Abstract
Infectious mononucleosis (IM), a manifestation of primary infection with EBV, is characterized by a massive expansion of the T cell population. In this study we examined this expanded T cell population regarding its EBV status, its proliferative and apoptotic activity, and its expression of apoptosis-related genes. Whereas previous studies were performed on ex vivo cultures or on peripheral blood, our investigations included in vivo analysis of IM tonsillectomy specimens (14 cases) by in situ hybridization for viral RNA (EBERs) combined with immunohistochemistry (IHC; CD3, CD45RO, CD20, CD79a, Ki-67, Bcl-2, Bax, Fas, FasL) and the TUNEL method. Of the EBER+ cells 50–70% showed expression of the B cell markers CD20/CD79a. The remainder of the EBER+ cells expressed neither B nor T cell antigens. No co-expression of EBERs and T cell antigens was detected in any of the specimens. In accordance with a high rate of apoptosis (up to 2·37%) within the expanded T cell population, Bcl-2 expression was drastically reduced and FasL expression remarkably increased. The levels of Bax and Fas expression showed no or moderate up-regulation. In conclusion, the massive expansion of IM T cells is not caused by EBV infection of these cells but merely represents an intense immune reaction. Through altered expression of Bcl-2/Bax and Fas/FasL, the activated T cells are subject to enhanced apoptosis while residing within the lymphoid tissue, which eventually allows the efficient silencing of this potentially damaging T cell response.
Keywords: infectious mononucleosis, Epstein–Barr virus, T cells, apoptosis
INTRODUCTION
EBV is the causative agent of infectious mononucleosis (IM) and has been strongly linked to the development of endemic Burkitt's lymphoma and other non-Hodgkin's lymphomas, Hodgkin's disease, and several malignant non-lymphoid neoplasias [1].
Since the initial discovery of the virus in Burkitt's lymphoma cell lines, EBV has been known as a B cell lymphotropic virus. To date however controversy exists as to which cellular compartments mediate primary EBV infection. Whereas observations from bone marrow transplant recipients [2] and histopathological analysis of IM tonsils and reactive lymph nodes [3–5] favour primary infection and persistence of EBV in B lymphocytes, recent reports on the association of EBV with T cell proliferation and lymphomas [6–8], together with evidence for in vitro infection of thymocytes by EBV [9], raise the question whether T lymphocytes may be a target of EBV infection. Indeed, during IM there is a massive proliferation of highly activated T cells which may either result from viral T cell infection or merely represent a vigorous immune reaction. Furthermore, recent investigations showed that this T cell proliferation is accompanied by increased susceptibility to apoptosis of T cells in the peripheral blood of IM patients [10,11]. Although down-regulation of Bcl-2 in these T cells was revealed by flow cytometric analysis [12], the in vivo situation in tonsillar tissue has not been investigated before. Therefore, the present study was undertaken to characterize in vivo the T cell population in IM tonsils regarding its EBV status, its proliferative and apoptotic activity, and its expression of apoptosis-related genes (Bcl-2, Bax, Fas, FasL).
MATERIALS AND METHODS
Materials
Formalin-fixed, paraffin-embedded bilateral tonsillectomy specimens of 14 patients with IM were reviewed. The diagnosis of primary EBV infection had been confirmed serologically in all but four patients. The tonsillectomies were performed at the same stage of the disease, as all patients had presented similar clinical symptoms (pharyngitis, cervical lymphadenopathy, absent to mild splenomegaly) for approximately 5 days. Clinical data of the IM patients are shown in Table 1. As controls, eight tonsillectomy specimens of chronic tonsillitis with lymphofollicular hyperplasia were used. None of the 22 patients had evidence of immunodeficiency.
Table 1.
Clinical data of infectious mononucleosis patients
| Case no. | Age/sex | Serology* |
|---|---|---|
| 1 | 17/M | H |
| 2 | 18/M | H |
| 3 | 14/F | VCA |
| 4 | 16/F | ND |
| 5 | 19/M | VCA |
| 6 | 17/F | ND |
| 7 | 15/F | VCA |
| 8 | 22/F | VCA |
| 9 | 34/M | ND |
| 10 | 22/M | H |
| 11 | 10/M | ND |
| 12 | 13/M | VCA |
| 13 | 16/F | VCA |
| 14 | 2/M | H |
H+,Positive detection of heterophilic antibodies (IgM); VCA+, positive detection of antibodies (IgM) against viral capsid antigen; ND, not done.
In situ hybridization
For in situ hybridization (ISH) of viral RNA, FITC-conjugated RNA probes complementary to EBER (EBV Early RNAs; Novocastra, Newcastle upon Tyne, UK) were hybridized to deparaffined sections of the tonsillectomy specimens. Detection was performed by alkaline phosphatase-conjugated rabbit anti-FITC antibody using 5‐bromo‐4‐chloro‐3‐indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) (Novocastra) as the chromogen. The sensitivity of the ISH was tested in two ways: (i) in a case of Hodgkin's disease, known to be EBV+, virtually all Hodgkin and Reed–Sternberg cells were found to be EBER+, and (ii) comparison of the number of EBER+ cells with the number of cells stained positive by immunohistochemistry (IHC; described below) for the viral proteins LMP-1 (Latent Membrane Protein 1) and EBNA2 (EBV Nuclear Antigen 2) showed equivalent results.
TUNEL
Free DNA-ends yielded by apoptosis were identified by TdT (terminal deoxyribonucleotidyl transferase; Promega, Madison, WI; 0·1 U/μl)-mediated tailing with biotin-labelled nucleotides (biotin-16-dUTP; Boehringer, Mannheim, Germany) and subsequent detection via a streptavidin–alkaline phosphatase complex (Jackson ImmunoResearch, West Grove, PA) and fast red (Boehringer) chromogen. All tissue sections were pretreated with proteinase K.
Immunohistochemistry
IHC was performed on paraffin-embedded tissue sections with an alkaline phosphatase-conjugated streptavidin-biotin detection system (Jackson ImmunoResearch) and fast red. The MoAbs and polyclonal antibodies used included CD20, CD79a, CD45RO, Bcl-2, LMP-1, EBNA2 (MoAbs; Dako, Glostrup, Denmark), CD3, CD4, CD8 (MoAbs; Novocastra), Ki-67 (MoAb; Dianova, Hamburg, Germany), Bax (polyclonal antibody; Dako), Fas and Fas ligand (polyclonal antibodies; Santa Cruz Biotechnology, Santa Cruz, CA). Antigen retrieval by standard microwaving was performed for all antibodies except anti-CD45RO and anti-LMP-1. For CD45RO detection, tissue sections were pretreated with pronase. For the negative control, the primary antibody was omitted. The specificity of FasL detection had been tested previously on testicular and placental tissue. For combined detection of proteins and EBER or apoptosis, IHC was performed subsequent to ISH and prior to TUNEL using BCIP/NBT as an additional chromogen in the latter. Double labelling was also performed for Ki-67/LMP-1 and Ki-67/CD3, using the Dako Envision TM+ Doublestain Kit for the latter combination.
Semiquantitative assessment and statistical analysis
Semiquantitative assessment of the proliferative and apoptotic activity, as well as the expression of Bcl-2, CD4 and CD8, was performed by scoring the percentage of labelled cells in at least 5 high-power fields (HPF) (×400). In combined ISH and IHC, the percentage of EBER+ cells co-expressing T or B cell markers was counted throughout the complete section surface.
Statistical correlation of the Bcl-2 score and CD8/CD4 ratio with the number of EBER+ cells was assessed by the Fisher's exact test.
Western blotting
Frozen tissue from three cases (cases 10, 11 and 13) was incubated in lysis buffer (20 mm Tris–HCl buffer pH 7·4; 150 mm NaCl; 1·5 mm EDTA; 3% glycerol; 1 mg/ml bovine serum albumin (BSA); 1% NP40; 1 μg/ml leupeptin; 5 μg/ml aprotinin; 2 μg/ml pepstatin A; 0·5 mm pefabloc) for 30 min at 0°C. SDS–PAGE (12%) and transfer to PVDF membranes (NEN Life Sciences, Boston, MA) was followed by incubation with 250-fold diluted anti-FasL polyclonal antibody. Immunoreaction was visualized by a secondary antibody conjugated to alkaline phosphatase (Jackson ImmunoResearch) and colorimetric detection (NBT/BCIP). Testicular and placental tissue samples were used as positive controls. For the negative control, BSA solution was analysed.
RESULTS
EBER+ cells with the morphology of lymphoid cells of various sizes or of Hodgkin/Reed–Sternberg (H/RS)-like cells, were detected in all cases of IM. All non-lymphoid cells were consistently EBER−. The number of EBER+ cells varied considerably (Table 2). There was no significant correlation between the number of EBER+ cells and the intensity or duration of clinical symptoms.
Table 2.
Results of EBER in situ hybridization, TUNEL reaction (AI), and immunophenotyping for Ki-67 (PI), Bcl-2, CD4, and CD8 in infectious mononucleosis (IM) and control tonsillar tissues
| Case no. | EBER+ cells* | PI† | AI‡ | Bcl-2§ | CD8/CD4¶ |
|---|---|---|---|---|---|
| IM | |||||
| 1 | 63 | 23·20 | 1·31 | 38·20 | 1·29 |
| 2 | 114 | 51·10 | 2·01 | 33·70 | 1·45 |
| 3 | 145 | 38·30 | 1·36 | 39·50 | 1·41 |
| 4 | 184 | 46·20 | 1·37 | 18·80 | 2·01 |
| 5 | 302 | 42·70 | 1·45 | 29·10 | 2·21 |
| 6 | 22 | 28·60 | 1·39 | 58·40 | 0·47 |
| 7 | 25 | 26·90 | 0·71 | 39·40 | 0·54 |
| 8 | 278 | 43·40 | 1·84 | 27·40 | 1·75 |
| 9 | 191 | 37·60 | 1·60 | 36·20 | 1·97 |
| 10 | 483 | 42·00 | 2·14 | 18·20 | 1·65 |
| 11 | 215 | 39·30 | 2·08 | 29·80 | 2·15 |
| 12 | 32 | 32·50 | 1·94 | 35·60 | 1·32 |
| 13 | 378 | 44·00 | 2·10 | 24·30 | 2·16 |
| 14 | 451 | 41·00 | 2·37 | 27·60 | 1·93 |
| Mean value | 206 | 38·34 | 1·69 | 32·58 | 1·59 |
| Control | |||||
| 15 | – | 11·00 | 0·10 | 67·70 | 0·19 |
| 16 | – | 9·70 | 0·25 | 54·10 | 0·21 |
| 17 | – | 9·00 | 0·15 | 42·70 | 0·22 |
| 18 | – | 16·90 | 0·26 | 55·30 | 0·29 |
| 19 | – | 5·40 | 0·30 | 35·40 | 0·28 |
| 20 | – | 9·40 | 0·34 | 44·90 | 0·21 |
| 21 | – | 12·50 | 0·30 | 75·50 | 0·17 |
| 22 | – | 4·90 | 0·27 | 59·80 | 0·18 |
| Mean value | – | 9·85 | 0·25 | 54·43 | 0·22 |
Number of EBER + cells per 10 000 lymphocytes.
Proliferation index, assessed exclusively in interfollicular T cells.
Apoptosis index, assessed exclusively in the interfollicular T cell zones.
Percentage of Bcl-2-expressing cells, counted exclusively in the interfollicular T cell zones.
Ratio of CD8- to CD4-expressing cells, counted exclusively in the interfollicular T cell zones.
EBER+ cells were not homogeneously distributed in the tonsillar tissue but were most frequently found adjacent to the crypts: within the crypt epithelium, in the subepithelial lymphoid tissue, and less numerously in the superficial interfollicular zones (Fig. 1). Only few EBER+ cells were found within the deeper interfollicular zones, remaining lymph follicles or peritonsillar soft tissue. In control tonsils (5/8) only very few, solitary EBER+ lymphoid cells of small to moderate size (< 3/section) were observed.
Fig. 1.
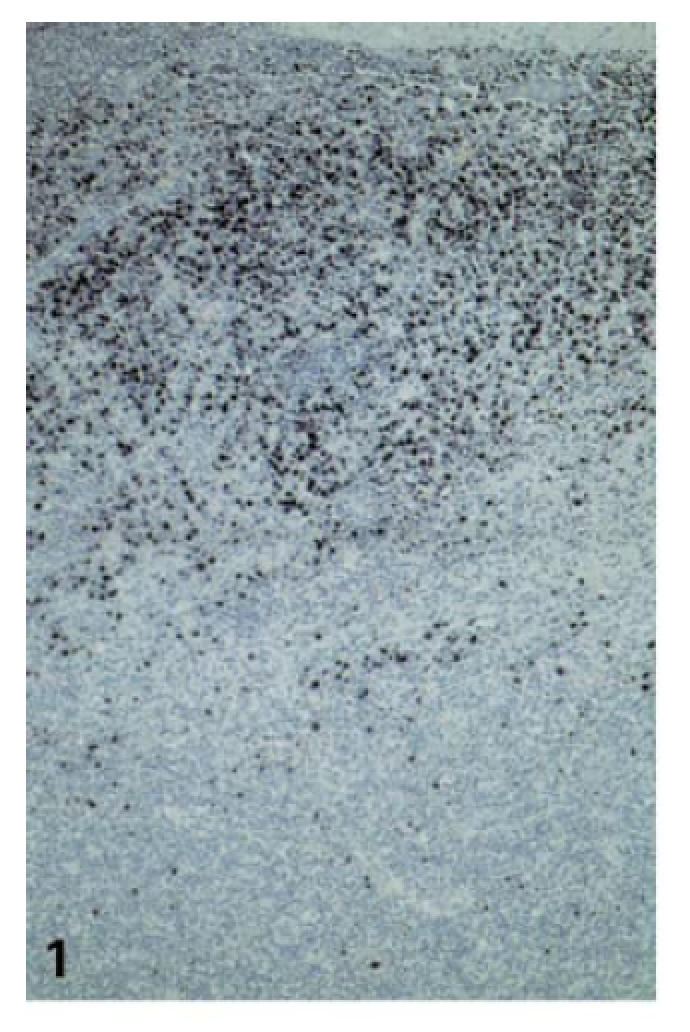
Numerous EBER+ cells with large, often bizarre nuclei within and underneath the crypt epithelium as well as in superficial interfollicular zones (case no. 2; ISH for EBER, ×100).
More than 50%, in some cases up to 70%, of all EBER+ cells expressed CD20/CD79a, independent of their morphology and size (Fig. 2). In no case could EBER+ cells co-expressing either CD3 or CD45RO be detected. Of the EBER+ 30% to <50% cells expressed neither CD20/CD79a nor CD3/CD45RO.
Fig. 2.
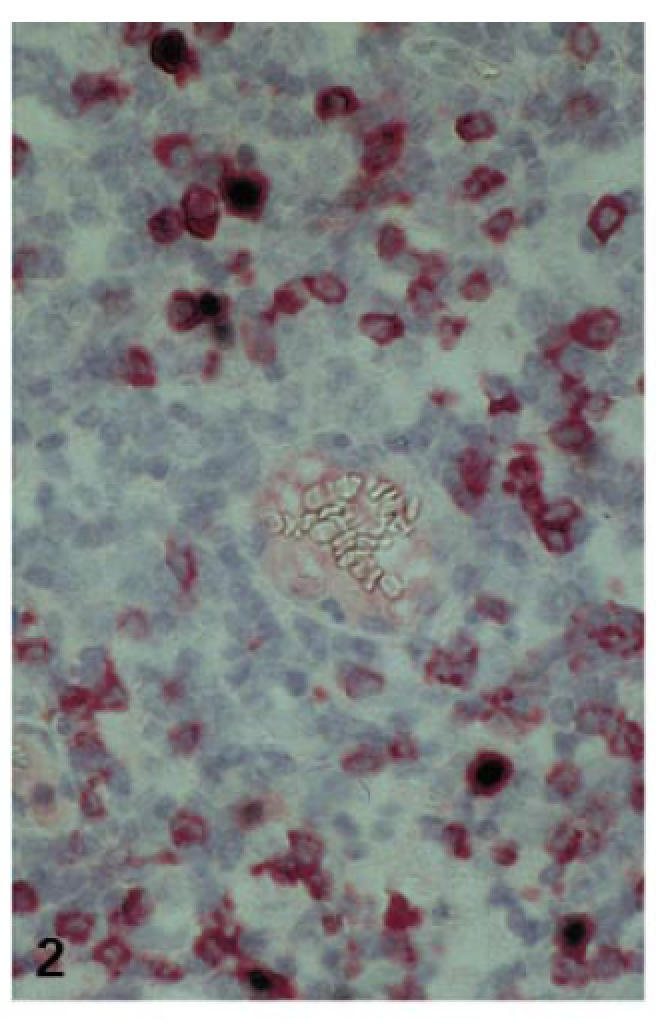
Expression of CD79a in EBV-infected lymphoid cells (case no. 2; combined ISH for EBER (NBT/BCIP) and IHC for CD79a (fast red); ×400).
Massive expansion of the interfollicular zones with progressive disappearance of pre-existent lymph follicles was due to dramatic activation and blastic transformation of EBER− or LMP− interfollicular cells (Fig. 3). Ki-67/CD3 double-labelling showed that most of the highly proliferating interfollicular cells were of a CD3+/CD45RO+ phenotype, corresponding to activated T cells. Their proliferative activity reached a mean proliferation index (PI) of 38·3%, which was significantly higher than that found within the interfollicular zones of control tonsils (PI 9·9%; Table 2; Figs 4 and 5). Immunostaining for CD4 and CD8 revealed a disproportionate T cell expansion as the CD8/CD4 ratio rose more than three-fold, especially in cases with many EBER+ cells (Table 2).
Fig. 3.
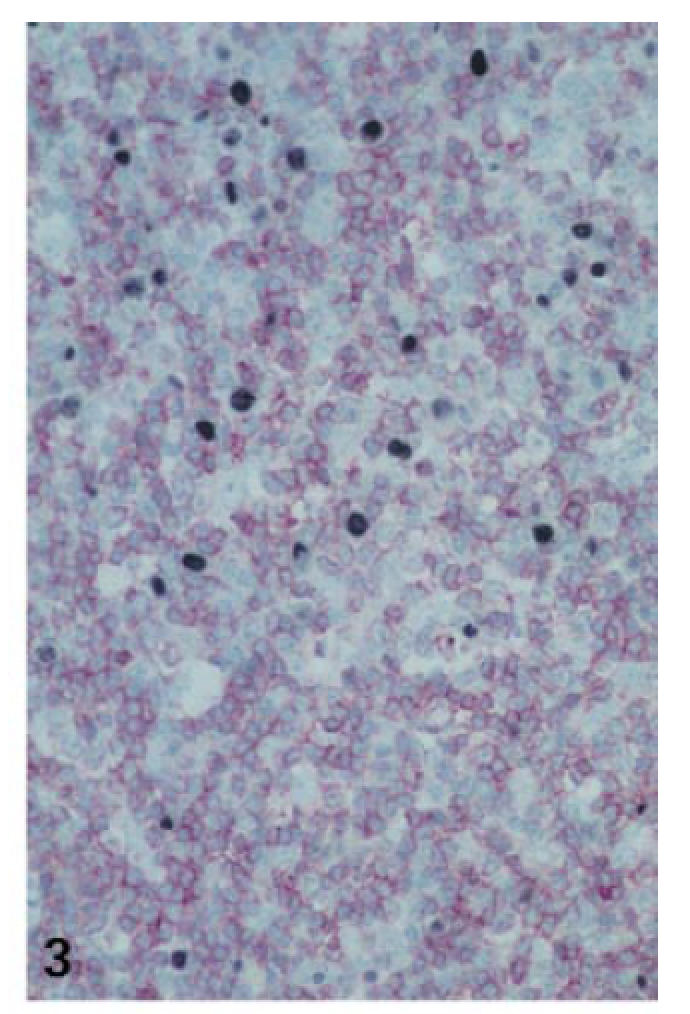
Expanded T zone underneath the EBV-infected cells close to the crypt surface (case no. 6; combined ISH for EBER (NBT/BCIP) and IHC for CD3 (fast red); ×200).
Fig. 4.
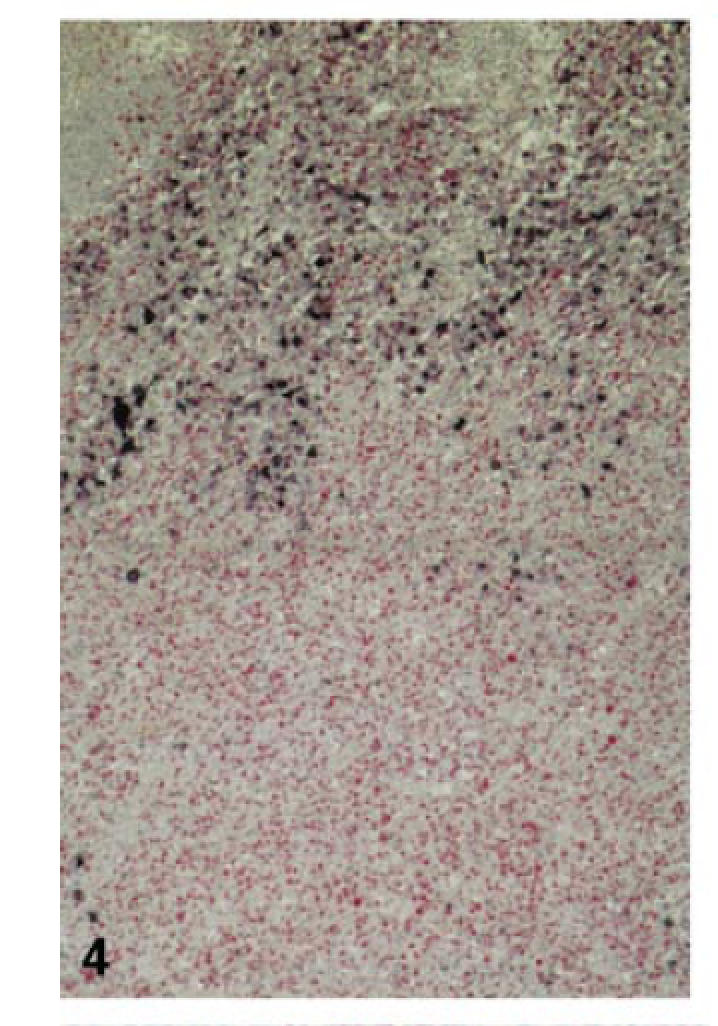
High proliferative activity in EBV− cells of the expanded T zone (case no. 2; double labelling for LMP (NBT/BCIP) and Ki-67 (fast red); × 100).
Fig. 5.
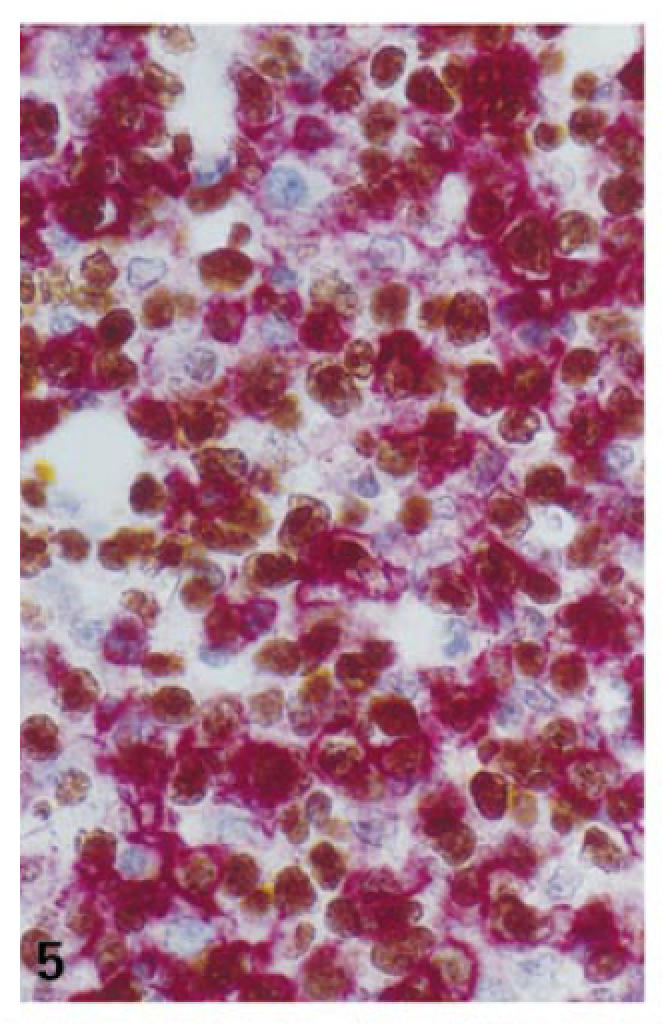
High proliferative activity in T cells of the expanded interfollicular zones (case no. 2; double labelling for Ki-67 (DAB) and CD3 (fast red); ×400).
Although the extent of necrosis was variable even among cases with high viral load, the apoptotic activity was constantly very high throughout the expanded interfollicular zones. The apoptosis index (AI) reached values up to 2·37% (mean 1·69%) and was thus comparable to the apoptotic activity in florid germinal centres of control tonsillar tissue (AI in germinal centres 1·8%; in interfollicular zones 0·3%; Table 2; Fig. 6).
Fig. 6.
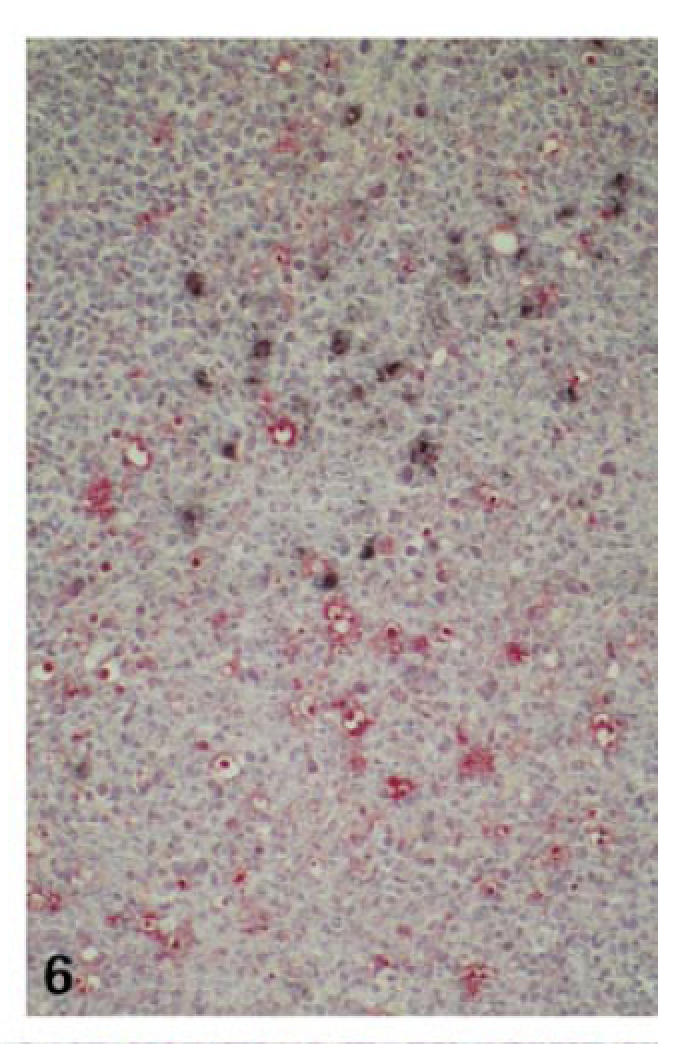
High apoptotic activity of EBV− interfollicular cells (case no. 6; combined TUNEL (fast red) and IHC for LMP (NBT/BCIP); ×100).
Compared with the T cells in control tonsils, the expression of Bcl-2 in the activated T cells of IM was markedly decreased (54·4% versus 32·6%). Down-regulation of Bcl-2 expression seemed to be time-dependent, since Bcl-2 expression was significantly lower in cases with abundant EBER+ cells (25·9%) than in tonsils with little viral load (cases 1, 6, 7 and 12: 42·9%; P < 0·015) (Table 2). In contrast, Bcl-2 expression within remaining lymph follicles in IM was not altered (Fig. 7).
Fig. 7.
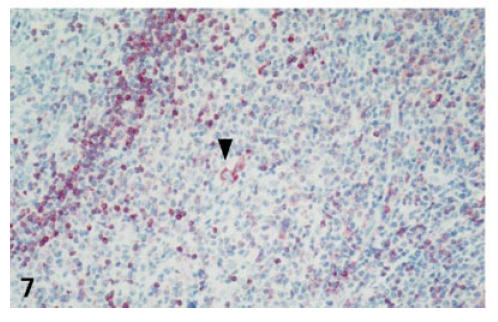
Down-regulation of Bcl-2 expression in interfollicular cells. Pre-existent small secondary lymph follicle (upper left corner) with strong Bcl-2 expression in the follicle mantle. Strong Bcl-2 expression in a single Hodgkin/Reed–Sternberg (H/RS)-like cell, compatible with an EBV-infected lymphocyte (arrowhead) (case no. 4; IHC for Bcl-2; ×200).
The level of Bax expression was not, or only slightly, increased in the interfollicular T zones of IM compared with control tonsils. H/RS-like cells in the vicinity of the tonsillar crypts showed weak to moderate Bax and strong Bcl-2 expression (not shown).
In accordance with previous reports [13], Fas expression was detected in germinal centre cells but not in mantle cells of control lymphoid tissues. In IM specimens, a moderate increase in staining intensity as well as in the number of interfollicular cells expressing Fas could be observed in all except two cases (nos 7 and 12). H/RS-like cells, representing EBV-infected lymphocytes, exhibited moderate Fas expression (not shown).
In all IM cases except nos 7 and 12, FasL IHC produced a very strong membranous signal in many lymphoid cells of the interfollicular zone (Fig. 8). As we described before [14], pre-existent lymph follicles showed a characteristic meshwork-like expression pattern within their germinal centres. In some H/RS-like cells a very strong membranous signal for FasL was found (Fig. 8, inset). In contrast, no significant positivity was seen in the interfollicular areas of control tonsils.
Fig. 8.
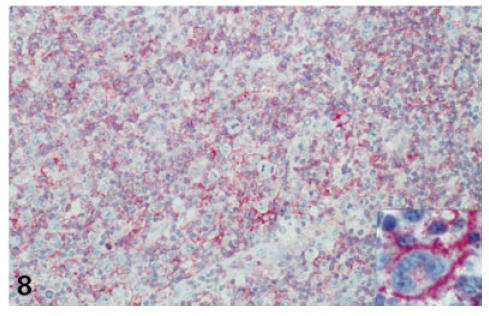
Strong membranous expression of FasL in activated, partially blast-like transformed interfollicular T cells. Inset: strong FasL expression in EBV-infected cells with Hodgkin/Reed–Sternberg (H/RS)-like morphology (case no. 11; IHC for FasL; ×200, inset ×400).
Western blot analysis of FasL expression in IM tonsils produced in all samples including the positive controls a protein band of 33 kD, compatible with membrane-bound FasL [15]. In addition, a protein band of 52 kD, probably representing dimeric soluble FasL [15], was detected in testis lysate. Negative control samples showed no protein bands (Fig. 9).
Fig. 9.
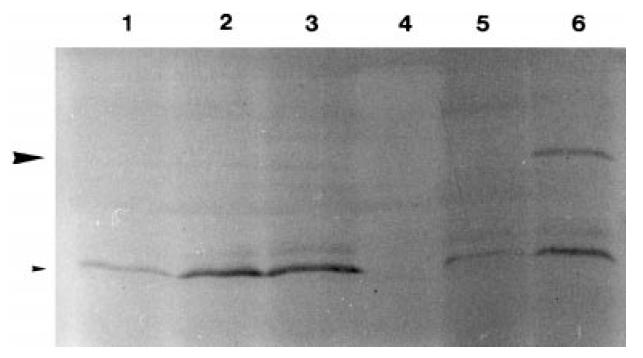
Western blot analysis of FasL expression: protein bands of 33 kD (small arrowhead) in infectious mononucleosis (IM) tonsils and an additional band of 52 kD (large arrowhead) in control testis (lanes 1–3, IM tonsil (cases 10, 11, 13); lane 4, negative control; lanes 5 and 6, positive control (5, placenta; 6, testis)).
DISCUSSION
IM represents a benign self-limiting lymphoproliferative disorder characterized by primary EBV infection of B lymphocytes and massive proliferation of activated T cells. Whether T cells are also a target of EBV infection or whether their massive expansion solely represents an immune reaction with activation and subsequent rapid elimination of T lymphocytes is controversial.
In our study we found no evidence of EBV infection in T cells. A number, varying from >50% to 70%, of the EBER+ cells showed expression of the B cell markers CD20/CD79a. The remainder of the EBER+ cells (30% to <50%) expressed neither CD20/CD79a nor CD3/CD45RO. The lineage allocation of these cells could thus not be determined definitively by our investigations. Two observations indicate that these cells may represent B cells, however: (i) former studies were able to label these cells with antibodies detecting B cellular or plasmocytoid differentiation antigens [4,5]; and (ii) a selective down-regulation of several B cellular markers including CD20 by EBV infection of (neoplastic) B cells has been observed in vitro and in vivo[16,17].
These data are in line with our observation that EBV+ T cells do not occur in IM. Hence, our findings imply that B cells represent the target of primary EBV infection and that EBV infection of T cells is at least an unusual event in IM. Previous studies addressing the possibility of EBV infection of T cells in IM have led to conflicting results, however. Some authors observed expression of T cell antigens in EBV-infected cells [18], the numbers of EBV+ T cells varying from very few in IM tonsils of Caucasians [5] up to 66% in Japanese individuals [19]. These discrepant findings may indicate that geographical and/or racial factors are possibly involved, as has been suggested for the different incidence of EBV association in peripheral (non-nasal) T cell lymphoma in Caucasians and Orientals [20,21]. Very recently however EBV was detected more frequently in reactive B cells within peripheral T cell lymphomas than in the neoplastic T cell population itself [22,23]. This interesting observation casts doubt on primary EBV infection of non-neoplastic T lymphocytes and infers the possibility of a secondary infection of neoplastic T cells.
Thus, primary EBV infection targets B cells and is accompanied by a prominent reactive expansion of CD45RO+ T cells, involving the cytotoxic CD8+ subset especially. This intense T cell proliferation is accompanied by a striking increase in apoptotic activity in the expanded T zones, which we found to be comparable to that in germinal centres. This observation implies that primed T cells in IM are eliminated at a high rate by apoptosis while residing within the lymphoid tissue. These findings are in line with experiments which reported on (i) high susceptibility of IM T cells to apoptosis after short-term culture ex vivo[24]; and (ii) in vivo apoptosis of T cells in the peripheral blood of IM patients [10,11]. The latter reports detected lower apoptotic rates in peripheral blood T cells of IM patients (0·6% [11]) than we observed within the tonsillar lymphoid tissue (up to 2·37%), however, which infers that the major T cell elimination takes place within the lymphoid tissue and that only a smaller proportion of the viable T cells leaving the lymphoid tissue later die through apoptosis while circulating in the peripheral blood. Thus, our observation of apoptosis of T cells residing within the lymphoid tissue is in contrast to the hypothesis that apoptosis arises when T cells leave the local lymphoid tissue, an area which actively produces soluble factors required for their survival [24]. Furthermore, as EBV-infected T cells were not detected, apoptotic T cell death is more likely to result from activation-induced cell death (AICD [25]) than from viral infection.
The large-scale T cell expansion followed by rapid cell death during primary EBV infection is thought to prevent immunopathogenesis caused by the persistence of activated T cells and to ensure the selection of the most appropriate candidates for immunological memory. The mechanisms of regulated death by apoptosis and selective survival of T cells after resolution of acute disease are unknown. In contrast to former studies which used IM T cells from peripheral blood or ex vivo cultures [12,26,27], we analysed the in vivo expression of apoptosis-related genes in the expanded T cell population in situ.
We observed that in conjunction with the progression of the viral infection and concomitant T cell activation, Bcl-2 expression was drastically reduced in the T cell population, as the Bcl-2 level correlated inversely with the number of EBER+ cells and the CD8/CD4 ratio (P < 0·06, P < 0·01, respectively). This observation confirms previous reports of Bcl-2 down-regulation in expanded CD45RO+ T cell populations after prolonged in vitro stimulation [26] or in peripheral blood T cells of patients with IM [12]. Unlike Bcl-2, expression of Bax was virtually unaltered. As the overall effect on cell survival depends on the Bcl-2/Bax ratio, the remarkable decrease in the level of Bcl-2 expression in combination with the minimal increase in Bax expression in IM T cells implies a switch towards cell death. A similar, quasi unaltered expression of Bax in short-term cultures of peripheral blood T cells during acute infections with other human herpesviruses has been reported before [27]. Among the cytokines playing a major role in preventing activated T cell apoptosis the main candidates are the IL-2 receptor (IL-2R) common γ chain (γc) family [28,29] and type I interferons (IFN) [30,31]. The former regulate T cell survival and apoptosis by co-ordinating the balance between pro-apoptotic (Bax, Bcl-xS) and anti-apoptotic (Bcl-2, Bcl-xL) genes. The decreased Bcl-2 expression and increased apoptosis in the IM T cell population as observed in the current study may result from inhibition of survival signal transduction through the γc pathway, since down-regulation of IL-2R on activated IM T cells has been described before [32,33].
Experiments with Bcl-2 transgenic mice however revealed unaffected T cell apoptosis during acute viral infections, indicating that other routes of cell death may be operative [34]. In this study we found a moderate increase in Fas expression and a remarkable increase in the expression of the ligand FasL in the T cell zones during IM. The up-regulation of Fas on T cells during acute herpesvirus infection has been documented previously by ex vivo analysis [27,35]. To the best of our knowledge however the in vivo expression of FasL by T cells during the resolution of primary EBV infection has not been described before. Western blot analysis revealed the expression of membrane-bound and dimeric soluble FasL protein forms which are both biologically active [15]. Thus, Western blot and IHC findings infer that the Fas/FasL system is involved in the extensive T cell apoptosis in IM, as has been suggested before by in vitro studies highlighting the critical role of Fas and FasL in T cell AICD [36,37]. Very recently type I IFNs were shown to rescue activated T cells from Fas-induced apoptosis [38,39] by a pathway distinct from that of the γc family [30]. As these cytokines are efficiently produced during a viral infection, they may to some extent counteract Fas-mediated AICD and play an important role in the generation and maintenance of immunological memory.
Taken together, our in vivo investigations in IM tonsils support the concept that the massive expansion of T cells is not caused by EBV infection of these cells but rather results from an intense cytotoxic immune reaction. The enhanced apoptosis of activated IM T cells seems to be regulated by multiple mechanisms, including a decrease of the Bcl-2/Bax ratio and an up-regulation of FasL and to a lesser extent of Fas. These apoptosis-inducing mechanisms impose constraints on the survival of activated T cells after IM and may thus act as a safeguard against chronic immune aggression. Further studies are needed to elucidate the triggers of this altered gene expression and to address the potential role of the γc family in the establishment of an effective immune response and specific immunological memory by the regulation of T cell death and survival in IM.
Acknowledgments
We are grateful to B. Hein for expert technical assistance and to U. Ackermann for photographic work. This work was supported by the Tumorzentrum Heidelberg/Mannheim (FSP I/I.4).
REFERENCES
- 1.Liebowitz D, Kieff E. Epstein-Barr virus. In: Roizman B, Whitley RJ, Lopez C, editors. The human herpesviruses. New York: Raven Press; 1993. pp. 107–72. [Google Scholar]
- 2.Gratama JW, Oosterveer MAP, Zwaan FE, Lepoutre J, Klein G, Ernberg I. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc Natl Acad Sci USA. 1988;85:8693–6. doi: 10.1073/pnas.85.22.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niedobitek G, Hamilton-Dutoit S, Herbst H, Finn T, Vetner M, Pallesen G, Stein H. Identification of Epstein-Barr virus-infected cells in tonsils of acute infectious mononucleosis by in situ hybridization. Hum Pathol. 1989;20:796–9. doi: 10.1016/0046-8177(89)90075-0. [DOI] [PubMed] [Google Scholar]
- 4.Niedobitek G, Agathanggelou A, Herbst H, Whitehead L, Wright DH, Young LS. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J Pathol. 1997;182:151–9. doi: 10.1002/(SICI)1096-9896(199706)182:2<151::AID-PATH824>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–50. [PubMed] [Google Scholar]
- 6.Jones JF, Shurin S, Abramowsky C, et al. T-cell lymphoma containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infection. N Engl J Med. 1988;318:733–41. doi: 10.1056/NEJM198803243181203. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi H, Miyashita T, Herbst H, et al. Epstein-Barr virus-infected T lymphocytes in Epstein-Barr virus-associated hemophagocytic syndrome. J Clin Invest. 1993;92:1444–50. doi: 10.1172/JCI116721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallesen G, Hamilton-Dutoit SJ, Zhou X. The association of Epstein-Barr virus (EBV) with T cell lymphoproliferations and Hodgkin's disease: two new developments in the EBV field. Adv Cancer Res. 1994;62:179–239. doi: 10.1016/s0065-230x(08)60319-x. [DOI] [PubMed] [Google Scholar]
- 9.Kelleher CA, Dreyfus DH, Jones JF, Gelfand EW. EBV infection of T cells: potential role in malignant transformation. Sem Canc Biol. 1996;7:197–207. doi: 10.1006/scbi.1996.0027. [DOI] [PubMed] [Google Scholar]
- 10.Fisher MMS, Guerra CG, Hickman JR, Hensley RE, Doe RH, Dunn CD, Hall RB. Peripheral blood lymphocyte apoptosis. A clue to the diagnosis of acute infectious mononucleosis. Arch Pathol Lab Med. 1996;120:951–5. [PubMed] [Google Scholar]
- 11.Matsuoka M, Hamada K, Saika T, Mizobuti N, Takahashi I. Apoptosis in infectious mononucleosis. Rinsho-Ketsueki. 1997;38:727–33. [PubMed] [Google Scholar]
- 12.Tamaru Y, Miyawaki T, Iwai K, Tsuji T, Nibu R, Yachie A, Koizumi S, Taniguchi N. Absence of bcl-2 expression by activated CD45RO+ T lymphocytes in acute infectious mononucleosis supporting their susceptibility to programmed cell death. Blood. 1993;82:521–7. [PubMed] [Google Scholar]
- 13.Leithäuser F, Dhein J, Mechtersheimer G, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993;69:415–29. [PubMed] [Google Scholar]
- 14.Verbeke CS, Wenthe U, Zentgraf H. Fas ligand expression in the germinal centre. J Pathol. 1999;189:155–60. doi: 10.1002/(SICI)1096-9896(199910)189:2<155::AID-PATH442>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–35. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai MC, Jiwa NM, Horstman A, et al. Decreased expression of cellular markers in Epstein-Barr virus-positive Hodgkin's disease. J Pathol. 1994;174:49–55. doi: 10.1002/path.1711740108. [DOI] [PubMed] [Google Scholar]
- 17.Garnier J-L, Cooper NR, Cannon MJ. Low expression of CD20 and CD23 in Epstein-Barr virus-induced B cell tumors in SCID/hu mice. Am J Pathol. 1993;142:353–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgarten E, Herbst H, Schmitt M, Seeger KH, Schulte-Overberg U, Henze G. Life-threatening infectious mononucleosis: is it correlated with virus-induced T cell proliferation? Clin Infect Dis. 1994;19:152–6. doi: 10.1093/clinids/19.1.152. [DOI] [PubMed] [Google Scholar]
- 19.Tokunaga M, Uemura Y, Tokudome T, Sato E. Epstein-Barr virus-infected T cells in infectious mononucleosis. Acta Pathol Japon. 1993;43:146–7. doi: 10.1111/j.1440-1827.1993.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostopoulos I, Hummel M, Stein H. Frequent presence of latent Epstein-Barr virus infection in peripheral T cell lymphomas. A review. Leuk Lymph. 1995;19:1–12. doi: 10.3109/10428199509059657. [DOI] [PubMed] [Google Scholar]
- 21.Zhou XG, Hamilton-Dutoit S, Yan QH, Pallesen G. High frequency of Epstein-Barr virus in Chinese peripheral T-cell lymphoma. Histopathology. 1994;24:115–22. doi: 10.1111/j.1365-2559.1994.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 22.Ho JWY, Ho FCS, Chan ACL, Liang RHS, Srivastava G. Frequent detection of Epstein-Barr virus-infected B cells in peripheral T-cell lymphomas. J Pathol. 1998;185:79–85. doi: 10.1002/(SICI)1096-9896(199805)185:1<79::AID-PATH52>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Hojo I, Takanashi M, Hirai K, Mori S. Increased number of Epstein-Barr virus latently infected B-cells in T-cell non-Hodgkin's lymphoma tissues. Arch Virol. 1995;140:1419–26. doi: 10.1007/BF01322668. [DOI] [PubMed] [Google Scholar]
- 24.Uehara T, Miyawaki T, Ohta K, Tamaru Y, Yokoi T, Nakamura S, Taniguchi N. Apoptotic cell death of primed CD45RO+ T lymphocytes in Epstein-Barr virus-induced infectious mononucleosis. Blood. 1992;80:452–8. [PubMed] [Google Scholar]
- 25.Russell JH, White CL, Loh DY, Meleedy-Rey P. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proc Natl Acad Sci USA. 1991;88:2151–5. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbar AN, Borthwick N, Salmon M, et al. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993;178:428–38. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borthwick NJ, Bofill M, Hassan I, Panayiotidis P, Janossy G, Salmon M, Akbar AN. Factors that influence activated CD8 (+) T cell apoptosis in patients with acute herpesvirus infections: loss of costimulatory molecules CD28, CD5 and CD6 but relative maintenance of Bax and Bcl-x expression. Immunology. 1996;88:508–15. [PMC free article] [PubMed] [Google Scholar]
- 28.Akbar AN, Borthwick NJ, Wickremasinghe RG, et al. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol. 1996;26:294–9. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 29.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–5. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilling D, Akbar AN, Girdlestone J, et al. Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–50. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt C, Burman K, Moss DJ, Ryan J, Burrows SR, Misko IS. Interleukin-2 receptors in infectious mononucleosis. Immunol Letters. 1989;23:139–42. doi: 10.1016/0165-2478(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 33.Ebihara T, Sakai N, Koyama S. CD8+ T cell subsets of cytotoxic T lymphocytes induced by Epstein-Barr virus infection in infectious mononucleosis. Tohoku J Exp Med. 1990;162:213–24. doi: 10.1620/tjem.162.213. [DOI] [PubMed] [Google Scholar]
- 34.Razvi ES, Jiang Z, Woda BA, Welsh RM. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and bcl-2 transgenic mice. Am J Pathol. 1995;157:79–91. [PMC free article] [PubMed] [Google Scholar]
- 35.Ito M, Watanabe M, Ihara T, Kamiya H, Sakura M. Fas antigen and bcl 2 expression on lymphocytes cultured with cytomegalovirus and varicella zoster virus antigen. Cell Immunol. 1995;160:173–7. doi: 10.1016/0008-8749(95)80024-d. [DOI] [PubMed] [Google Scholar]
- 36.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T cell suicide mediated by APO-1 (Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 37.Ju S-T, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 38.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–50. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 39.Scheel-Toellner D, Pilling D, Akbar AN, Hardie D, Lombardi G, Salmon M, Lord JM. Inhibition of T cell apoptosis by IFN-beta rapidly reverses nuclear translocation of protein kinase C-delta. Eur J Immunol. 1999;29:2603–12. doi: 10.1002/(SICI)1521-4141(199908)29:08<2603::AID-IMMU2603>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]


