Abstract
We set out to examine the effect of gold treatment on the Th2-dependent antibodies IgG4 and IgE in relation to other IgG subclasses in patients with rheumatoid arthritis (RA). Eighty-five gold-treated RA patients and 82 RA controls were studied. Serum IgG subclass concentrations were measured by ELISA, IgE was measured by automated enzyme immunoassay. Samples were studied serially in 13 gold-treated patients and in 11 patients with gold-induced adverse events. There was a significant reduction in the concentration of IgG1, IgG2 and IgG3 in gold-treated RA patients compared with RA controls (P 0·004–0·019), whereas IgG4 was less significantly reduced in gold-treated patients (P = 0·044) and there was no difference in IgE. In serial samples there was a significant fall in the concentration of IgG1 (P = 0·001), IgG2 (P = 0·001) and IgG3 (P = 0·026) with time but no change in IgG4 and IgE. The development of gold-induced adverse events was not associated with any change in the concentration of each IgG subclass or IgE. Deficiencies of IgG subclasses were found in 30% of gold-treated RA patients and 8·5% of RA controls, and were associated in gold-treated patients with a longer disease duration (P = 0·003) and with erosive disease (P = 0·03). IgG2 was affected most frequently and in the majority of these cases subnormal specific IgG2 binding to widespread polysaccharide antigens (Pneumovax II) was found. Gold induces an overall immunosuppressant effect on IgG subclasses, with a deficiency in 21·5%, adjusted for controls. The effect on the Th2-dependent antibodies IgG4 and IgE is less marked, suggesting a sparing of Th2 responses.
Keywords: gold, rheumatoid arthritis, IgG subclasses, IgE, Th2
INTRODUCTION
The role of the Th1–Th2 model in inflammatory arthritis is controversial, but the weight of evidence points towards involvement of Th1-like processes in rheumatoid arthritis (RA) [1,2]. This has led to the concept that enhancement of Th2 responses might be of benefit in the treatment of RA [1].
Gold has been used in the treatment of RA for over 40 years [3] but the mechanisms of its therapeutic and adverse effects remain poorly understood. It is intriguing that in susceptible animals gold induces an autoimmune syndrome characterized by hallmarks of Th2 activation, namely up-regulation of IL-4 and high concentrations of IgE [4]. In humans there is limited evidence that gold may have an enhancing effect on Th2 responses [5].
A study of membranous nephropathy, which occurs in 2–10% of gold-treated patients [6], reveals that the glomerular deposits are rich in IgG4 [7], the rarest of circulating IgG subclasses and the only subclass, in addition to IgE, that is induced by the Th2 cytokine IL-4 [8]. This has led to the hypothesis that Th2 activation and IgG4 immune complex deposition are central processes in the pathogenesis of membranous nephropathy [9]. If the immunopathogenesis of gold-induced nephropathy is similar to the immune sequelae of gold treatment then one might expect to find other evidence of Th2 activation in gold-treated patients. This is of potential relevance to the management of gold treatment, as the emergence of a Th2 response might be a useful marker of efficacy or toxicity.
This study examines the effect of gold treatment on the Th2-dependent antibodies IgG4 and IgE in comparison with IgG1, IgG2 and IgG3 (i) in a cross section of RA patients compared with RA controls, (ii) in gold-treated RA patients prospectively with time, and (iii) in a subgroup with gold-induced adverse events.
PATIENTS AND METHODS
Patients
Serum samples were collected from 167 patients with RA (1987 ARA criteria) attending the rheumatology out-patients of St George's Healthcare and St Helier NHS Trusts between July 1996 and July 1998. The study was approved by the Merton and Sutton Local Research Ethics Committee. Demographic details are shown in Table 1. Patients in the RA control group (n = 82) were treated with standard disease-modifying drugs (DMARD), 73% with sulphasalazine or methotrexate, and none were treated with gold, d-penicillamine or prednisolone. Patients in the gold group (n = 85) were all treated with intramuscular sodium aurothiomalate (myocrisin) at doses varying between 50 mg weekly and 20 mg monthly. None was treated with any other DMARD, but 23 (27%) were also receiving low-dose prednisolone (< 10 mg/day). The cumulative dose of gold that had been given at the time of each serum sample was recorded for all patients. No patient had any other condition or drug therapy known to be associated with immunoglobulin deficiency. In 13 randomly selected patients between two and five serial serum samples were collected over the 2-year period.
Table 1.
Demographic characteristics of rheumatoid arthritis (RA) patients
| Patient group | Number | Mean age (years) | Percent male | Percent RF+ | Percent with erosions | Median disease duration, years |
|---|---|---|---|---|---|---|
| Controls | 82 | 62 | 27 | 87 | 71 | 8 |
| Gold | 85 | 64 | 29 | 73 | 87 | 10 |
| P | NS** | NS* | 0·03* | 0·01* | 0·01** |
RF, Rheumatoid factor.
NS, P =0·05.
Fisher's exact test.
Mann Whitney U-test.
IgG subclass analysis
Serum samples were stored at −80°C and coded by an observer unaware of patient group prior to analysis. The concentration of serum IgG1, IgG2, IgG3 and IgG4 was measured by ELISA [10]. Microtitre plates were coated with either standards, controls or serum samples (100 μl per well) diluted 1:25 600 in carbonate–bicarbonate buffer (C-3041; Sigma, Poole, UK) and the plates were incubated at 4°C overnight. Plates were washed twice with wash solution (0·05% Tween-20 in PBS) and incubated for 1 h at 37°C with blocking solution (0·1% Tween-20 in PBS, 100 μl per well). Plates were washed twice and incubated for 3 h at 37°C with polyclonal affinity-purified anti-human IgG1, IgG2, IgG3 or IgG4 peroxidase-conjugated antibody (AP006/7/8/9; The Binding Site, Birmingham, UK) 100 μl per well diluted 1:1000 (anti-IgG1 and 3) or 1:500 (anti-IgG2 and 4) in wash solution. Plates were washed twice and incubated at room temperature with tetramethylbenzidine (TMB) solution (100 μl per well; Sigma). The absorbances from standards were plotted against log concentration and the concentration in samples calculated from this. Within assay coefficient of variation (CV) at each concentration was <7%.
IgE analysis
The concentration of total IgE was measured in serum samples by an automated enzyme immunoassay system (Cobas Core; Roche, Lewes, UK). Serum samples were loaded neat, automatically diluted and analysed in relation to a series of standard dilutions of IgE.
IgG2 anti-pneumococcal polysaccharide analysis
Serum samples from patients with deficiencies of IgG2 were measured for specific IgG2 anti-pneumococcal polysaccharide binding. Standards, controls and serum samples were preabsorded with pneumococcal cell wall polysaccharide (1 μg/ml; Staten Seruminstitut, Copenhagen, Denmark) overnight at 4°C to remove non-specific antibodies. Microtitre plates were coated with 12 μg/ml Pneumovax II (Pasteur Merieux MSD Ltd, Maidenhead, UK) in carbonate–bicarbonate buffer and incubated at 4°C overnight. Plates were washed three times with wash solution and incubated for 1 h at 37°C with blocking solution. Plates were washed three times and incubated for 4 h at 37°C with either standards, controls or serum samples diluted in wash solution (100 μl per well). Plates were washed three times and incubated for 3 h at 37°C with polyclonal affinity-purified anti-human IgG2 peroxidase-conjugated antibody (AP007) diluted 1:500 in wash solution. Plates were washed three times and incubated at room temperature with TMB solution. The absorbances from standards were plotted against log concentration and the concentration in samples calculated from this. Within assay CV at each concentration was <12%.
Statistical analysis
Demographic data in the control and gold-treated patient groups were compared with the Fisher's exact and Mann–Whitney U-tests. IgG subclass and IgE concentrations were log transformed to reduce skew and make the data more suitable for analysis. Multiple regression analysis was used to compare each IgG subclass and IgE between the gold-treated and control groups taking disease duration, rheumatoid factor and erosive status into account. Where repeated serum samples were collected from individual patients the immunoglobulin concentrations in the most recent sample, i.e. that corresponding to the highest cumulative dose of gold, were used in the comparative analysis with control RA patients.
In gold-treated patients the relationship between immunoglobulin concentrations and the cumulative dose of gold was examined using Spearman correlation. In the subgroup followed prospectively the paired Wilcoxon signed rank test was used to compare immunoglobulin concentrations in the first and last samples taken from individual patients during gold treatment.
RESULTS
Demography
The age, sex, rheumatoid factor, erosive status and duration of RA in the control and gold-treated groups are shown in Table 1. In both groups the majority of patients had long-standing seropositive, erosive rheumatoid disease. However in the control group there was a significantly higher proportion of seropositive and non-erosive patients and the disease duration was significantly shorter. In the gold-treated group the median cumulative dose of gold at the time of the serum sample was 2·6 g (range 100 mg to 13 g) representing a median duration of treatment of approximately 4·5 years. The majority of these patients were therefore ‘gold survivors’, as adverse reactions most commonly occur within the first year of treatment.
IgG subclass and IgE concentrations in gold-treated and control RA patients
Table 2 shows the median serum IgG subclass and IgE concentrations in gold-treated compared with control RA patients. In each case the concentration was lower in the gold-treated group, and this difference was highly significant for IgG1, IgG2 and IgG3, less significant for IgG4 and not significant for IgE.
Table 2.
Median serum IgG subclass and total IgE concentration in control and gold-treated rheumatoid arthritis (RA) patients, range in parentheses
| Patient group | Number | IgG1, g/l | IgG2, g/l | IgG3, g/l | IgG4, g/l | IgE, g/l |
|---|---|---|---|---|---|---|
| Controls | 82 | 8·5 | 3·2 | 0·7 | 0·4 | 21 |
| (3·38–21·5) | (0·48–8·1) | (0·15–1·7) | (0·06–5·16) | (1–934) | ||
| Gold | 85 | 7·2 | 2·5 | 0·6 | 0·3 | 15 |
| (1·47–29·3) | (0·48–10·9) | (0·07–2·27) | (0·02–3·15) | (1–1963) | ||
| P* | 0·004 | 0·008 | 0·019 | 0·044 | 0·3 |
Multiple regression analysis: gold-treated compared with control group taking into account disease duration, rheumatoid factor and erosive status.
There was a weak negative correlation between the concentration of each IgG subclass and the cumulative dose of gold, but this did not reach significance. There was also no significant correlation between the IgE concentration and the cumulative dose of gold (data not shown).
In the gold-treated group 23 patients (27%) were also treated with low-dose prednisolone. The concentration of IgG3 was significantly lower in this subgroup compared with the gold-treated patients who did not receive prednisolone. There were no significant differences in the concentrations of IgG1, IgG2, IgG4 or IgE between these groups (see Table 3).
Table 3.
Median serum IgG subclass and total IgE concentration in gold-treated rheumatoid arthritis (RA) patients ±low dose-prednisolone, range in parentheses
| Patient group | Number | IgG1, g/l | IgG2, g/l | IgG3, g/l | IgG4, g/l | IgE, g/l |
|---|---|---|---|---|---|---|
| Gold + | 62 | 7·0 | 2·46 | 0·68 | 0·3 | 14 |
| alone | (1·47–15·6) | (0·56–10·9) | (0·13–2·27) | (0·02–2·47) | (1–1963) | |
| gold + | 23 | 6·4 | 2·48 | 0·49 | 0·3 | 16 |
| prednisolone | (3·5–29·3) | (0·48–6·0) | (0·07–2·0) | (0·02–3·15) | (1–686) | |
| P* | NS | NS | 0·04 | NS | NS |
Mann-Whitney U-test, gold alone compared with gold + prednisolone group.
Prospective analysis of IgG subclass and IgE concentrations
In 13 gold-treated patients serial samples were collected over 3–8 months (median 6 months). During this time the cumulative injected dose of gold was 150–1300 mg (median 650 mg). In five patients the first sample was taken at the initiation of gold treatment and in two others after a total dose of 150–200 mg of gold. The remaining six patients had received between 600 and 4110 mg gold at the time of the first sample.
In this prospective analysis the behaviour of IgG1, IgG2 and IgG3 contrasted with that of IgG4 and IgE in the same patients. The concentration of IgG1, IgG2 and IgG3 fell in 10/13 and was unchanged in 3/13 patients. In contrast, IgG4 fell in 7/13, rose in 5/13 and was unchanged in one patient, and IgE fell in 5/13, rose in 6/13 and was unchanged in two patients with time. Paired analysis of each subclass concentration at the start and end of this prospective period revealed a significant fall in IgG1, 2 and 3 but no significant change over time in IgG4 and IgE (see Table 4).
Table 4.
Median serum IgG subclass and total IgE concentration in 13 gold-treated rheumatoid arthritis (RA) patients in a prospective analysis, range in parentheses
| Time | IgG1, g/l | IgG2, g/l | IgG3, g/l | IgG4, g/l | IgE g/l |
|---|---|---|---|---|---|
| Start | 9·0 | 3·4 | 0·56 | 0·41 | 23 |
| (5·6–19·6) | (1·4–5·5) | (0·24–2·0) | (0·1–2·19) | (1–1266) | |
| End | 6·7 | 2·5 | 0·52 | 0·32 | 15 |
| (4·6–15·6) | (0·87–5·2) | (0·23–1·3) | (0·13–1·56) | (1–1009) | |
| P* | 0·001 | 0·001 | 0·026 | 0·15 | 0·47 |
Paired Wilcoxon signed rank.
IgG subclass and IgE concentrations in patients with gold-induced adverse effects
Adverse effects attributable to gold treatment occurred in 11 patients (13%) over the study period. Eight patients developed a typical gold-induced rash or mucositis and three developed nephrotic syndrome. The concentration of each IgG subclass and IgE was in the normal range in all patients at the time of the adverse event and did not differ significantly from the rest of the gold-treated group (data not shown).
IgG subclass deficiency
In 26 (30%) gold-treated patients and in seven (8·5%) RA controls there was either an isolated or combined deficiency of one or more of the IgG subclasses (see Table 5). Deficiency was defined as less than the 95th centile from a population study of 172 healthy UK adults: IgG1 < 3·2 g/l, IgG2 < 1·2 g/l, IgG3 < 0·2 g/l, IgG4 < 0·03 g/l [11]. The disease duration in gold-treated RA patients with an isolated or combined subclass deficiency was significantly longer than that of the rest of the gold-treated group (median 15 years versus 9 years, P = 0·003, Mann–Whitney U-test) and a higher proportion had erosive disease (100% versus 82%, P = 0·03, Fisher's exact), but the rheumatoid factor status, age and the cumulative dose of gold did not distinguish these two groups. In contrast, there were no significant differences in disease duration, erosive status or other demographic factors between the RA control subclass deficiency patients and the rest of the RA control group.
Table 5.
Numbers of rheumatoid arthritis (RA) patients with an isolated or combined deficiency of IgG subclasses
| Patient group | IgG1 alone | IgG2 alone | IgG3 alone | IgG4 alone | IgG 2 + 3 | IgG 2 + 4 | IgG 1 + 2 | IgG 1 + 2 + 3 | IgG 2 + 3 + 4 |
|---|---|---|---|---|---|---|---|---|---|
| Controls | 0 | 4 | 2 | 0 | 1 | 0 | 0 | 0 | 0 |
| Gold | 1 | 8 | 6 | 4 | 2 | 2 | 1 | 1 | 1 |
Deficiency defined as IgG1 = 3·2 g/l, IgG2 = 1·2 g/l, IgG3 = 0·2 g/l, IgG4 = 0·1 g/l.
Deficiency most frequently affected IgG2, occurring in 15 (18%) gold-treated patients and five (6%) RA controls. Because this subclass is produced in response to carbohydrate antigens [12], we studied the binding of IgG2 in each IgG2-deficient sample to a mixture of polysaccharide pneumococcal antigens (Pneumovax II, containing 23 serotypes). Binding to pneumococcal antigens equivalent to that of pooled normal human serum was found in only 4/14 gold-treated and 1/5 RA control IgG2-deficient patients. Binding was subnormal in the majority of samples and did not correlate with the total concentration of IgG2 (see Fig. 1).
Fig. 1.
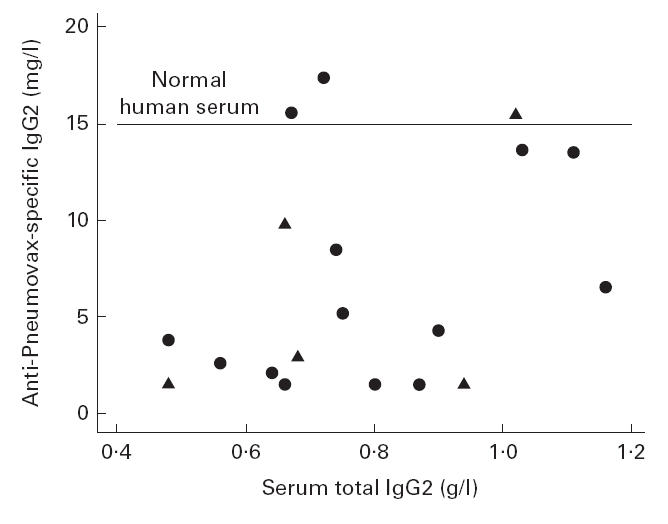
Relationship between serum total IgG2 concentration (g/l) and specific IgG2 anti-Pneumovax II concentration (mg/l) by ELISA in 14 gold-treated rheumatoid arthritis (RA) patients (•) and five RA controls (▴) with IgG2 deficiency. Specific IgG2 anti-Pneumovax II concentration in a pool of normal human serum shown by the horizontal line.
DISCUSSION
Immunoglobulin deficiency is a widely described complication of gold treatment [13,14]. This study provides the first extensive analysis of the effect of gold treatment on IgG subclasses. Gold treatment is associated with a significant reduction of the concentration of serum IgG1, IgG2 and IgG3 both in a cross-section analysis of RA patients compared with RA controls and also in a prospective analysis. This effect is not related to the cumulative dose of gold. In contrast, the effect of gold on IgG4 and IgE, the Th2-dependent antibodies [8], is less marked than that on IgG1, 2 and 3. In the cross-section analysis the degree of reduction of IgG4 was less significant than that of other IgG subclasses and gold treatment had no significant effect on the concentration of IgE compared with RA controls. Furthermore, this pattern was also found in individual gold-treated patients followed prospectively, where there was no significant change in the concentration of IgG4 or IgE.
In all patients the decision to select gold or a different DMARD reflected the practice of five separate clinicians and was not based on any preset criteria. Gold was the first-line DMARD in 68% of the patients in this group, the remainder having failed previously to respond to penicillamine, sulphasalazine or methotrexate. It is difficult to rule out selection bias, but the recruitment of patients from five different clinicians' practices in two hospitals should reduce any such effect.
The gold-treated and RA control groups differed with respect to steroid treatment as 27% of patients in the gold-treated group were also treated with low-dose prednisolone. This may have exaggerated the differences in IgG4 and IgE between the groups, as corticosteroids are reported to induce a Th2 shift in immune responses [15]. However, there was no evidence of this in our patients as there was no significant difference in the concentration of IgG4 and IgE in patients treated with gold and prednisolone compared with those treated with gold alone.
From a clinical perspective both groups were identical, in having long-standing RA with a high proportion of patients with rheumatoid factor and erosions (see Table 1). However, in statistical terms in the gold-treated group the disease duration was significantly longer (median 8 versus 10 years) and a higher proportion had erosive disease, both indirect markers of disease severity. For this reason multiple regression analysis was employed to exclude the effect of disease duration, rheumatoid factor and erosive status on immunoglobulin concentrations. Nonetheless, as RA is thought to be a Th1-driven disease [1,2] the tendency for the gold-treated group to have more active disease might be predicted to induce a bias against Th2-dependent antibodies. However, this is the opposite of our observations, which show an apparent sparing of IgG4 and IgE from the global reduction of IgG subclasses in the gold-treated group.
One interpretation of these data is that the relative sparing of Th2-dependent antibodies from gold-induced immunosuppression is a direct effect of gold treatment itself. This interpretation is supported by evidence that gold induces a Th2-like response in animal models characterized by increased secretion of IL-4 and IgE [4], and by a report of an increase in total IgE and eosinophilia (dependent on the Th2 cytokine IL-5) in patients with gold-induced adverse events [5]. This later effect was not found in our subgroup with predominantly mucocutaneous adverse events, but the group was small (because the patients were ‘gold survivors’) and the effect of other susceptibility factors, such as the HLA-DR3 allele [16], was not investigated in this study.
Other evidence for a direct Th2-like effect of gold comes from the glomerular deposits in membranous nephropathy, which occur in 2–10% of gold-treated patients [6]. The presence of IgG4 and C3 at the site of pathology is striking [7,9], since aggregated IgG4 can fix C3 and activate complement by the alternate pathway. Therefore the co-localization of IgG4 and C3 in membranous nephropathy may be of functional significance to pathogenesis. In this study very few patients developed gold-induced membranous nephropathy; however, a study of serum IgG4 and IgE in such patients is being undertaken.
In addition to the induction of a general reduction of IgG subclasses, this study reveals a high prevalence of IgG subclass deficiencies amongst gold-treated patients. The prevalence of either an isolated or combined IgG subclass deficiency is 3·4-fold higher in gold-treated patients than in controls. In our study the adjusted prevalence of 21·5% in the gold-treated group (allowing for the prevalence in RA controls) is considerably higher than an estimated 2% prevalence of gold-induced total IgG, IgA or IgM deficiency in a separate report where the population size was unknown [14]. Subclass deficiency was not related to the cumulative dose of gold but was associated with two markers of severity (disease duration and erosive status) in the gold-treated patients. IgG2 was the most commonly affected subclass and in the majority of these cases there was subnormal specific IgG2 binding to Pneumovax II, a mixture of widespread polysaccharide antigens to which all UK adults would be expected to react [17]. A recent study has also reported similar IgG2 antibody defects to Pneumovax II immunization in two gold-treated RA patients, and in three non-gold-treated RA patients with bronchiectasis [19]. Although none of the patients in our series had had recurrent or invasive bacterial infection, these findings are intriguing, as increased susceptibility to infection is widely recognized in RA [14,18] and a larger study of IgG2 functional activity in RA would be of interest.
In conclusion, this study has shown that gold treatment is associated with a global reduction of IgG subclasses and a high prevalence of subclass deficiency. The effect on the Th2-dependent IgG4 and IgE antibodies is less marked than that on IgG1, 2 or 3, and this provides evidence supporting a sparing of Th2-like responses. The current data do not support a role for the routine measurement of IgG4 or IgE in the monitoring or assessment of gold-induced adverse events. However, it may be prudent to measure IgG subclasses in gold-treated patients with long-standing erosive disease who are at increased risk of severe deficiencies and consequent infection.
Acknowledgments
This study was funded by The St Helier NHS Trust Fund for Research. We thank Mr A. Wise and staff of the Protein Reference Unit at St George's Hospital Medical School for technical help, and Professor J. M. Bland for statistical help. We thank Drs J. Axford, B. Bourke, F. Bruckner, O. Duke and S. Patel for access to their patients.
REFERENCES
- 1.Miossec P, van den Berg W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997;40:2105–15. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- 2.Kiely PDW. The Th1-Th2 model—what relevance to inflammatory arthritis? Ann Rheum Dis. 1998;57:328–30. doi: 10.1136/ard.57.6.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forestier J. Rheumatoid arthritis and its treatment by gold salts. Lancet. 1934;2:646–8. [Google Scholar]
- 4.Goldman M, Druet P, Gleichmann E. Th2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–7. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 5.Davis P, Ezeoke A, Munro J, Hobbs JR, Hughes GRV. Immunological studies on the mechanism of gold hypersensitivity. Brit Med J. 1973;III:676–8. doi: 10.1136/bmj.3.5882.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall CL, Fothergill NJ, Blackwell MM, Harrison PR, MacKenzie JC, MacIver AG. The natural course of gold nephropathy: long term study of 21 patients. Br Med J. 1987;295:745–8. doi: 10.1136/bmj.295.6601.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doi T, Mayumi M, Kanatsu K, Suehiro F, Hamashima Y. Distribution of IgG subclasses in membranous nephropathy. Clin Exp Immunol. 1984;58:57–62. [PMC free article] [PubMed] [Google Scholar]
- 8.Lundgren M, Persson U, Larsson P, et al. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989;19:1311–5. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira DBG. Membranous nephropathy: an IgG4-mediated disease. Lancet. 1998;351:670–1. doi: 10.1016/S0140-6736(97)04122-6. [DOI] [PubMed] [Google Scholar]
- 10.Miles J, Riches PGR. The determination of IgG subclass concentrations in serum by enzyme linked immunosorbent assay: establishment of age related reference range for cord blood samples, children aged 5–13 years and adults. Ann Clin Biochem. 1994;31:245–8. doi: 10.1177/000456329403100305. [DOI] [PubMed] [Google Scholar]
- 11.French AH, Harrison G. Serum IgG subclass concentrations in healthy adults: a study using monoclonal antisera. Clin Exp Immunol. 1984;56:473–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Siber GR, Schur PH, Aisenberg AC, et al. Correlation between serum IgG2 concentrations and the antibody response to bacterial polysaccharide antigens. N Eng J Med. 1990;303:178. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- 13.Lorber A, Simon T, Leeb J, Peter A, Wilcox S. Chrysotherapy, suppression of immunoglobulin synthesis. Arthritis Rheum. 1978;21:785–91. doi: 10.1002/art.1780210708. [DOI] [PubMed] [Google Scholar]
- 14.Snowden N, Dietch DM, Teh LS, Hilton RC, Haeney MR. Antibody deficiency associated with gold treatment: natural history and management in 22 patients. Ann Rheum Dis. 1996;55:616–21. doi: 10.1136/ard.55.9.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rook GAW, Hernandez-Pando R, Lightman SL. Hormones, peripherally activated pro-hormones and regulation of the Th1/Th2 balance. Immunol Today. 1994;7:301–3. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 16.Wooley PA, Griffin J, Panayi GS, Batchelor JR, Welsh KJ, Gibson TJ. HLA DR antigens and toxic reactions to sodium aurothiomalate and d-penicillamine in patients with rheumatoid arthritis. N Eng J Med. 1980;303:300–2. doi: 10.1056/NEJM198008073030602. [DOI] [PubMed] [Google Scholar]
- 17.Hazlewood M, Nusrat R, Kumararatne DS, et al. The acquisition of anti-pneumococcal capsular polysaccharide, Haemophilus influenzae type b and tetanus toxoid antibodies, with age, in the UK. Clin Exp Immunol. 1993;93:157–64. doi: 10.1111/j.1365-2249.1993.tb07959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson TM, Bevan M, Linton S, Williams BD. Serious opportunistic infection associated with gold-induced panhypogammaglobulinaemia. Brit J Rheumatol. 1998;37:914–6. doi: 10.1093/rheumatology/37.8.914b. [DOI] [PubMed] [Google Scholar]
- 19.Snowden N, Moran A, Booth J, Haeney MR, Swinson DR. Defective antibody production in patients with rheumatoid arthritis and bronchiectasis. Clin Rheumatol. 1999;18:132–5. doi: 10.1007/s100670050070. [DOI] [PubMed] [Google Scholar]


