+ T cells producing either IFN-γ or IL-4 in the cytosol were visualized by multicolour flow cytometric analysis (Fig. 1a). We did not find a significant percentage of CD4+ T cells producing both IFN-γ and IL-4 in either group. CD4+ T cells from the elderly subjects were found to contain more cells producing IFN-γ than those from the young (Fig. 1b,c). As a group, elderly subjects included significantly more high producers of IFN-γ than the young subjects (Fig. 1b,c, P =0·0009). Moreover, in the elderly group, high IFN-γ producers appeared also to be low IL-4 producers, although the results were not statistically significant. Thus, CD4+ T cells from elderly subjects contained a greater proportion of precursor cells which had the potential to produce IFN-γ upon activation than those from young subjects.
Fig. 1.
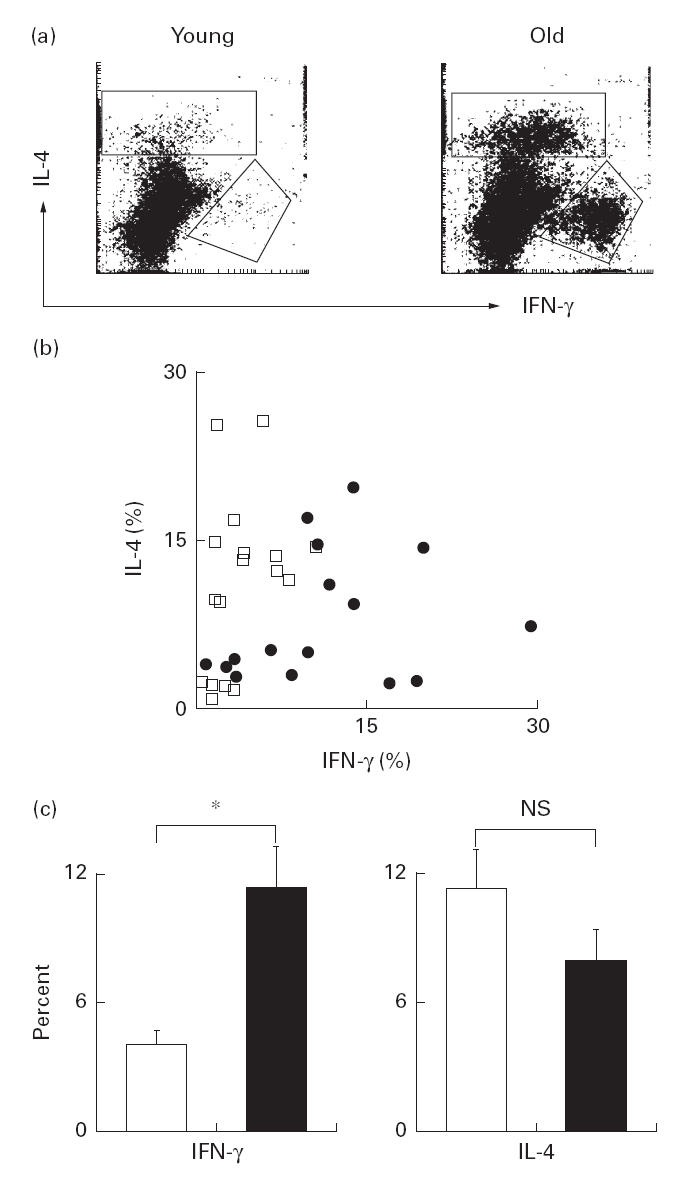
Multicolour flow cytometric analysis of CD4+ T cells producing IL-4 and IFN-γ in the cytosol. CD4+ T cells purified from young and elderly subjects were stimulated in the culture containing phorbol myristate acetate and ionomycin for 6 h and stained with antibodies to CD4, IL-4 and IFN-γ. Histograms show representative results obtained from the total of 33 subjects. CD4+ cells were gated out for the analysis of IL-4 and IFN-γ in the cytosol (a). Percentage of cells containing IL-4 and IFN-γ in CD4+ T cells for each individual was determined by multicolour flow cytometry. The overall results were shown as a dot plot graph (□, young; •, old) (b). The mean percentages of CD4+ T cells producing IL-4 and IFN-γ in the young (□) and elderly subjects (▪) are shown (mean +s.e.m.) (c). *P < 0·01; NS, not significant.
Cytokine secretion by CD4+ T cells stimulated via TCR and CD28
We next assessed the capability for lymphokine secretion of CD4+ T cells. In order to activate the CD4+ T cells, we double cross-linked the CD3 receptor and CD28 by co-culturing the CD4+ T cells with LCD32/CD80 cells, which co-express FcrR (CD32) and CD80. In the presence of the costimulation provided by CD80 expressed on LCD32/CD80 cells, a suboptimal dose of anti-CD3 effectively activated the CD4+ T cells by binding to both CD3 and CD32 molecules [22]. To control the costimulation, we used LCD32 cells, which do not express CD80. After 36 h of co-culture in media containing anti-CD3, cytokines that had been secreted into each culture supernatant were measured. Costimulation was effective in both young and old CD4+ T cell specimens, as we saw a significant increase of cytokine secretion in the co-culture with LCD32/CD80(Fig. 2). Interestingly, CD4+ T cells from the old subjects secreted significantly more IL-2 than those from the young subjects, even without costimulation (P =0·0104) (Fig. 2e,f). Although anti-CD3 stimulation alone did not lead to a significant difference in IFN-γ and IL-4 secretion between the two groups (Fig. 2a,c), the addition of costimulation led to a higher secretion level of IFN-γ (P =0·0345) and less of IL-4 (P =0·036) by CD4+ T cells from the old subjects than from the young subjects (Fig. 2b,d).
Fig. 2.
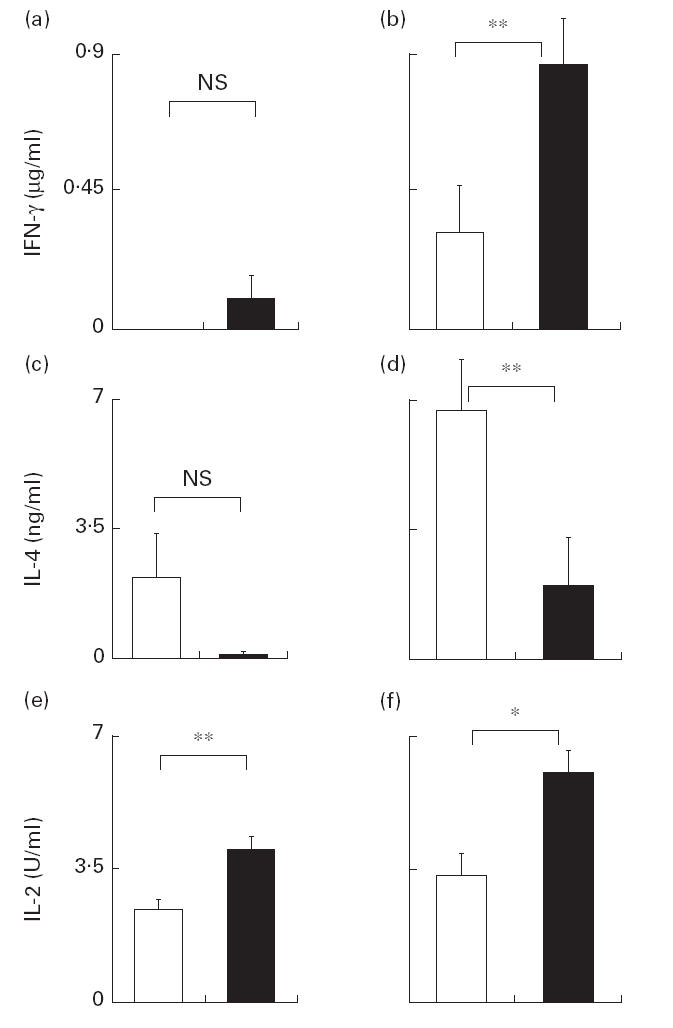
ΙIFNΝ-γ (a,b), IL-4 (c,d) and IL-2 (e,f) secretion by purified CD4+ T cells stimulated in the co-culture. CD4+ T cells (5 × 104/well) were activated by anti-CD3 alone (a,c,e) or together with CD80 stimulation (b,d,f). IL-2, IFN-γ and IL-4 secreted by CD4+ T cells from young subjects (□) and elderly subjects (▪) at 36 h after stimulation are shown. Results are expressed as mean +s.e.m. *P < 0·01; **P < 0·05; NS, not significant.
Expression of CD26 and CD30 in CD4+ T cells
Having obtained evidence that ageing affects the secretion of IFN-γ and IL-4, which are surrogate lymphokines for Th1 and Th2, respectively, we next studied the expression of CD26 and CD30 in CD4+ T cells. CD26 and CD30 are known to be associated, respectively, with the Th1 and Th2 differentiation of CD4+ T cells. CD4+ T cells were activated by co-culture with LCD32/CD80 in medium containing anti-CD3 (0·01 μg/ml) for 60 h. We observed the emergence of a cell population with an increased CD26 expression in CD4+ T cells from the elderly subjects, compared with that from the young subjects (Fig. 3a,c). In contrast, up-regulation of CD30 in the CD4+ T cells from the elderly subjects was virtually non-existent, whereas a significant increase was seen in those from young subjects (Fig. 3b,d).
Fig. 3.
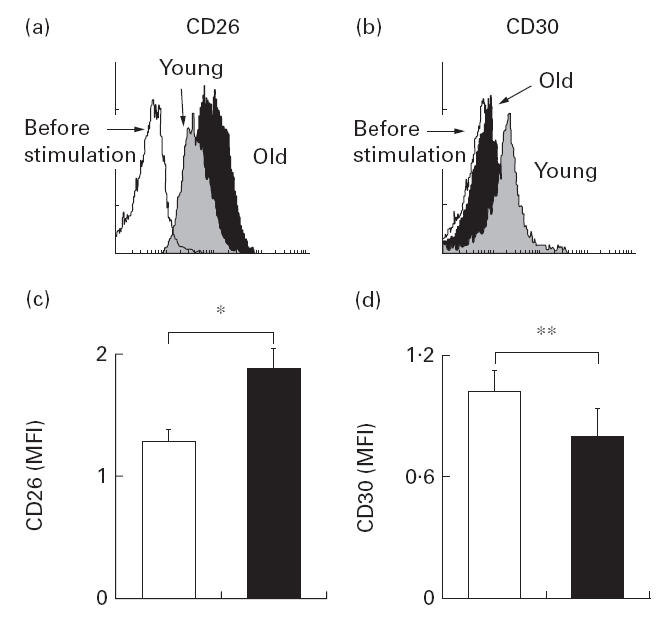
Flow cytometric analysis of CD26 and CD30 expression in the CD4+ T cells from young (grey) and elderly subjects (▪). CD4+ T cells were co-cultured with LCD32/CD80 in the medium containing anti-CD3 (0·01 μg/ml) for 60 h. Up-regulation of CD26 (a) and CD30 (b) expression in CD4+ T cells was assessed by flow cytometric analysis. Mean fluorescence intensity (MFI) of CD26 and CD30 was determined in each sample. Overall results were expressed as mean +s.e.m. (c,d) in the young (□) and the elderly (▪). *P < 0·01; **P < 0·05.
Expression of CD40L
Since a CD40/CD40L signal is involved in the up-regulation of IFN-γ secretion by T cells, we compared CD40L induction in CD4+ T cells upon activation between the young and elderly groups. In this experiment, CD4+ T cells were activated by co-culture with LCD32/CD80 in the presence of anti-CD3 (0·01 μg/ml) in medium for 60 h. The mean fluorescence intensity of CD40L in CD4+ cells was found to be much greater with the elderly group than with the young (Fig. 4).
Fig. 4.
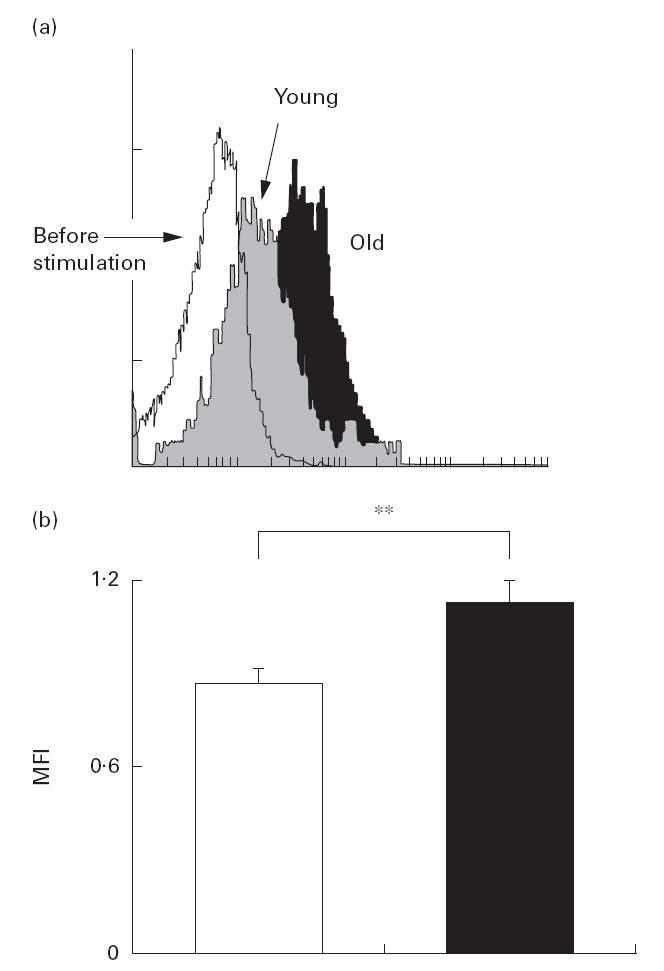
Flow cytometric analysis of CD40L expression in the CD4+ T cells from young (□) and elderly (▪) subjects. CD4+ T cells were co-cultured with LCD32/CD80 for 60 h in the medium containing anti-CD3 (0·01 μg/ml). Induction of CD40L by this co-culture in each sample was assessed by measuring mean fluorescence intensity (MFI) of CD40L in flow cytometric analysis (a). Mean +s.e.m. of MFI in the young (□) and elderly (▪) groups are shown (b). **P < 0·05.
Effects of ageing on LFA-1 and CD45RO expression
We next studied the effect of ageing on the expression of cell adhesion molecules in CD4+ T cells using LFA-1 (CD11a) and CD45RO. Alterations of LFA-1 expression by ageing are important, since LFA-1/intercellular adhesion molecule (ICAM) interactions suppress Th2 development [25]. In this experiment, T cells were co-cultured with LCD32/CD80 in medium containing 0·01 μg/ml of anti-CD3. Anti-CD3 with CD80 stimulation induced the emergence of LFA-1bright cells. Moreover, the induction of LFA-1bright cells was significantly greater in the elderly subjects than in the young (Fig. 5). Without in vitro stimulation, fresh CD4 T cells obtained from the elderly subjects contained more CD45RO+ cells than those obtained from the young (Fig. 6).
Fig. 5.
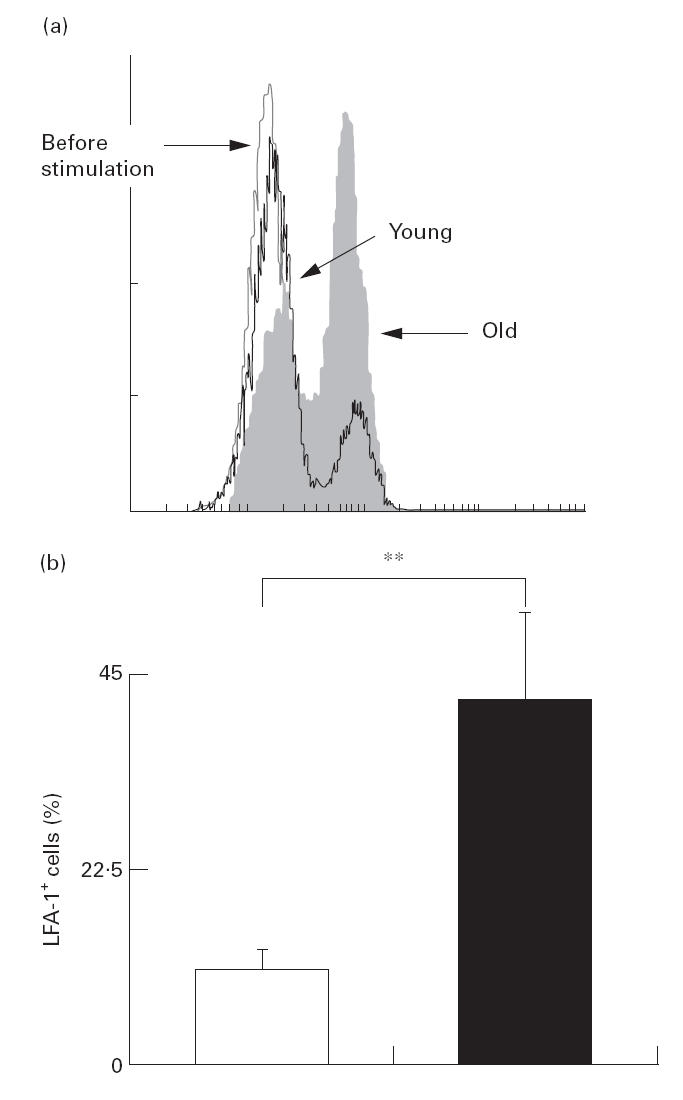
Multicolour flow cytometric analysis of LFA-1 (CD11a) expression in the CD4+ T cells of young and elderly subjects. After CD4+ T cells were co-cultured with LCD32/CD80 for 60 h in the medium containing anti-CD3 (0·01 μg/ml), emergence of LFA-1+ cells induced by the co-culture in each sample was assessed by measuring percentage of LFA-1+ cells in CD4+ T cells (a). The mean percentages +s.e.m. of LFA-1+ cells in CD4+ T cells of the young (□) and elderly (▪) groups are shown (b). **P < 0·05.
Fig. 6.
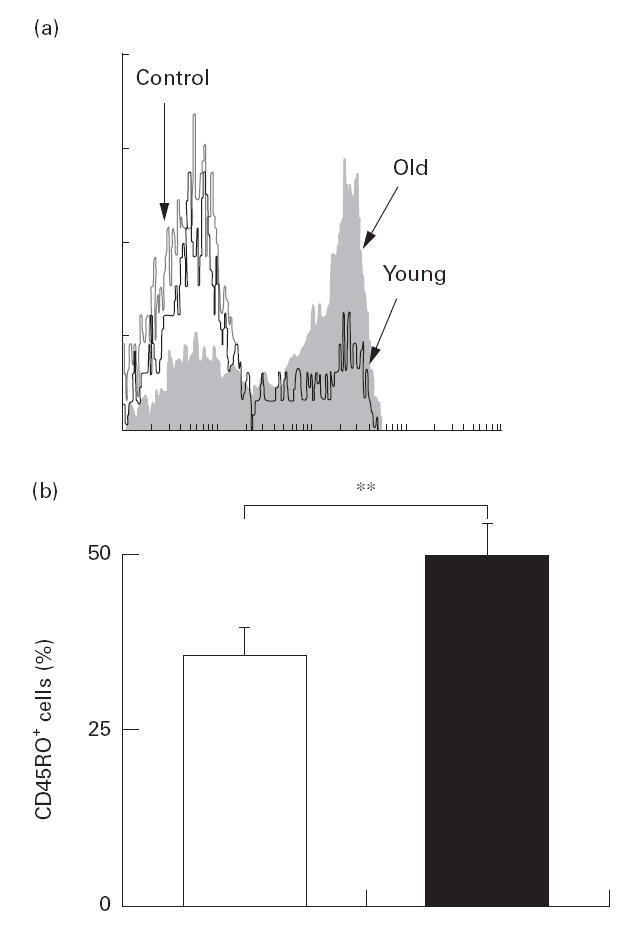
Flow cytometric analysis of CD45RO expression in the CD4+ T cells from young (n = 17) and elderly (n = 16) subjects. Peripheral blood mononuclear cells were stained with anti-CD4 and anti-CD45RO antibodies and analysed by flow cytometry. (a) A representative result showing CD45RO expression in CD4+ T cells. Percentage of CD45RO+ cells in CD4 T cells is shown in the young (□) and elderly (▪) groups. Overall results were expressed as the mean +s.e.m. (b). **P < 0·05.
DISCUSSION
Our study showed distinctive age-associated alterations in the function of human CD4+ T cells. Immune response is regulated at multiple levels, from an induction phase to cell migration and then to a final effector phase, which involves cells of various lineages, multiple cytokines/lymphokines and various cell adhesion molecules. Because of the complexity of immune regulation and the difficulty in obtaining appropriate aged subjects, studies of immunosenescence are sometimes contradictory. In order to overcome these difficulties, we utilized purified CD4+ T cells from healthy donors and a co-culture system which activated CD4+ T cells via an antigen–receptor complex and a costimulation receptor.
Stimuli mediated via CD28 stabilize IL-2 mRNA, thereby increasing IL-2 secretion. Contrary to our prediction, cell activation by this co-culture induced more IL-2 secretion by CD4+ T cells in elderly subjects than in the young subjects. Since our preliminary studies showed no significant difference in CD28 and CTLA-4 expression in CD4+ T cells between young and elderly subjects (data not shown), we postulated that CD4+ T cells from the elderly subjects had already received several activation signals in vivo (memory T cells), which facilitate signalling in the TCR/CD3 pathway leading to IL-2 secretion. This notion was corroborated by the fact that, even without CD80 costimulation, CD4+ T cells from elderly subjects secreted significantly more IL-2 upon activation by anti-CD3 alone. In fact, the proportion of CD45RO+ cells in CD4+ T cells was greater in elderly subjects than in the young subjects, indicating a greater proportion of CD4+ T cells with a memory cell phenotype. Since both anti-CD3-mediated calcium signalling and protein kinase C activation in humans are higher in CD45RO+ T cells than in CD45RA+ T cells [26], and CD45RO molecules couple with CD3-mediated signalling, the greater secretion of IL-2 by CD4+ T cells may indicate a more efficient TCR signalling in CD4+ T cells in elderly subjects than in young subjects. This greater expression of CD45RO seen in elderly subjects may also explain the greater amount of IFN-γ secretion by CD4+ T cells observed in the co-culture [27].
In the present study, polyclonal cell activation, either by PMA or anti-CD3 plus CD80 stimulation, led to the appearance of more Th1-like cells in CD4+ T cells from the elderly group than in those from the young. Further, we observed that CD4+ T cells from the elderly group produced more IFN-γ and IL-2, which expressed greater levels of CD40L, CD26 and LFA-1, than those from the young subjects. CD40L and IFN-γ are known to activate monocytes and macrophages to produce reactive nitrogen intermediates or various monokines [28], which play an indispensable role in CMI. CD40L and IFN-γ also activate antigen-presenting cells (APC) to up-regulate class II molecules and costimulation molecules, which in turn further activate T cells in an antigen-specific manner.
As seen in multicolour flow cytometric analysis, even without antigenic stimulation, a considerable proportion of CD4+ T cells in peripheral blood are already committed to differentiate to either Th1- or Th2-like cells upon cell activation. In an in vivo situation, CD4+ T cells activated by APC back-stimulate APC by secreting lymphokines and expressing various ligand molecules, such as CD40L, and this interaction collectively determines the Th1 or Th2 differentiation of CD4+ T cells. By utilizing irradiated LCD32/CD80 cells as surrogate APC, we controlled the costimulation provided by APC, which may be altered with ageing and have an influence on Th differentiation. We analysed the cytokine production response by T cells to antigenic stimuli. This is important, because cytokines produced in the early stage of antigenic activation determine subsequent Th differentiation [10].
If ageing is associated with a defect in Th1 cell development and subsequent cell-mediated immunity to an intracellular infection, as suggested by the higher frequency of tuberculosis recurrence in the elderly population [1,2], the apparently discrepant results in this study could be explained by the following. First, the present study is not a clonal analysis. Ageing CD4+ T cells may have limitations in their TCR repertoire or possess a limited frequency of antigen-specific T cells. Second, an effective expansion of differentiated Th1 clones may require Th2-like cells. Alternatively, the presence of polyclonal Th1-like cells may inhibit the expansion of antigen-specific Th1 clones. Third, as discussed below, the decline of CMI in the old may be due to a defect in the effector phase of immune response.
CD40L expression by CD4+ T cells would augment the IL-12 expression by an APC [29], which in turn up-regulates IFN-γ secretion by T cells [30]. Therefore, our assay system, which has CD40L but lacks IL-12, could underestimate the capability of CD4+ T cells to produce IFN-γ in the co-culture. Since the interaction of LFA-1 with its counter ligand would activate T cells and promote Th1 development by suppressing Th2 development [25,31], an increase of LFA-1 expression may explain the suppression of IL-4 secretion by CD4+ T cells from the elderly. An augmentation of CD26 expression and almost no induction of CD30 expression in the CD4+ T cells are both compatible with the notion that a net effect of ageing on the commitment of Th differentiation is more likely to produce Th1-like cells than Th2-like cells in elderly subjects [32,33].
Many other factors are also involved in the age-associated functional alterations of cell-mediated immunity. For instance, a recent report indicated a reduction of perforin activity at the cell level in elderly individuals when compared with all age groups [34]. Another study noted a decrease of CD28 in the CD8+ subset of T cells in elderly subjects, suggesting an age-related diminution of cytotoxic T cell responsiveness to mitogenic signals [35]. This may imply a deficiency in anti-viral and anti-tumour immunity in elderly persons. A recent study with ageing mice has also suggested a decrease in the numbers of mature functional natural killer (NK) cells along with age, which may be caused by an impairment in signal transduction [36,37], as well as decreases in the production of lytic enzymes and other cytolytic proteins [38,39] in NK cells. Further, another report showed age-associated phenotypic changes in human monocytes from elderly subjects [40] as well as a decrease of macrophage activation and a failure to enhance cytotoxic activity in old mice. Thus, these results taken together with our study suggest that CD4 T cells in elderly subjects still retain the potential to differentiate to Th1-like cells in vitro. Our study results argue for the possibility that an age-associated decline of CMI may due to a defect in APC–T cell interaction which in turn induces Th1 development in vivo and/or an alteration in immune effector mechanisms, events which are downstream of T cell activation.
Finally, our results differ from some of the previous studies done with SENIEUR and non-SENIEUR protocols, which have shown a decrease of IFN-γ production in elderly subjects [18,19]. However, in contrast to those, a study done with a SENIEUR protocol reported an increase of IFN-γ production in elderly subjects that was normalized by the number of CD3+ T cells [21]. Since these three studies did not separate CD4 T cells and measure IL-4 simultaneously, a direct comparison with ours seems to be difficult. In a study done with old mice, anti-CD3/CD28 stimulated CD4+ T cells produced more IFN-γ than those in young controls, with no changes in IL-4 or IL-2 production [41]. Thus, ageing was not associated with a defect in IFN-γ production in these studies [21,41]. Although both the young and elderly subjects in the present study were younger than those recommended by the SENIEUR protocol [17], we still saw age-associated differences in several parameters with CD4+ T cells. This may correlate with a recent finding that the decline of T cell replacement starts at a much earlier time point than usually considered, and could also explain the accelerated rate of disease progression in older patients with HIV infection [42]. Thus, age-associated functional alterations in CD4+ T cells may precede immunosenescence in the elderly population.
In conclusion, we analysed the effect of ageing on the responsiveness of human CD4+ T cells to polyclonal stimuli. At the time of antigenic stimulation, and subsequent induction of lymphokine secretion and cell adhesion molecule expression, the CD4+ subset in the peripheral blood of aged individuals was found to contain a greater proportion of cells committed to Th1-type cells than that from the young individuals. These results may provide new clues for an understanding of age-associated alterations in cell-mediated immunity.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Science and Culture, Japan, the Japan Society for the promotion of Science (JSPS) and the Dr Shimizu Foundation for the Promotion of Immunology Research Grant. We would like to thank Mr T. Yasuda (Japan Red-Cross Blood Centre, Kyoto) for providing blood samples, and Miss Naoko Sakanashi for her secretarial work.
REFERENCES
- 1.Alvarez S, Shell C, Berk SL. Pulmonary tuberculosis in elderly men. Am J Med. 1987;82:602–6. doi: 10.1016/0002-9343(87)90106-9. [DOI] [PubMed] [Google Scholar]
- 2.Nagami P, Yoshikawa TT. Aging and tuberculosis. Gerontology. 1984;30:308–15. doi: 10.1159/000212650. [DOI] [PubMed] [Google Scholar]
- 3.Vissinga CS, Dirven CJ, Steinmeyer FA, et al. Deterioration of cellular immunity during aging. The relationship between age-dependent impairment of delayed-type hypersensitivity reactivity, interleukin-2 production capacity, and frequency of Thy-1+, Lyt-2− cells in C57BL/Ka and CBA/Rij mice. Cell Immunol. 1987;108:323–34. doi: 10.1016/0008-8749(87)90216-4. [DOI] [PubMed] [Google Scholar]
- 4.Vissinga C, Nagelkerken L, Zijlstra J, et al. A decreased functional capacity of CD4+ T cells underlies the impaired DTH reactivity in old mice. Mech Ageing Dev. 1990;53:127–39. doi: 10.1016/0047-6374(90)90065-n. [DOI] [PubMed] [Google Scholar]
- 5.Davila DR, Edwards CKD, Arkins S, et al. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990;4:2906–11. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- 6.Beharka AA, Wu D, Han SN, et al. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev. 1997;93:59–77. doi: 10.1016/s0047-6374(96)01819-2. [DOI] [PubMed] [Google Scholar]
- 7.Chang MP, Makinodan T, Peterson WJ, et al. Role of T cells and adherent cells in age-related decline in murine interleukin 2 production. J Immunol. 1982;129:2426–30. [PubMed] [Google Scholar]
- 8.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 9.Powrie F, Coffman RL. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today. 1993;14:270–4. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- 10.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 11.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 12.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 13.Hosken NA, Shibuya K, Heath AW, et al. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constant S, Pfeiffer C, Woodard A, et al. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–6. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Prete G, De Carli M, D'Elios MM, et al. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp Med. 1995;182:1655–61. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annunziato F, Manetti R, Cosmi L, et al. Opposite role for interleukin-4 and interferon-gamma on CD30 and lymphocyte activation gene-3 (LAG-3) expression by activated naive T cells. Eur J Immunol. 1997;27:2239–44. doi: 10.1002/eji.1830270918. [DOI] [PubMed] [Google Scholar]
- 17.Ligthart GJ, Corberand JX, Fournier C, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 18.Kita M, Shiozawa S, Yamaji M, et al. Production of human alpha- and gamma-interferon is dependent on age and sex and is decreased in rheumatoid arthritis: a simple method for a large-scale assay. J Clin Lab Anal. 1991;5:238–41. doi: 10.1002/jcla.1860050403. [DOI] [PubMed] [Google Scholar]
- 19.Cakman I, Rohwer J, Schutz RM, et al. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech Ageing Dev. 1996;87:197–209. doi: 10.1016/0047-6374(96)01708-3. [DOI] [PubMed] [Google Scholar]
- 20.Sindermann J, Kruse A, Frercks HJ, et al. Investigations of the lymphokine system in elderly individuals. Mech Ageing Dev. 1993;70:149–59. doi: 10.1016/0047-6374(93)90066-z. [DOI] [PubMed] [Google Scholar]
- 21.Born J, Uthgenannt D, Dodt C, et al. Cytokine production and lymphocyte subpopulations in aged humans. An assessment during nocturnal sleep. Mech Ageing Dev. 1995;84:113–26. doi: 10.1016/0047-6374(95)01638-4. [DOI] [PubMed] [Google Scholar]
- 22.Sakata Kaneko S, Wakatsuki Y, Usui T, et al. Lysophosphatidylcholine upregulates CD40 ligand expression in newly activated human CD4+ T cells. FEBS Letters. 1998;433:161–5. doi: 10.1016/s0014-5793(98)00898-9. [DOI] [PubMed] [Google Scholar]
- 23.Gillis S, Ferm MM, Ou W, et al. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–32. [PubMed] [Google Scholar]
- 24.Hu Li J, Ohara J, Watson C, et al. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S) J Immunol. 1989;142:800–7. [PubMed] [Google Scholar]
- 25.Salomon B, Bluestone JA. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161:5138–42. [PubMed] [Google Scholar]
- 26.Robinson AT, Miller N, Alexander DR. CD3 antigen-mediated calcium signals and protein kinase C activation are higher in CD45RO+ than in CD45RA+ human T lymphocyte subsets. Eur J Immunol. 1993;23:61–68. doi: 10.1002/eji.1830230111. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Kristofic C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO− and CD45RO+ subsets. J Immunol. 1995;155:3322–8. [PubMed] [Google Scholar]
- 28.Stout RD, Suttles J, Xu J, et al. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 29.Mackey MF, Gunn JR, Maliszewsky C, et al. Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161:2094–8. [PubMed] [Google Scholar]
- 30.McDyer JF, Goletz TJ, Thomas E, et al. CD40 ligand/CD40 stimulation regulates the production of IFN-gamma from human peripheral blood mononuclear cells in an IL-12- and/or CD28- dependent manner. J Immunol. 1998;160:1701–7. [PubMed] [Google Scholar]
- 31.Wang J, Lenardo MJ. Essential lymphocyte function associated 1 (LFA-1): intercellular adhesion molecule interactions for T cell-mediated B cell apoptosis by Fas/APO-1/CD95. J Exp Med. 1997;186:1171–6. doi: 10.1084/jem.186.7.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura T, Lee RK, Nam SY, et al. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-gamma. J Immunol. 1997;158:2090–8. [PubMed] [Google Scholar]
- 33.Willheim M, Ebner C, Baier K, et al. Cell surface characterization of T lymphocytes and allergen-specific T cell clones: correlation of CD26 expression with T(H1) subsets. J Allergy Clin Immunol. 1997;100:348–55. doi: 10.1016/s0091-6749(97)70248-3. [DOI] [PubMed] [Google Scholar]
- 34.Rukavina D, Laskarin G, Rubesa G, et al. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood. 1998;92:2410–20. [PubMed] [Google Scholar]
- 35.Boucher N, Dufeu Duchesne T, Vicaut E, et al. CD28 expression in T cell aging and human longevity. Exp Gerontol. 1998;33:267–82. doi: 10.1016/s0531-5565(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 36.Mariani E, Mariani AR, Meneghetti A, et al. Age-dependent decreases of NK cell phosphoinositide turnover during spontaneous but not Fc-mediated cytolytic activity. Int Immunol. 1998;10:981–9. doi: 10.1093/intimm/10.7.981. [DOI] [PubMed] [Google Scholar]
- 37.Borrego F, Alonso MC, Galiani MD, et al. NK phenotypic markers and IL-2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 38.Mariani E, Sgobbi S, Meneghetti A, et al. Perforins in human cytolytic cells: the effect of age. Mech Ageing Dev. 1996;92:195–209. doi: 10.1016/s0047-6374(96)01829-5. [DOI] [PubMed] [Google Scholar]
- 39.Dussault I, Miller SC. Decline in natural killer cell-mediated immunosurveillance in aging mice—a consequence of reduced cell production and tumor binding capacity. Mech Ageing Dev. 1994;75:115–29. doi: 10.1016/0047-6374(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 40.Stohlawetz P, Hahn P, Koller M, et al. Immunophenotypic characteristics of monocytes in elderly subjects. Scand J Immunol. 1998;48:324–6. doi: 10.1046/j.1365-3083.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 41.Engwerda CR, Fox BS, Handwerger BS. Cytokine production by T lymphocytes from young and aged mice. J Immunol. 1996;156:3621–30. [PubMed] [Google Scholar]
- 42.Adler WH, Baskar PV, Chrest FJ, et al. HIV infection and aging: mechanisms to explain the accelerated rate of progression in the older patient. Mech Ageing Dev. 1997;96:137–55. doi: 10.1016/s0047-6374(97)01888-5. [DOI] [PubMed] [Google Scholar]


