Abstract
A number of adhesion molecules participate in the recruitment of inflammatory cells to the site of inflammation, and selectins together with their ligands are important in the early transient adhesion phase. In this study, we evaluated the role of l-selectin in T lymphocyte alveolitis in patients with active pulmonary sarcoidosis. We measured serum and bronchoalveolar lavage fluid (BALF) concentrations of soluble (s)l-selectin using an ELISA. Serum and BALF concentrations of sl-selectin were significantly elevated in patients with sarcoidosis compared with control healthy subjects and idiopathic pulmonary fibrosis (IPF) patients (P < 0·05 and P < 0·01, respectively). The lymphocyte surface marker was also examined in peripheral blood and BALF by flow cytometric analysis. The percentage of CD3+CD62L+ cells (l-selectin-bearing T lymphocytes) was significantly lower in peripheral blood of sarcoidosis than in that of healthy subjects (P < 0·01). In contrast, the percentage of CD3+CD62L− cells (l-selectin-negative T lymphocytes) in BALF of patients with sarcoidosis was significantly higher than in healthy subjects (P < 0·05) and IPF patients (P < 0·01). Furthermore, there was a significant correlation between serum concentrations of sl-selectin and the number of l-selectin-negative T lymphocytes in BALF (r = 0·535, P < 0·01). Our results suggest that l-selectin may be involved in T lymphocyte alveolitis in patients with active pulmonary sarcoidosis.
Keywords: sarcoidosis, l-selectin, T lymphocytes
INTRODUCTION
Sarcoidosis is a chronic systemic disorder characterized by the presence of non-caseating granulomas and accumulation of T lymphocytes and macrophages in multiple organs [1]. The lungs in this disease show infiltration of activated CD4+ memory T lymphocytes [2,3]. However, the underlying mechanisms of lymphocyte accumulation in the lung remain to be determined, although it is likely that a number of adhesion molecules are involved in this process, such as selectins, integrins and the immunoglobulin superfamily [4]. Among these, selectins play a key role in the initial attachment of peripheral blood circulating leucocytes to the endothelium and are responsible for the rolling of leucocytes along the vascular walls. This process is followed by a firm adhesion of lymphocytes to endothelial cells, a process mediated through integrins and the immunoglobulin superfamily. Finally, leucocytes extravasate into the inflamed tissues and directly participate in the host defence process [5]. In this regard, it has been reported that cell-bound adhesion molecules such as β1-integrin and CD11a are highly expressed on T lymphocytes in bronchoalveolar lavage fluid (BALF) in sarcoidosis [3,6]. Furthermore, the concentrations of the soluble form of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) are increased in serum obtained from patients with sarcoidosis, reflecting disease activity [6–9].
l-selectin, a member of the selectin family, is a highly glycosylated protein of 95–105 kD on neutrophils and 74 kD on lymphocytes, and is constitutively expressed on most of leucocytes [10,11]. The role of l-selectin in lymphocytes has been recently demonstrated in vivo by injection of MEL-14 MoAb, which blocked their migration to sites of inflammation [12,13], and by studies of l-selectin knockout mouse, which showed impaired lymphocyte homing to peripheral lymph nodes, leucocyte rolling and leucocyte migration to the site of inflammation [14]. The surface expression of l-selectin on both neutrophils and lymphocytes is down-regulated after activation by proteolytic cleavage in the extracellular domain of the molecule [15], which results in the formation of its soluble form. It is also speculated that the loss of l-selectin allows leucocytes to de-adhere from the luminal surface of the vessel wall and transmigrate between endothelial cells and across the vessel wall into the underlying tissues [16].
In this study, we investigated the role of l-selectin in T lymphocyte alveolitis of active pulmonary sarcoidosis. For this purpose, we determined the concentration of soluble l-selectin and expression of CD62L (l-selectin) on CD3 lymphocytes both in BALF and serum samples obtained from patients with active pulmonary sarcoidosis. The results were compared with those of patients with idiopathic pulmonary fibrosis (IPF) and control healthy subjects.
PATIENTS AND METHODS
Patient population
We investigated 20 untreated Japanese patients with active sarcoidosis (mean age 54 years, range 24–76 years; 10 women), 10 patients with IPF (mean age 64 years, range 53–70 years; all men) and seven healthy subjects (mean age 26 years, range 22–38 years; two women). At the time of study, five patients with sarcoidosis, seven patients with IPF and two healthy volunteers were smokers. The normal subjects had no previous history of pulmonary diseases or airway infection. The diagnosis of pulmonary sarcoidosis was based on histological examination of biopsy samples from the lungs, lymph nodes, skin, or liver. There was no evidence of inorganic material known to cause granulomatous diseases. All patients had clinically active disease, as indicated by the presence of non-caseating epithelioid cell granulomas, new pulmonary or generalized symptoms and signs, chest radiographic abnormalities, and positive biochemical findings. The clinical classification of lung involvement in each patient was determined by chest radiography. Fifteen patients were stage 1 with bilateral hilar lymphadenopathy (BHL) but clear lung fields, while the remaining patients were classified as stage 2 with BHL and parenchymal infiltration. IPF was histologically diagnosed as usual interstitial pneumonia by open lung biopsy or video-assisted thoracoscopic lung biopsy. None of the patients with IPF was on corticosteroids at the time of the study. Patients seropositive for human T lymphotrophic virus type-1 (HTLV-1) were excluded from the study since HTLV-1 carriers are known to have elevated levels of T cell activation markers [17]. The study was approved by the local ethics committee and all individuals gave informed consent.
Bronchoalveolar lavage
BAL was performed in all individuals using a standard technique. For this purpose, the subject was first premedicated with atropine (0·5 mg intramuscularly), followed by local anaesthesia of the upper airway with 4% lidocaine. A flexible fibreoptic bronchoscope (BF-P200; Olympus, Tokyo, Japan) was wedged into a subsegment of the right middle lobe for lavage. An aliquot of 50 ml sterile physiological saline solution at body temperature was instilled through the bronchoscope and the fluid was immediately retrieved by gentle suction using a sterile syringe. The process of instillation of saline solution and retrieval was carried out four times. The lavage fluid was passed through two sheets of gauze and centrifuged at 400 g for 10 min at 4°C and the supernatant was stored at −80°C until use.
After washing twice with PBS, cells were suspended with 10% heat-inactivated fetal calf serum (FCS) and counted using a haemocytometer. The aliquot was then adjusted to 2 × 105 cells/ml and 0·2 ml sample of each cell suspension was spun down onto a glass slide at 160 g for 2 min using a cytocentrifuge (Cytospin 2; Shandon Instruments, Sewickly, PA). The slides were later dried, fixed, and then stained by the May–Giemsa method. Two hundred cells were identified with a photomicroscope. The remaining cells were resuspended in PBS, supplemented with 10% FCS, and incubated in plastic flasks for 90 min at 37°C in humidified 5% CO2–air to deplete alveolar macrophages. The cells were then centrifuged at 500 g for 5 min at 4°C, the supernatant was discarded, and cells were resuspended in PBS. Cells were then washed twice in PBS, passed through a 100-μ m nylon mesh, and finally adjusted to a concentration of 1 × 106/ml. More than 90% of non-adherent cells collected for flow cytometric analysis were viable by the trypan blue exclusion test.
Monoclonal antibodies
FITC-conjugated anti-CD62L (Leu-8) antibody, a MoAb to l-selectin, and PE-conjugated anti-CD3 (Leu-4) antibody were purchased from Becton Dickinson (Mountain View, CA). Mouse IgG1 and IgG2a conjugated with FITC or PE were purchased from Becton Dickinson and used to determine the borderline between stained and unstained cells in flow cytometric analysis.
Two-colour direct immunofluorescence staining
A total of 100 μ l of whole blood collected by venepuncture with EDTA was placed into a 12 × 5 mm polystyrene tube (Falcon Plastics, Oxnard, CA) and 5 μ l of each MoAb were added. The tubes were incubated for 15 min at room temperature in darkness and 2 ml of 1 × FACS lysing solution (Becton Dickinson) were added to destroy erythrocytes. The cells were mixed vigorously, then incubated for 10 min at room temperature followed by washing once in cold PBS containing 0·1% sodium azide. Cells were finally resuspended in cold PBS containing 0·5% paraformaldehyde. Serum samples were prepared from clotted blood obtained at the same time as flow cytometric analysis, and stored in aliquots at −80°C until use. Peripheral blood samples were simultaneously obtained from all patients with sarcoidosis, IPF and seven healthy volunteers at the time of BALF sampling.
BALF cells were adjusted to 1 × 106/ml. A total of 5 μ l of each MoAb was placed into a polystyrene tube and 100 μ l of the cell suspension (1 × 105 cells) were added. Cells were incubated for 30 min on ice in darkness, washed once in cold PBS containing 0·1% sodium azide, and then resuspended in cold PBS containing 0·5% paraformaldehyde. The fixed cells were kept in darkness at 4°C until analysis.
Two-colour flow cytometry
Stained cells were analysed on a flow cytometer equipped with an argon ion laser set at 488 nm (FACScan; Becton Dickinson, FACS Division), and a computer system (Consort 30; Becton Dickinson) was used for data acquisition and analysis. A minimum of 10 000 events was collected for each sample. A cell gate containing lymphocytes was established based on forward and side light scatter. To determine the borderline between stained and unstained cells, cells were stained with mouse PE-conjugated IgG1 and FITC-conjugated IgG2a. The percentages were calculated based on the number of lymphocytes found in each quadrant. Interassay reproducibility was checked using beads (CaliBRITE; Becton Dickinson) and a software program (AutoCOMP; Becton Dickinson). The absolute number of CD3+CD62L− cells in BALF was calculated using the formula: (total cell count (× 105/ml) × percentage CD3+CD62L− cells/100).
Measurement of soluble l-selectin
Soluble (s)l-selectin was measured in serum and BALF samples obtained from patients with sarcoidosis, IPF and healthy controls using a commercially available sandwich ELISA kit (R&D Systems Europe, Abingdon, UK). Briefly, 80 μ l of standard lavage fluid and serum were transferred to microtitre wells coated with the MoAb. They were then incubated with 20 μ l of biotin-conjugated second MoAb at room temperature for 60 min. After washing five times with TBS containing 0·05% Tween 20, binding of biotinylated antibody was detected by incubation with streptavidin–peroxidase complexes at room temperature for 30 min, followed by reaction with a specific substrate. The optical density (OD) of each well was read at 492 nm using a microplate reader (Flow Labs, Rickmansworth, UK). The concentration of human sl-selectin was calibrated from a dose–response curve based on reference standards and expressed in ng/ml.
Statistical analysis
All values were expressed as median (range). Significant differences were identified by non-parametric testing, using the Statview 4.0 statistical package. The Kruskal–Wallis test was used to examine differences between three groups. Pearson correlation was used to examine the relationship between various parameters. P <0·05 was considered significant.
RESULTS
Total and differential cell counts in BALF
The total cell counts in BALF of patients with sarcoidosis and IPF were significantly higher than in healthy controls; the count was also significantly higher in IPF than in sarcoidosis (Table 1). Significantly higher percentages of lymphocytes and lower percentages of macrophages were also observed in sarcoidosis relative to those in IPF and healthy controls (Table 1).
Table 1.
Comparison of total and differential cell counts in bronchoalveolar lavage fluid from healthy subjects, patients with sarcoidosis and idiopathic pulmonary fibrosis (IPF)
| Total cells (× 105/ml) | Macrophages (%) | Lymphocytes (%) | Neutrophils (%) | Eosinophils (%) | |
|---|---|---|---|---|---|
| Healthy subjects (n = 7) | 1·2 (0·6–2·4) | 89·0 (79·8–93·6) | 9·2 (3·0–19·1) | 1·4 (0·2–4·5) | 0·2 (0·0–2·0) |
| Sarcoidosis (n = 20) | 2·6* (1·1–5·5) | 64·8†‡ (44·8–83·5) | 31·8§ (11·6–51·4) | 1·2 (0·2–12·1) | 0·6 (0·0–2·4) |
| IPF (n = 10) | 6·2** (3·4–8·2) | 82·8 (43·6–95·0) | 5·6 (2·7–21·9) | 5·1 (0·9–36·7) | 1·0 (0·0–12·8) |
Values are expressed as median (range).
P < 0·05 compared with healthy subjects;
P < 0·01 compared with healthy subjects and sarcoidosis.
P < 0·01 compared with healthy subjects
P < 0·05 compared with IPF.
P < 0·01 compared with healthy subjects and IPF.
Soluble l-selectin levels in serum and BALF
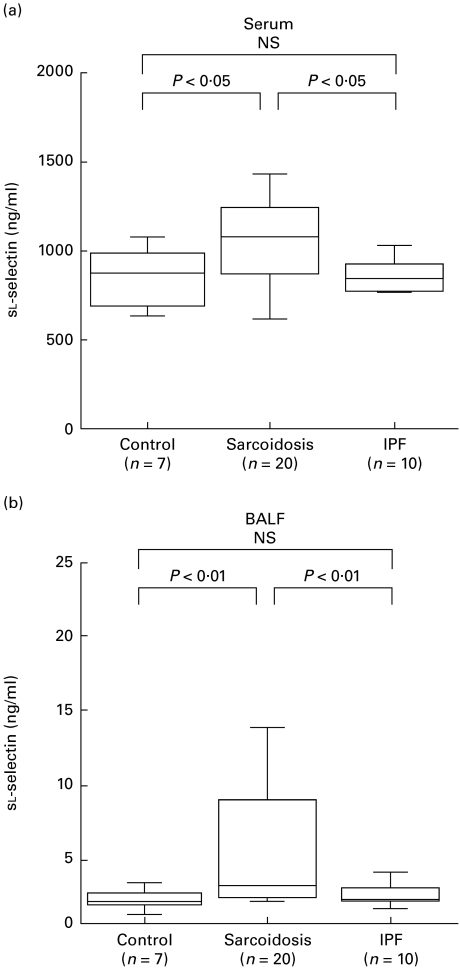
Serum concentrations of sl-selectin were significantly higher in patients with sarcoidosis (1068 ng/ml (655–1434 ng/ml)) relative to those in IPF patients (840 ng/ml (762–1028 ng/ml)) and healthy subjects (899 ng/ml (737–1046 ng/ml)) (P < 0·05, Fig. 1a). Furthermore, BALF concentrations of sl-selectin were significantly higher in patients with sarcoidosis (2·4 ng/ml (1·5–13·0 ng/ml)) than IPF (1·9 ng/ml (1·7–3·8 ng/ml)) and control (2·1 ng/ml (0·2–3·1 ng/ml)) (P < 0·01, Fig. 1b).
Fig. 1.
Concentrations of soluble l-selectin in serum (a) and bronchoalveolar lavage fluid (BALF) (b). The whisker box plots represent the 25th to 75th percentile of results inside the box, the median is indicated by the horizontal bar inside the box, and the whiskers on each box represent the 10th to 90th percentiles. Control, Healthy subjects; IPF, idiopathic pulmonary fibrosis; NS, not significant.
Expression of l-selectin on surface of T lymphocytes in peripheral blood and BALF
The percentage of CD3+CD62L+ and CD3+CD62L− cells in paired samples was compared in all healthy subjects and patients. As shown in Table 2, the percentage of CD3+CD62L+ cells in peripheral blood was significantly lower in sarcoidosis patients than in healthy subjects (P < 0·01). However, the percentages of these cells were similar in BALF of the three groups. The percentage of CD3+CD62L− cells in BALF of sarcoidosis patients was significantly higher than in healthy subjects (P < 0·05) and IPF patients (P < 0·01), but there was no difference between healthy subjects and IPF patients. There was also no significant difference in the percentage of these cells in peripheral blood among the three groups.
Table 2.
Expression of l-selectin on the surface of T lymphocytes from peripheral blood and bronchoalveolar lavage fluid (BALF)
| CD3+CD62L+ (%) | CD3+CD62L− (%) | |||||
|---|---|---|---|---|---|---|
| Healthy subjects (n = 7) | Sarcoidosis (n = 20) | IPF (n = 10) | Healthy subjects (n = 7) | Sarcoidosis (n = 20) | IPF (n = 10) | |
| Peripheral blood | 39·5 | 13·1* | 15·8 | 28·1 | 41·5 | 36·0 |
| (29·8–44·8) | (4·4–26·6) | (8·3–41·4) | (21·2–29·1) | (24·0–60·9) | (17·4–56·1) | |
| BALF | 5·2 | 3·6 | 5·1 | 76·5 | 86·2**† | 83·7 |
| (2·9–7·4) | (1·4–13·2) | (1·2–8·9) | (75·0–79·4) | (61·5–94·3) | (72·1–88·5) | |
Values are expressed as median (range).
IPF, Idiopathic pulmonary fibrosis.
P < 0·01 compared with healthy subjects;
P < 0·05 compared with healthy subjects.
P < 0·01 compared with IPF.
Correlation between serum sl-selectin levels and the absolute number of CD3+CD62L− cells in bronchoalveolar lavage fluid
In patients with sarcoidosis, a significant correlation was noted between serum sl-selectin concentrations and the absolute number of CD3+CD62L− cells in BALF (r = 0·535, P < 0·01, Fig. 2). However, there was no significant correlation between serum sl-selectin concentrations and various parameters of disease activity such as serum angiotensin converting enzyme (ACE) and lysozyme (data not shown).
Fig. 2.
Correlation between serum sl-selectin and absolute number of CD3+CD62L− cells in bronchoalveolar lavage fluid of patients with sarcoidosis.
DISCUSSION
The main findings of the present study were the following: (i) the presence of high concentrations of sl-selectin in the serum and BALF of patients with sarcoidosis relative to those of healthy subjects and IPF patients (Fig. 1); (ii) a high percentage of lymphocytes in BALF of patients with sarcoidosis compared with the other two groups (Table 1). We also found a low percentage of l-selectin-bearing T lymphocytes in peripheral blood of sarcoidosis patients compared with healthy subjects in contrast to a high percentage of l-selectin-negative T lymphocytes in BALF of sarcoidosis patients compared with healthy subjects and IPF patients (Table 2); (iii) a significant correlation between serum sl-selectin and l-selectin-negative T lymphocyte counts in the lungs of patients with sarcoidosis (Fig. 2).
l-selectin shows a unique property of being proteolytically cleaved in the membrane-proximal extracellular region to yield a soluble fragment. l-selectin on T lymphocytes is down-regulated by cross-linking CD3 or by mitogenic stimulation [18]. This suggests that the high soluble form of l-selectin in serum of sarcoidosis patients may be due to its cleavage on the surface of T lymphocytes activated by unknown antigen, hence explaining the low expression of l-selectin on peripheral T lymphocytes. In addition, the cleavage of l-selectin may also occur in the lung of sarcoidosis patients, since significantly higher levels of sl-selectin and percentages of CD3+CD62L−cells were observed in BALF.
It has also been proposed that shedding or loss of l-selectin occurs when cells migrate through the endothelium to the inflammatory site [16,19]. Furthermore, the lack of l-selectin on antigen-activated lymphocytes is thought to underlie altered migration of these lymphocytes away from lymph nodes and into non-lymphoid tissues [20]. In our study, a significantly low percentage of l-selectin-bearing T lymphocytes was found in peripheral blood, in parallel with the finding of a high percentage of l-selectin-negative T lymphocytes in BALF. Our results also showed a significant correlation between serum levels of sl-selectin and the number of l-selectin-negative T lymphocytes in BALF in patients with sarcoidosis. Considered together with these findings, we suggest that shedding of l-selectin occurs when T lymphocytes transmigrate from the circulation into the lung of sarcoidosis patients, and that l-selectin contributes to the accumulation of T lymphocytes in pulmonary sarcoidosis. However, it is necessary to define whether this event is specific to sarcoidosis by comparing our findings not with IPF patients but with other diseases known to be associated with T lymphocyte alveolitis.
Berlin et al. [6] reported that serum concentrations of sE-selectin and sICAM-1 were significantly higher in active than in inactive sarcoidosis. In addition, Ishii & Kitamura [9] also reported a similar relationship between sICAM-1 concentrations both in serum and BALF and disease activity. In contrast, our study demonstrated no correlation between serum concentrations of sl-selectin and various clinical laboratory parameters of sarcoid disease activity. This is in agreement with the report of Berlin et al. [6], who showed no difference in serum sl-selectin between active and inactive sarcoidosis. Although we can not explain the discrepancy in the concentrations of various soluble adhesion molecules, our findings suggest that serum levels of endothelium-derived soluble adhesion molecules may be more reflective of disease activity of sarcoidosis than those of lymphocyte-derived molecules. Another explanation is that a number of parameters used to assess sarcoid disease activity are conventional and not completely satisfactory. However, since a significant correlation was observed between serum levels of sl-selectin and the number of l-selectin-negative T lymphocytes in BALF, it remains to be determined whether the level of circulating l-selectin reflects an aspect of the disease that would make it a useful marker for clinical and pathological activity of sarcoidosis.
In conclusion, we found higher levels of l-selectin in serum and BALF of patients with sarcoidosis than in healthy subjects and IPF patients. We also found fewer l-selectin-bearing peripheral T lymphocytes in peripheral blood of sarcoidosis than in control and higher l-selectin-negative T lymphocytes in BALF of sarcoidosis than in control and IPF patients. Furthermore, we demonstrated that serum levels of sl-selectin correlated with pulmonary T lymphocytosis negative for l-selectin. These findings suggest a possible role of l-selectin with T lymphocyte alveolitis in sarcoidosis. Recently, l-selectin was found to be important in the immune response to antigens such as primary T cell proliferation and cytokine production in experiments using l-selectin-deficient mice [21]. Taken all data together, examination of the role of l-selectin in the pathophysiology of sarcoidosis deserves further investigation.
Acknowledgments
The authors thank Mr Atsushi Yokoyama for his excellent technical support and Dr F. G. Issa (Word-Medex, Sydney, Australia) for his assistance in editing the manuscript.
REFERENCES
- 1.Thomas PD, Hunninghake GW. Current concepts of the pathogenesis of sarcoidosis. Am Rev Respir Dis. 1987;135:747–60. doi: 10.1164/arrd.1987.135.3.747. [DOI] [PubMed] [Google Scholar]
- 2.Hunninghake GW, Crystal RG. Pulmonary sarcoidosis. A disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981;305:429–34. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- 3.Iida K, Kadota J, Kawakami K, Matsubara Y, Shirai R, Kohno S. Analysis of T cell subsets and β chemokines in patients with pulmonary sarcoidosis. Thorax. 1997;52:431–7. doi: 10.1136/thx.52.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamacher J, Schaberg T. Adhesion molecules in the lung. Lung. 1994;172:189–213. doi: 10.1007/BF00164437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 6.Berlin M, Lundahl J, Skold CM, Grunewald J, Eklund A. The lymphocytic alveolitis in sarcoidosis is associated with increased amounts of soluble and cell-bound adhesion molecule in bronchoalveolar lavage fluid and serum. J Intern Med. 1998;244:333–40. doi: 10.1046/j.1365-2796.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 7.Southcott AM, Hemingway I, Lorimer S, et al. Adhesion molecule expression in the lung: a comparison between normal and diffuse interstitial lung disease. Eur Respir J. 1998;11:91–98. doi: 10.1183/09031936.98.11010091. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin AS, Shakoor Z, Kapahi P, Haskard D. Circulating adhesion molecules in sarcoidosis. Clin Exp Immunol. 1994;96:335–8. doi: 10.1111/j.1365-2249.1994.tb06563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii Y, Kitamura S. Elevated levels of soluble ICAM-1 in serum and BAL fluid in patients with active sarcoidosis. Chest. 1995;107:1636–40. doi: 10.1378/chest.107.6.1636. [DOI] [PubMed] [Google Scholar]
- 10.Griffin JD, Spertini O, Ernst TJ, et al. GM-CSF and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes and their precursors. J Immunol. 1990;145:576–84. [PubMed] [Google Scholar]
- 11.Tedder TF, Matsuyama T, Rothstein DM, Schlossman SF, Morimoto C. Human antigen-specific memory T cells express the homing receptor necessary for lymphocyte recirculation. Eur J Immunol. 1990;20:1351–5. doi: 10.1002/eji.1830200622. [DOI] [PubMed] [Google Scholar]
- 12.Dawson J, Sedgwick AD, Edwards JC, Lees P. The monoclonal antibody MEL-14 can block lymphocyte migration into a site of chronic inflammation. Eur J Immunol. 1992;22:1647–50. doi: 10.1002/eji.1830220646. [DOI] [PubMed] [Google Scholar]
- 13.Pizcueta P, Luscinskas FW. Monoclonal antibody blockade of l-selectin inhibits mononuclear leukocyte recruitment to inflammatory sites in vivo. Am J Pathol. 1994;145:461–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Arbones ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in l-selectin deficient mice. Immunity. 1994;1:247–60. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 15.Kahn J, Ingraham RH, Shirley F, Migaki GI, Kishimoto TK. Membrane proximal cleavage of l-selectin: identification of the cleavage site and a 6-kD transmembrane peptide fragment of l-selectin. J Cell Biol. 1994;125:461–70. doi: 10.1083/jcb.125.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–41. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 17.Sei Y, Yasuda K, Hara A, Yasuda K, Yokoyama MM. Cell surface antigen expression in cultured lymphocytes derived from healthy HTLV-1 carriers. J Clin Lab Immunol. 1986;19:71–75. [PubMed] [Google Scholar]
- 18.Buhrer C, Berlin C, Jablonski-Westrich D, Holzmann B, Thiele HG, Hamann A. Lymphocyte activation and regulation of three adhesion molecules with supposed function in homing. LECAM-1 (MEL-14 antigen), LPAM-1/2 (alpha 4-integrin) and CD44 (Pgp-1) Scand J Immunol. 1992;35:107–20. doi: 10.1111/j.1365-3083.1992.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 19.Georas SN, Liu MC, Newman W, Beall LD, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial cell activation accompany the recruitment of human granulocytes to the lung after segmental antigen challenge. Am J Respir Cell Mol Biol. 1992;7:261–9. doi: 10.1165/ajrcmb/7.3.261. [DOI] [PubMed] [Google Scholar]
- 20.Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–7. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med. 1996;183:589–98. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]