Abstract
The purpose of this study was to characterize the clinical features and components of 30 patients with isolated cryofibrinogen (CF) versus those of 19 patients with combined CF and cryoglobulins (CG). Secondary forms of cryofibrinogenaemia associated with collagen disorders, infectious or malignant diseases, were significantly more frequent in patients with combined CF and CG than those with isolated CF (79 versus 47%, P = 0·02). Both groups of CF patients presented predominantly cutaneous symptoms (77% in isolated CF; 58% in combined CF + CG), and less frequently venous and/or arterial thrombosis (13% in isolated CF; 3% in combined CF + CG). Patients with idiopathic forms of CF, and particularly those without CG, suffered essentially from recurrent painful skin ulcers, mainly triggered by cold exposure. Patients with isolated CF had higher mean plasma concentrations of CF than those with combined CF + CG (1·61 ± 1·26 versus 0·82 ± 1·18 g/l, respectively; P = 0·004), but there was no correlation between the CF plasma level and either the severity of symptoms or the sensitivity to cold. In patients with isolated CF, fibronectin was suggested (by precipitation analysis) to be a major component of the cryoprecipitate, whereas immunoglobulins were rarely present (in only three out of 30 patients). By contrast, in the majority of patients (78%) with combined CF and CG, the CF consisted mainly of immunoglobulins of the same class as those characterizing the associated CG. Analysis of the CG precipitate revealed the presence of fibronectin but not fibrinogen, α1-antitrypsin and α2-macroglobulin. In conclusion, isolated and combined cryofibrinogenaemia are associated with different clinical signs requiring different clinical management, but there is no evidence as yet for a causal role of the cryoprecipitates in the differences observed.
Keywords: cryofibrinogenaemia, cryofibrinogen, cryoglobulinaemia
INTRODUCTION
Cryofibrinogen (CF), originally characterized in 1955 by Korst & Kratochvil [1], refers to the presence of a cryoprecipitate in plasma that has been anti-coagulated with oxalate, citrate, or edetic acid. This cryoprecipitate is composed of a complex including: fibrin, fibrinogen, fibrin split products with albumin, fibronectin (cold-insoluble globulin), factor VIII, immunoglobulins and other plasma proteins [2]. CF precipitates in plasma cooled to 4°C and redissolves upon warming to 37°C. CF is consumed in the clotting process and therefore does not precipitate in cooled serum [1]. This is in contrast to cryoglobulins (CG), which precipitate in both cooled plasma and serum.
Cryofibrinogenaemia (the presence of CF in blood) may be primary (i.e. idiopathic or essential) [3–5] or secondary to an underlying disorder including: carcinoma, acute infections, inflammatory processes, collagen vascular or thromboembolic diseases, and other miscellaneous conditions such as chronic lung disease, acute myocardial infarction, and hypothyroidism [2,3,6–10]. CF itself is often asymptomatic [7]; however, cold intolerance, thrombotic and/or haemorrhagic phenomena, purpura, skin necrosis, ulcers and gangrene have been reported [4,11], as well as systemic manifestations (arthralgias, glomerulonephritis) [12,13]. In addition to cold exposure avoidance, cryofibrinogenaemia patients may be treated with numerous drugs, including heparin, warfarin, streptokinase, anabolic steroids, immunosuppressive therapies, and plasmapheresis, all with varying degrees of success [3,4,14–16]. Although it is generally agreed that CF is produced in a diverse group of diseases, the mechanism of production and whether it is actually pathogenic remain unclear.
The present study was designed to determine: (i) the relative frequency of CF and/or CG in patients either presenting signs commonly ascribed to cryoproteinaemia or referred for an evaluation of unexplained miscellaneous disorders compared with healthy adult subjects; (ii) the relative frequencies of CF alone (isolated CF) versus combined CF and CG in CF patients; (iii) the disease categories with which CF is associated; (iv) the clinical features of patients of both groups; and lastly (v) the CF components in both groups.
PATIENTS AND METHODS
Healthy subjects
Sera and plasma from 72 healthy blood donors were collected to establish the control range for CG and CF.
Patients
Between February 1990 and February 1994, 220 consecutive patients, referred to our department of Internal Medicine for an evaluation of symptoms commonly ascribed to cryoproteinaemia (cold intolerance, recurrent arterial or venous thrombosis, purpura, skin necrosis, skin ulcers, or other miscellaneous conditions), were screened for CF and CG. The patients were then followed up until August 1997. Clinical and biological data were collected retrospectively from their medical files.
Analysis of cryoproteins and plasma fibrinogen
Using prewarmed equipment, 10 ml of blood were collected into either anti-coagulant-free tubes for CG detection or into citrated tubes for CF detection. Both sera (for CG screening) and plasma (for CF screening) were prepared by centrifugation at 2000 g for 30 min at 37°C, an antiseptic (sodium azide, 0·1 g/l) added, the sera/plasma chilled to 4°C for 8 days and finally analysed for precipitate or gel formation. By warming to 37°C we checked the reversible nature of the CF precipitate before proceeding with a new precipitation at 4°C and purification. CG and CF were isolated by centrifugation (2000 g for 15 min) and then extensively washed in a minimal volume of 0·15 m NaCl pH 7·4. A fraction of the washed CG and CF was redissolved in 0·1 m NaOH and the absorbance was read in a spectrophotometer at 280 nm. Quantification of CG was calculated according to a standard curve (obtained using a purified human gamma globulin preparation supplied by the Centre National de Transfusion Sanguine, Paris, France). Similarly a purified fibrinogen preparation was used for CF quantification.
Characterization of purified cryoprecipitate components was performed by Western blotting after thin-layer agarose electrophoresis as previously described [17]. Briefly, CG and CF, at a concentration of 0·2 mg/ml in 0·15 m NaCl pH 7·4, were separated by electrophoresis at 37°C on thin-layer agarose gels (Paragon; Beckman, Gagny, France), and transferred onto nitrocellulose sheets (Schleicher & Schull, Dassel, Germany; BA85; 0·45 μ m) by pressure blotting (pressure of 1 kg and then 5 kg/cm2, each for 5 min). The blots were then oven-dried. After saturation with 50 mg/ml skimmed milk (Régilait, Lyon, France) in Tris-buffered saline−0·1% Tween 20 (pH 7·4) for 1 h at 37°C, the blots were probed with polyclonal antibodies specific for either fibrinogen (rabbit antibodies, diluted 1:3000), the light and heavy chains of human immunoglobulin (goat antibodies, diluted 1:1000) or fibronectin, α1-antitrypsin and α2-macroglobulin (sheep antibodies) (Sebia, Issy les Moulinaux, France). After four 5-min washes in 0·15 m NaCl−0·1% Tween 20, the strips were incubated for 30 min with a 10 000-fold dilution of the appropriate detection antibody (i.e. anti-rabbit, anti-goat or anti-sheep antibody labelled with alkaline phosphatase) (EC 3.1.3.1; Jackson ImmunoResearch Labs Inc., West Grove, PA). Following further washes, enzyme activity was revealed using the appropriate substrate (5‐bromo‐4‐chloro indoxyl phosphate/nitroblue tetrazolium (BCIP/NBT); Sigma, St Louis, MO), prepared just before use. Concentrations as low as 20 μg/ml of a monoclonal immunoglobulin of the various isotypes (IgG, IgA, IgM) were easily detected, as were other studied proteins (fibrinogen, fibronectin, α1-antitrypsin, α2-macroglobulin).
We determined the plasma fibrinogen level by the clotting rate method of Von Clauss [18] and plasma α1-antitrypsin, α2-macroglobulin and fibronectin levels were detected by the nephelemetric method (BNA; Behring, Marburg, Germany).
RESULTS
CG were detected in the sera of two of the 72 controls (2·8%) at a concentration of 0·01 g/l and 0·03 g/l. The immunoglobulin composition of the CG isolated from these two controls was found to be polyclonal IgG + IgA + IgM in the first case and oligoclonal IgM in the second case. Accordingly, CG levels >0·05 g/l were used as the criterion of CG positivity. CF in association with fibrinogen was detected in the plasma of five of the 72 controls (7%), three of which were polyclonal IgA + IgG + IgM and two were polyclonal IgM. The mean protein concentration was 0·023 ± 0·005 g/l. Therefore, levels >0·05 g/l were used as the criterion for CF positivity.
Among the 220 patients, plasma and sera analysis revealed the presence of: 37 cases (16·8%) of isolated CF, 10 cases (4·5%) of isolated CG and 23 cases (10·5%) of combined CF + CG. Among the 60 CF-positive patients only 49 patients had sufficient clinical and biological information available to warrant further investigation; of these 49 patients, 30 had isolated CF and 19 combined CF + CG.
Clinical features
Tables 1 and 2 describe the clinical features, treatment and outcome of the subjects with isolated CF or combined CF + CG. The mean age of the patients with isolated CF (47·5 ± 18 years) was similar to that noted in patients with both CF and CG (53 ± 15 years). The sex ratio was equal in both groups, i.e. 14 M/16 F in patients with isolated CF and 10 M/9 F in patients with CF + CG.
Table 1.
Clinical characteristics of the 30 patients presenting with isolated cryofibrinogen (CF). CF and plasma fibrinogen (Fg) levels and immunoglobulin (Ig) composition of plasma cryoprecipitates are also given.
| No. | Age (years) | Sex | Diagnosis | Signs or symptoms | CF level (g/l) | Fg level (g/l) | Ig CF | Cold sensitivity | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | M | Zona + arterial thrombosis | Herpes zoster. Arteritis | 1·5 | 3·1 | 0 | No | Acyclovir, coumadin | CF present only during viral illness |
| 2 | 69 | F | Infectious colitis | Generalized urticaria,diarrhoea | 0·7 | 3·55 | 0 | No | Antibiotics | Remission |
| 3 | 32 | F | Chlamydia infection | Skin nodules,arthralgia, fever | 0·22 | 4·5 | 0 | No | Antibiotics | CF present only during febrile illness |
| 4 | 69 | F | Erysipelas—giant cellarteritis | Erysipelas—haemorrhagic ulcers ofthe lower limbs | 1 | 10·4 | 0 | No | Antibiotics and prednisone | Clinical remission |
| 5 | 55 | F | Mycosis fungoide | Mycosis fungoide | 1·2 | ND | 0 | No | UV therapy | Remission |
| 6 | 60 | F | Adenocarcinoma | Recurrent venousthrombosis | 1·2 | 4·3 | 0 | No | Chemotherapy | Died |
| 7 | 41 | M | Leukaemia | Generalized urticaria—swelling of legs | 0·52 | 8·6 | 0 | No | Chemotherapy | Died |
| 8 | 30 | M | Crohn's disease | Haemorrhagic diarrhoea. Necrotic ulcerationson legs | 0·64 | ND | 0 | Yes | Prednisone | Remission |
| 9 | 37 | M | Behçet's disease | Haemoptysis | 0·23 | 7·4 | 0 | No | Prednisone, azathioprine | Remission |
| 10 | 39 | F | Polymyositis | Muscle weakness andpains | 1·1 | ND | IgG | Yes | Prednisone | Improvement |
| 11 | 75 | M | Leukocytoclastic vasculitis. CMV hepatitis | Purpura and livedo of legsand feet | 0·2 | 7 | 0 | No | Coumadin | Died |
| 12 | 67 | M | Periarteritis nodosa | Neuromyositis | 0·19 | 7·1 | 0 | No | Prednisone | Remission |
| 13 | 38 | F | Tuberculosis. Leukocytoclastic vasculitis | Skin nodules of thelower limbs. Arthralgia | 0·12 | 4·5 | 0 | No | Antibiotics | CF present only during febrile illness |
| 14 | 50 | F | Periarteritis nodosa—dermalvascular thrombi—hepatitis C | Purpura and livedo of legs | 0·1 | 3·9 | IgG, IgA and IgM | Yes | Colchicine, aspirin, IFN-α | Recurrence of skin lesions |
| 15 | 69 | M | Essential cryofibrinogenaemiaLeukocytoclastic vasculitis | Purpura, painful ulcerativelesions with necrosis | 6·2 | ND | 0 | No | Prednisone, aspirin | Recurrence of skin lesions |
| 16 | 8 | M | Essential cryofibrinogenaemia | Recurrent acral ulcerations | 0·06 | 3·5 | 0 | Yes | Occlusive dressing | Recurrence of skin lesions |
| 17 | M | Essential cryofibrinogenaemia | Purpura, livedo and painfululcerative lesions of legs | 0·9 | 2·9 | 0 | Yes | Prednisone, aspirin, colchicine, coumadin | Recurrence of skin lesions | |
| 18 | 36 | M | Essential cryofibrinogenaemia | Purpura, livedo of legs and feet, Raynaud's phenomenon | 0·7 | 2·9 | 0 | Yes | Prednisone, aspirin, colchicine | Recurrence of skin lesions |
| 19 | 34 | F | Essential cryofibrinogenaemia | Livedo and necroticulcerations on legs and feet | 0·22 | 3·2 | 0 | Yes | Prednisone, aspirin, colchicine, coumadin | Recurrence of skin lesions |
| 20 | 51 | F | Essential cryofibrinogenaemia | Generalized purpura | 0·16 | 3·4 | 0 | Yes | Prednisone, aspirin, colchicine, coumadin, | Recurrence of skin lesions |
| 21 | 53 | F | Essential cryofibrinogenaemia | Purpuric and necrotic lesions on legs | 0·47 | 3·9 | 0 | Yes | Prednisone, aspirin, colchicine | Recurrence of skin lesions |
| 22 | 74 | F | Essential cryofibrinogenaemia | Recurrent arterial thromboses | 0·46 | 4·7 | 0 | No | AVK | Remission |
| 23 | 28 | F | Essential cryofibrinogenaemia | Necrotic ulcerations on legs and feet | 0·45 | 3·1 | 0 | Yes | Prednisone, aspirin, colchicine, coumadin | Recurrence of skin lesions |
| 24 | 70 | F | Essential cryofibrinogenaemia | Recurrent venous thromboses | 0·4 | 4·8 | IgG, IgA and IgM | Yes | Coumadin, prednisone | Remission |
| 25 | 42 | M | Essential cryofibrinogenaemia | Peripheral neuropathy with recurrent attacks | 0·31 | 3·7 | 0 | Yes | Prednisone, aspirin, colchicine | Recurrence of neuropathy attacks |
| 26 | 63 | F | Essential cryofibrinogenaemia | Necrotic ulcerations and swelling on legs and feet | 0·3 | 3·6 | 0 | Yes | Heparin, coumadin | Recurrence of skin lesions |
| 27 | 32 | F | Essential cryofibrinogenaemia | Raynaud's phenomenon, necrotic ulcerations swelling on legs and feet. Arthralgia | 0·25 | 2·8 | 0 | Yes | Prednisone, aspirin, colchicine, coumadin | Recurrence of skin lesions |
| 28 | 61 | M | Essential cryofibrinogenaemia | Necrotic ulcerations on legs Raynaud's phenomenon | 0·68 | 3·5 | 0 | Yes | Prednisone, aspirin, colchicine, coumadin | Recurrence of skin lesions |
| 29 | 40 | M | Essential cryofibrinogenaemia | Ulcerative lesions of legs and feet. Arterial thrombosis | 2·5 | 7·1 | 0 | Yes | Coumarin | Relapse after coumadin withdraw |
| 30 | 14 | M | Essential cryofibrinogenaemia | Purpura, livedo of legs and feet, Raynaud's phenomenon | 1·9 | ND | 0 | Yes | Prednisone, aspirin, colchicine | Recurrence of skin lesions |
Table 2.
Clinical characteristics of the 19 patients presenting with both cryofibrinogen (CF) and cryoglobulins (CG). The levels of CF, CG and plasma fibrinogen (Fg) and immunoglobulin (Ig) composition of plasma and serum cryoprecipitates are also given
| No. | Age (years) | Sex | Diagnosis | Signs or symptoms | CF (g/l) | Fg (g/l) | CG (g/l) | CF | CG | Cold sensitivity | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | F | Sjögren's disease | Necrotic and purpuric lesions on legs and feet | 4·6 | 4·6 | 5·6 | IgMκ and IgG | IgMκ and IgG | No | Prednisone | Septic shock. Died |
| 2 | 62 | F | Scleroderma (CREST) | Raynaud's phenomenon, skin necrotic ulcerations | 1·7 | 3·5 | 0·26 | 0 | IgG, IgA and IgM | No | Prostacyclin, calcium blockers | Recurrence skin lesions |
| 3 | 76 | F | Polymyositis. Pyocyanic infection | Fever and muscle weakness | 2·8 | 4·6 | 0·01 | IgMλ, IgG and IgA | IgMλ, IgG and IgA | No | Prednisone | Septic shock. Died |
| 4 | 57 | F | Polymyositis | Muscle weakness | 0·32 | 2·9 | 0·06 | IgM | IgM | No | Prednisone | Clinical remission |
| 5 | 34 | M | Systemic lupus erythematosus | Kidney involvement of systemic lupus | 1·4 | 3·1 | 0·1 | IgMλ | IgMλ | No | Cyclophos- phamide, prednisone, aspirin | Clinical remission |
| 6 | 45 | F | Lupus-like arthritis | Arthralgia | 0·12 | 5 | 0·12 | IgG, IgA and IgM | IgG, IgA and IgM | No | Prednisone, cyclophos-phamide | Clinical remission |
| 7 | 37 | F | Hashimoto hypothyroidism | Perineal skin nodules | 3·4 | 3·7 | 0·09 | IgG, IgA and IgM | IgG, IgA and IgM | No | Coumadin, prednisone | Clinical remission |
| 8 | 47 | F | Osteitis | Livedo, ulcerations ofthe legs, gangrene | 3 | 4·6 | 0·08 | IgG, IgA and IgM | IgG, IgA and IgM | No | Coumadin Feet amputation | ND |
| 9 | 50 | M | Erysipelas | Swelling and ulcerations of the lower extremities | 1·3 | 3·6 | 0·01 | IgGκ | IgG, IgA and IgM | No | Antibiotics | CF present only during febrile illness |
| 10 | 29 | M | CMV infection | Weakness, fever, purpuric lesionsof the legs | 0·9 | 2·7 | 0·9 | IgG, IgA and IgM | IgG, IgA and IgM | No | Dressingchanges, wound care | Recurrence during febrile illness |
| 11 | 47 | M | Hepatitis C. Vasculitis. Waldenström | Painful ulcer of the legs. Myocardial infarction | 0·39 | 3·7 | 0·25 | IgMκ, IgG and IgA | IgMκ, IgG and IgA | No | – | Died |
| 12 | 60 | F | Hepatitis C Arthralgia | Purpura of the legs | 0·9 | 4·5 | 0·73 | IgM | IgM | No | IFN-α, ribavirin, plasma-pheresis | Recurrence |
| 13 | 56 | M | Myeloprolifer- ative disease | Mucosal lesions | 0·5 | 5·4 | 0·12 | 0 | IgG | No | Chemo- therapy. IFN-α | Died |
| 14 | 80 | M | Factor XII deficiency | Painful ulcers of the legs Extradural haematoma | 1·28 | 5 | 0·03 | IgG, IgA and IgM | IgG, IgA and IgM | No | Vasodilators | Recurrence of skin lesions |
| 15 | 50 | M | Severe atherosclerosis. Idiopathic? | Non-healing ulcerations on the legs. Alcoholism | 2 | 4·6 | 0·07 | IgG, IgA and IgM | IgG, IgA and IgM | Yes | Occlusive dressing | ND |
| 16 | 35 | M | Idiopathic | Skin necrotic ulcerations, Raynaud's phenomenon, gangrene | 0·49 | 8·6 | 0·07 | IgMλ | IgMλ, IgG and IgA | No | Cyclophos-phamide, prednisone,coumadin | Recurrence of skin lesions |
| 17 | 48 | F | Idiopathic | Recurrent pulmonary embolisms. Renal veinthrombosis | 1·2 | 3·5 | IgMλ | IgMλ, IgG and IgA | No | Heparine, coumadin | Clinical remission | |
| 18 | 52 | M | Idiopathic | Stroke | 0·47 | 5·25 | 0·02 | 0 | IgMλ, IgG and IgA | Yes | Coumadin | Pain of legs and feet |
| 19 | 35 | M | Idiopathic | Purpuric lesions, pulmonary fibrosis | 3·36 | 7·3 | 0·01 | 0 | 0 | No | Prednisone | Purpura |
Underlying diseases were less frequent in patients with isolated CF, i.e. 14/30 cases (47%), than in patients with combined CF and CG, i.e. 15/19 cases (79%) (P = 0·02). These diseases included, in the CF versus CF + CG groups, respectively: five (17%) versus eight cases (43%) of a collagen disorder, six (20%) versus six cases (31%) of an infectious disease and three (10%) versus two cases (10%) of a malignant disease. One patient with combined CF and CG suffered from a chronic hepatitis C virus infection associated with malignant disease.
The most common clinical symptoms in both groups were cutaneous in nature. They were noted in 23 cases (77%) in the isolated CF group and in 11 cases (58%) in the combined CF and CG groups. Less frequent symptoms included, in the CF versus combined CF and CG groups, respectively: venous thrombosis (three (10%) versus one case (5%)), arterial thrombosis (one (3%) versus one case (5%)), arthralgia (three (10%) versus two cases (10%)), and neurological symptoms (three (10%) versus two cases (10%)). In one patient with both CF and CG (5%) respiratory symptoms were observed. In patients with isolated CF, especially those with essential cryofibrinogenaemia, the cutaneous lesions were predominantly recurrent and necrotic. These lesions included (in decreasing order): painful ulcerations (11/23 (48%)), purpura (8/23 (26%)), livedo (6/23 (26%)), urticaria of the lower extremities (2/23 (8%)), Raynaud's phenomenon (4/23 (15%)), segmental swelling (2/23 (8%)) and nodules located on the leg or foot (2/23 (8%)). Hypersensitivity to cold was more common in patients with isolated CF than in those with combined CF and CG (17/30 (43%) versus 2/19 (10%), respectively, P = 0·03). Hypersensitivity was particularly prominent in patients with essential isolated cryofibrinogenaemia (14/16 (87%)).
In mild cases of essential isolated cryofibrinogenaemia, the treatment was purely symptomatic, essentially based on cold avoidance. In more severe cases, such as those presenting skin ulceration and/or necrosis, specific treatments were applied. In secondary cryofibrinogenaemia, the treatment was directed against the underlying cause, for example prednisone in cases of collagen vascular disorders. Prednisone was effective in four out of five patients with isolated CF. In eight patients with combined CF + CG, immunosuppressive therapy proved efficient in four. When the underlying diseases were adequately treated the CF precipitates disappeared accordingly.
In primary cutaneous forms of CF, topic or systemic steroids (three cases), anti-coagulants or platelet-active drugs (aspirin) (10 cases) were always ineffective. Cold exposure avoidance and early antibiotic treatment of supra infections helped reduce the recurrence and severity of cutaneous attacks. Treatments with fibrinolytic properties were not used in this series.
Anti-coagulants were effective in the two cases of cutaneous thrombosis, but did not prevent relapses during the following winter months. Long-term treatments with anti-coagulants were effective against venous thrombosis and arterial thrombosis in both groups. In two patients with isolated CF however a relapse occurred soon after treatment withdrawal.
In most cases the absence of any histological evaluation of skin lesions prevented any differentiation between vasculopathy and vasculitis.
Analysis of CF precipitates
The CF plasma levels of the 60 CF-positive patients exceeded 1 g/l in 20 cases, ranging from 0·1 g/l to 1 g/l in 35 cases and 0·05 g/l to 0·1 g/l in five cases. Details of the level and composition of the CF proteins found in the 30 patients with isolated CF and the 19 patients with CF + CG are presented in Tables 1 and 2. The mean CF concentration was higher in the patients with isolated CF (1·61 ± 1·26 g/l) than in those with combined CF + CG (0·82 ± 1·18 g/l) (P = 0·004).
Each CF sample from both groups was tested with specific anti-fibronectin, anti-fibrinogen, anti-α1-antitrypsin and anti-α2-macroglobulin antibodies. Analysis of the CG precipitate revealed the presence of fibronectin but the absence of fibrinogen, α1-antitrypsin and α2-macroglobulin (Fig. 1). In only 13 patients (all with isolated CF) was sufficient cryoprecipitate (> 1 g/l) available for quantitative analysis. The mean concentration of plasma fibronectin, α1-antitrypsin and α2-macroglobulin before and after precipitation is shown in Table 3. α1-antitrypsin and α2-macroglobulin plasma levels were unaffected by precipitation, whereas the plasma fibronectin level dropped significantly (Table 3). In only three out of 30 patients with isolated CF (10%) were polyclonal immunoglobulins associated with CF. In patients with combined CF and CG, the CF consisted mostly of immunoglobulins (14/18, 78%) of the same as those characterizing the associated CG (13/18, 72%) (Table 2).
Fig. 1.
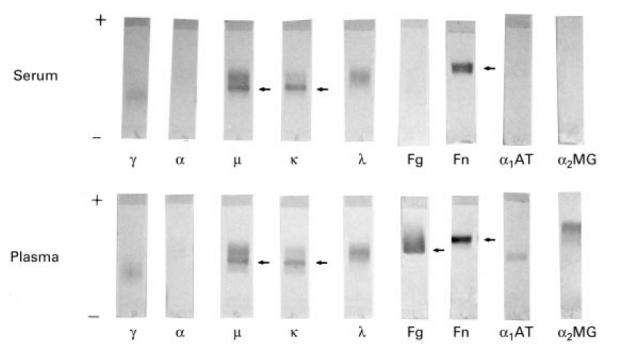
Identification of the plasma and serum cryoprecipitated components by immunoblot analysis in a patient (no. 1 in Table 2) with mixed cryoglobulin with monoclonal component (IgMκ). The cryoprecipitated components were immunoreacted with different antibodies. Lane 1, anti-γ heavy chain; lane 2, anti-α heavy chain; lane 3, anti-μ heavy chain; lane 4, anti-κ light chain; lane 5, anti-λ light chain; lane 6, anti-fibrinogen; lane 7, anti-fibronectin; lane 8, anti-α1-antitrypsin; lane 9, anti-α2-macroglobulin.
Table 3.
Quantitative analysis of components of plasmas of 13 patients with cryofibrinogen (CF) (> 1 g/l) without cryoglobulin (CG), before and after CF precipitation
| Before precipitation (n = 13) | After precipitation (n = 13) | Normal range | |
|---|---|---|---|
| α1-antitrypsin (g/l) (mean ±s.d.) | 3·87 ± 1·16 | 3·65 ± 1·02 | 1·4–3·2 |
| (g/l) (mean ±s.d.) | (2·71–6·51) | (2·4–5·4) | |
| α2-macroglobulin | |||
| Men (g/l) | 2·52 ± 0·91(1·7–5·3) | 2·20 ± 0·41(1·5–2·8) | 1·2–2·7 |
| Women (g/l) | 2·65 ± 0·93(1·8–4·2) | 2·63 ± 0·91(2·3–3·4) | 1·4–3·2 |
| Fibronectin (g/l) | 0·31 ± 0·08 (0·18–0·46) | 0·16 ± 0·11 (< 0·008–0·38) | 0·25–0·4 |
| Total cryofibrinogen (g/l) | 0·25–0·4 (1·1–6·2) | ||
As shown in Table 1, there was no obvious correlation between CF plasma levels and either the severity of symptoms or the sensitivity to cold.
Plasma fibrinogen levels and underlying disease
Compared with the normal range of plasma fibrinogen (1·5–4·5 g/l), its levels were high in patients both with isolated CF (5·6 ± 2·4 g/l) and with combined CF + CG (5·0 ± 2·4 g/l). Although plasma fibrinogen levels were not significantly different in primary and secondary forms of CF, they were lower in primary CF patients suffering from cutaneous lesions than in the other studied patients (3·5 ± 0·6 g/l versus 6·0 ± 2·8 g/l).
DISCUSSION
Cryofibrinogenaemia has so far received little attention in the medical literature but nevertheless it is not such an infrequent condition. We found the prevalence of CF to be 2·8% in healthy blood donors and 27·3% in patients presenting symptoms commonly ascribed to cryoproteinaemia (16·8% with isolated CF and 10·5% with combined CF + CG). In other studies cryofibrinogenaemia was reported in 3% of healthy subjects [6], 3·4–8·5% of out-patients [19], 3–15% of hospitalized patients [6,8] and in 75% of patients with biopsy-proven IgA nephropathy [13]. In most cases however CF was only an accidental finding [4,5,20,21]. Furthermore, improper blood sample collection may result in a false-negative result for CF [4], and this raises several important points regarding exploration of CF. First, heparin may form a cryoprecipitate with fibronectin, fibrin, and fibrinogen (named the ‘heparin-precipitable fraction’), thereby requiring the use of blood anti-coagulated with citrate, oxalate, or EDTA for CF detection [9,22,23]. Second, plasma (not sera) has to be used to test for CF whereas sera are necessary to detect CG [11]. Collected blood must be kept at 37°C until centrifuged, which preferably should be done immediately. Finally, it may require >72 h for the precipitate to form.
In the present study the five healthy blood donors with CF had cryoprecipitate levels of <0·05 g/l. Conversely, the 60 CF-positive patients contained CF levels >1 g/l in 20 cases, and ranging from 0·1 g/l to 1 g/l in 35 cases and from 0·05 g/l to 0·1 g/l in five cases. These findings suggest that not just CF levels exceeding 1 g/l are associated with symptoms commonly ascribed to cryoproteinaemia. The clinical descriptions and cryoprecipitate analyses of 49 patients, as detailed in Tables 1 and 2, support the hypothesis that there is no correlation between the levels of CF and either disease intensity or cold sensitivity [11,24].
There are at least three reasons, based on the different clinical and biological features we describe, that patients with isolated cryofibrinogenaemia must be distinguished from those with associated cryoglobulinaemia. First, collagen, infectious or malignant diseases were encountered more frequently in patients with combined CF and CG than in those with isolated CF. Second, patients with isolated CF, particularly those without an underlying disease, suffered more frequently from recurrent and necrotic skin lesions (mainly triggered by cold exposure) than patients with both CF and CG. Topical treatments, platelet-active drugs, anti-coagulants, and immunosuppressive therapy were ineffective in these patients with isolated CF. Third, the mean concentration of plasma cryoprecipitate was found to be higher in patients with isolated CF than in those with combined CF and CG. It should be noted however that there is no evidence as yet for a causal role of the cryoprecipitates in the differences observed between isolated and combined cryofibrinogenaemia.
The analysis of plasma cryoprecipitates suggests that several of these clinical and biological differences could result from different pathogenic mechanisms. We noted that CF reacted with specific anti-fibrinogen and anti-fibronectin antibodies in both groups [11]. The decreased serum levels of fibronectin following precipitation suggests that it is one of the major components of the CF. We found that serum fibrinogen levels were elevated in patients of both groups, except in those with essential isolated CF (characterized by recurrent necrotic cutaneous lesions), suggesting that in this group CF is not merely a reflection of elevated plasma fibrinogen levels [8]. Analysis of CF revealed the presence of two protease inhibitors: α1-antitrypsin and α2-macroglobulin, which both inhibit plasmin, a fibrinolytic agent [8]. These findings suggest that fibrinolysis inhibition by α1-antitrypsin and α2-macroglobulin may lead to the accumulation of CF. CF may cause thrombotic occlusion of small vessels, especially in the skin, via either reflex vasospasm, vascular stasis, and/or hyperviscosity [6,8]. This could explain the histopathology of the most common skin lesion associated with CF, namely eosinophilic thrombi of the small intradermal vessels. Streptokinase, streptodornase, urokinase, and more recently stanazolol (an androgenic steroid with fibrinolytic activity), have shown promising results in treating a subgroup of patients with essential isolated CF and painful leg ulcers [5,25–28]. By analogy with congenital dysfibrinogenaemia, a mutated fibrinogen with conformational changes or altered thrombin-binding capacities may also cause clot formation upon exposure to cold or other stimuli [29].
In addition to its implication in the coagulation/clotting mechanisms, an immunological role for CF is suggested by the frequent presence of immunoglobulins, along with fibronectin, α1-antitrypsin and α2-macroglobulin, within the CF complex when associated with CG. Indirect evidence for this immunological role has been suggested by the presence of either the granular deposition of IgM, IgA and C3 [14] or endothelial immune complexes [29] in the vessel walls of patients with CF and leukocytoclastic vasculitis. CG and CF associated with neutrophilic skin infiltrate and myelodysplastic syndrome [30], as well as in association with homocystinuria [21], have also been reported. The interaction between fibronectin and circulating immunoglobulins or immune complexes, which are produced excessively in several conditions (including infectious, malignant or collagen vascular diseases), could be a key in the vascular deposition of CF [31,32]. The precipitation of complexes including fibrin, fibrinogen, and fibronectin may also lead to the formation of small vessel thrombi. Vasculitis appears to be the consequence rather than the cause of tissue damage and thrombosis. The relatively rapid resolution of skin manifestations observed in patients with CF when either the underlying disease has been adequately treated or when immunosuppressive therapy, plasmapheresis [33] and/or fibrinolytic drugs have been used [21,29] supports this hypothesis.
Further studies are needed to confirm whether insufficient fibrinolysis exists in all patients with CF and where it is manifest (i.e. the circulating blood or merely within these abnormal precipitates). Additional knowledge regarding the composition of CF precipitates and the histology of cutaneous lesions is needed in order to elucidate further the pathological mechanisms involved in the production of CF alone or in association with CG.
In conclusion, although it is rare, clinicians should be aware of potentially symptomatic CF. Patients presenting purpura, skin necrosis, ulceration of unknown aetiology, or otherwise unexplained cold intolerance should be screened for plasma CF and serum CG. Due to specific practical implications, a distinction should be drawn between patients presenting associated CF and CG and those with isolated CF, and furthermore whether that CF is essential or secondary.
Acknowledgments
The authors thank Agnes P Dauphin (MD) and My-M Cao (MD) for their assistance in data analysis, Jean-Marie Lecomte for his helpful comments and Evelyne Colopi for her secretarial expertise.
REFERENCES
- 1.Korst DR, Kratochvil CH. ‘Cryofibrinogen’ in a case of lung neoplasm associated with thrombophlebitis migrans. Blood. 1955;10:945–53. [PubMed] [Google Scholar]
- 2.Stathakis NE, Karamanolis D, Koukoulis G, Tsianos E. Characterization of cryofibrinogen isolated from patient's plasma. Haemostasis. 1981;10:195–202. doi: 10.1159/000214404. [DOI] [PubMed] [Google Scholar]
- 3.Rachmilewitz EA, Sacks MI, Zlotnick A. Essential cryofibrinogenemia. Clinical, pathological and immunological studies. Isr J Med Sci. 1970;6:32–43. [PubMed] [Google Scholar]
- 4.Beightler E, Diven DG, Sanchez RL, Solomon AR. Thrombotic vasculopathy associated with cryofibrinogenemia. J Am Acad Dermatol. 1991;24:342–5. doi: 10.1016/0190-9622(91)70048-7. [DOI] [PubMed] [Google Scholar]
- 5.Jantunen E, Soppi E, Neittaanmäki H, Lahtinen R. Essential cryofibrinogenaemia, leukocytoclastic vasculitis and chronic purpura. J Int Med. 1993;234:331–3. doi: 10.1111/j.1365-2796.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 6.McKee PA, Kalbfleisch JM, Bird RM. Incidence and significance of cryofibrinogenemia. J Lab Clin Med. 1963;61:203–10. [Google Scholar]
- 7.Zlotnick A, Shahin W, Rachmilewitz EA. Studies in cryofibrinogenemia. Acta Haematol. 1969;42:8–17. doi: 10.1159/000208756. [DOI] [PubMed] [Google Scholar]
- 8.Smith SB, Arkin C. Cryofibrinogenemia. Incidence, clinical correlations, and a review of the literature. Am J Clin Pathol. 1972;58:524–30. doi: 10.1093/ajcp/58.5.524. [DOI] [PubMed] [Google Scholar]
- 9.Moroz LA, Rose B. The cryopathies. In: Samter M, editor. Immunological diseases. Boston: Little, Brown; 1978. pp. 570–91. [Google Scholar]
- 10.Robinson MG, Troiano G, Cohen H, et al. Acute transient cryofibrinogenemia in infants. J Pediatr. 1966;69:35–39. doi: 10.1016/s0022-3476(66)80358-x. [DOI] [PubMed] [Google Scholar]
- 11.Ritzmann SE, Levin WC, Ivers JB, Koch JL. 1. Cryoproteinemias. 2. Primary cryofibrinogenemia. Its association with cryopathy and telangiectasis. Tex Rep Biol Med. 1963;21:567–80. [PubMed] [Google Scholar]
- 12.Ireland TA, Werner DA, Rietschel RL, Patterson JH, Spraker MK. Cutaneous lesions in cryofibrinogenemia. J Pediatr. 1984;10:67–70. doi: 10.1016/s0022-3476(84)80363-7. [DOI] [PubMed] [Google Scholar]
- 13.Nagy J, Ambrus M, Paal M, Trinn C, Burger T. Cryoglobulinaemia and cryofibrinogenaemia in IgA nephropathy: a follow-up study. Nephron. 1987;46:337–42. doi: 10.1159/000184385. [DOI] [PubMed] [Google Scholar]
- 14.Brungger A, Brulisauer M, Mitsuhashi Y, Schneider BV, Bollinger A, Schnyder UW. Cryofibrinogenemic purpura. Arch Dermatol Res. 1987;279:S24–29. doi: 10.1007/BF00585916. [DOI] [PubMed] [Google Scholar]
- 15.Griswold WR, Simon G, McIntosh RM. Anaphylactoid purpura, cold exposure, and cryofibrinogenemia. Ann Intern Med. 1973;78:611. doi: 10.7326/0003-4819-78-4-611_1. [DOI] [PubMed] [Google Scholar]
- 16.Barrett MC, Prendiville JS, Pardy BJ, Cream JJ. Cryofibrinogenaemia and acute gangrene in systemic sclerosis. Postgr Med J. 1986;62:935–6. doi: 10.1136/pgmj.62.732.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musset L, Diemert MC, Taibi F, et al. Characterization of cryoglobulins by immunoblotting. Clin Chem. 1992;38:798–802. [PubMed] [Google Scholar]
- 18.Von Clauss A. Gerinnungsphysiologische Schnellmethod zur Bestimmung des Fibrinogens. Acta Haematol (Basel) 1957;17:237–46. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 19.Kellett J. Thyrotoxicosis, cryofibrinogenemia, sinutab maximum strength, and purple ears. Ann Intern Med. 1989;110:668. doi: 10.7326/0003-4819-110-8-668. (letter) [DOI] [PubMed] [Google Scholar]
- 20.Rubegni P, Flori ML, Fimiani M, Andreassi L. A case of cryofibrinogenemia responsive to stanozolol. Br J Haematol. 1996;93:217–9. doi: 10.1046/j.1365-2141.1996.4791016.x. [DOI] [PubMed] [Google Scholar]
- 21.Williamson AE, Cone LA, Il SH, Mirage R. Spontaneous necrosis of the skin associated with cryofibrinogenemia, cryoglobulinemia, and homocystinuria. Ann Vasc Surg. 1996;10:365–9. doi: 10.1007/BF02286781. [DOI] [PubMed] [Google Scholar]
- 22.Jager BV. Cryofibrinogenemia. N Engl J Med. 1972;266:579–83. doi: 10.1056/NEJM196203222661202. [DOI] [PubMed] [Google Scholar]
- 23.Martin S. Cryofibrinogenemia, monoclonal gammopathy, and purpura. Arch Dermatol. 1979;115:208–11. [PubMed] [Google Scholar]
- 24.Kuipers JG, Kellett J, May D. Low levels of cryofibrinogenaemia and peripheral circulatory dysfunction. Ir Med J. 1991;84:68–69. [PubMed] [Google Scholar]
- 25.Ayres ML, Jarrett PEM, Browse NL. Blood viscosity, Raynaud's phenomenon and the effect of fibrinolytic enhancement. BJS. 1981;68:51–54. doi: 10.1002/bjs.1800680117. [DOI] [PubMed] [Google Scholar]
- 26.Kluft C, Preston FE, Malia RG, et al. Stanozolol induced changes in fibrinolysis and coagulation in healthy adults. Throm Haemost. 1984;51:157–64. [PubMed] [Google Scholar]
- 27.Klein AD, Kerdel FA. Purpura and recurrent ulcers on the lower extremities. Essential cryofibrinogenemia. Arch Dermatol. 1991;127:113–8. doi: 10.1001/archderm.127.1.115. [DOI] [PubMed] [Google Scholar]
- 28.Falanga V, Kirsner RS, Eaglstein WH, Katz MH, Kerdel FA. Stanozolol in treatment of leg ulcers due to cryofibrinogenaemia. Lancet. 1991;338:347–8. doi: 10.1016/0140-6736(91)90483-6. [DOI] [PubMed] [Google Scholar]
- 29.Wulffraat N, Meyer JT, Zegers BJM, Kuis W. Familial presence of primary cryofibrinogenemia. A report of three cases. Br J Rhumatol. 1996;35:102–4. doi: 10.1093/rheumatology/35.1.102. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe R, Iijima M, Otsuka F. A case of neutrophilic dermatosis (ND) complicated by cryofibrinogenemia (CFGN) and myelodysplastic syndrome (MDS) J Dermatol. 1992;19:181–5. doi: 10.1111/j.1346-8138.1992.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 31.Strevey J, Beaulieu AD, Menard C, Valet JP, Latulippe L, Hebert J. The role of fibronectin in the precipitation of monoclonal cryoglobulins. Clin Exp Immunol. 1984;55:340–5. [PMC free article] [PubMed] [Google Scholar]
- 32.Brandau DT, O'Donnell R, Kimmel-Truitt VL. Lack of binding between cryoimmunoglobulins, immunoglobulins and fibronectin: implication for immune complex vasculitis. Clin Exp Immunol. 1991;83:58–63. doi: 10.1111/j.1365-2249.1991.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copeman PW. Cryofibrinogenaemia and skin ulcers: treatment with plasmapheresis. Br J Dermatol. 1979;101:57–58. [PubMed] [Google Scholar]


