Abstract
The reaction of ANCA with ANCA antigens on the surface of neutrophils may play a critical role in the pathogenesis of ANCA vasculitis. Therefore, an understanding of the circumstances that result in surface expression of these antigens is important for an understanding of pathogenic mechanisms. In this study we investigated the surface expression of ANCA antigens on quiescent, primed, and apoptotic neutrophils. ANCA antigens and other granule constituents were not detected on the surface of neutrophils in freshly heparinized blood. ANCA antigens were on the surface of neutrophils primed by in vitro incubation for 4 h and 8 h. These cells did not show evidence of apoptosis. After 24 h incubation, about 30% of the neutrophils were apoptotic, and ANCA antigens and other granule constituents were present on the surface of both apoptotic and non-apoptotic cells. Our data indicate that there are no ANCA antigens on the surface of quiescent neutrophils, but that they are on the surface of primed neutrophils before the cells become apoptotic, and remain on the surface of cells after they become apoptotic. Based on these observations, we hypothesize that ANCA can react in vivo with primed but not quiescent neutrophils. Previously published observations indicate that the interaction of ANCA with primed neutrophils results in neutrophil activation, which may be involved in the pathogenesis of ANCA vasculitis.
Keywords: ANCA antigen, surface expression, neutrophil, primed apoptosis
INTRODUCTION
ANCA are specific for proteins in the cytoplasmic granules of neutrophils and the lysosomes of monocytes [1–4]. The two major ANCA antigens are myeloperoxidase (MPO) and proteinase 3 (PR3), although there are other less frequent ANCA antigens, such as elastase, cathepsin G, anti-bactericidal/permeability-increasing protein, lactoferrin, azurocidin, and lysozyme [1–4]. ANCA, especially MPO-ANCA and PR3-ANCA, are closely associated with a spectrum of vasculitides, including Wegener’s granulomatosis, microscopic polyangiitis, and Churg–Strauss syndrome [1–4].
There is increasing evidence that ANCA are involved in the pathogenesis of ANCA vasculitis [1–4]. Most of the proposed pathogenic mechanisms require that ANCA antigens are translocated from the cytoplasm to the surface of neutrophils in order for ANCA to bind to the antigens and activate neutrophils. We and other investigators have concluded that ANCA antigens are displayed at the surface of viable neutrophils that have been primed by cytokines, such as tumour necrosis factor (TNF) [5–9]. Gilligan and associates have contended that ANCA antigens occur on the surface of apoptotic neutrophils in the absence of prior neutrophil priming [10]. The theoretical pathogenic potential of ANCA is very different if ANCA antigens are expressed only on apoptotic neutrophils rather than primed viable neutrophils. This study addresses two major controversies pertaining to the surface expression of ANCA antigens by neutrophils: (i) whether or not ANCA antigens are expressed by quiescent neutrophils, and (ii) whether or not ANCA antigens are expressed by neutrophils that are primed or have become apoptotic. In the following study we demonstrate that ANCA antigens are not on the surface of quiescent neutrophils, but are on the surface of incubated (primed and activated) neutrophils as well as cells that have become apoptotic.
MATERIALS AND METHODS
Preparation of antibodies
Human anti-PR3 IgG and human anti-MPO IgG were isolated from PR3-ANCA+ or MPO-ANCA+ sera by ammonium sulphate precipitation and chromatographed on HiTrap Protein G column by fast protein liquid chromatography (FPLC; Pharmacia Biotech, Uppsala, Sweden) [9]. Mouse monoclonal anti-human PR3, rabbit anti-human PR3, and rabbit anti-human azurocidin antibodies were prepared in our laboratory. The specificities of these antibodies were confirmed by ELISA, Western blot and indirect immunofluorescence assay. Monoclonal mouse and polyclonal rabbit anti-human MPO antibodies (Dako, Carpinteria, CA), rabbit anti-human elastase antibody (Calbiochem, San Diego, CA), rabbit anti-human cathepsin G antibody (Calbiochem), sheep anti-human lysozyme antibody (Calbiochem), and rabbit anti-human lactoferrin antibody (Biodesign Int., Kennebunk, ME) were purchased. Normal human IgG was obtained from Pierce (Rockford, IL) and normal rabbit serum from Sigma (St Louis, MO).
Incubation of whole blood
To avoid neutrophil priming during isolation procedures, 2-ml aliquots of heparinized venous blood from 12 healthy volunteers were directly pipetted into 12 × 75 mm polypropylene culture tubes (Fisher, Fair Lawn, NJ) and maintained in a humidified tissue culture incubator at 37°C in an atmosphere of 5% CO2/95% air for 0, 4, 8 or 24 h.
Immunofluorescence staining
For immunofluorescence staining, 100-μ l aliquots of whole blood from each time point were added to the bottom of 12 × 75 mm tubes and kept in ice. Primary antibodies, either human anti-MPO IgG (25 μg/ml), polyclonal anti-MPO antibody (1:100), monoclonal anti-MPO antibody (1:40), human anti-PR3 IgG (25 μg/ml), rabbit anti-PR3 serum (1:20), monoclonal anti-PR3 (1:2), rabbit anti-elastase antibody (1:20), rabbit anti-azurocidin serum (1:20), rabbit anti-cathepsin G antibody (1:20), sheep anti-lysozyme antibody (1:10), or rabbit anti-lactoferrin antibody (1:20) were directly added to the blood and incubated for 30 min at 4°C. The samples were washed with ice-cold PBS and pelleted at 200 g for 5 min at 4°C. The samples were resuspended to 100 μl with FITC-conjugated affinipure F(ab′)2 fragment donkey anti-human IgG + IgM (H + L), donkey anti-rabbit IgG (H + L), donkey anti-mouse IgG (H + L), or donkey anti-sheep IgG (H + L) (Jackson, West Grove, PA) that was diluted 1:200 with 0·2% goat serum/PBS and incubated for 20 min at 4°C. After washing twice with PBS, erythrocytes were lysed and leucocytes were fixed with Dako Uti-Lyse reagent (Dako). Cells treated with 2 ng/ml of recombinant TNF-α for 30 min to cause surface expression of ANCA antigens were used as positive control. Negative controls included normal human IgG (25 μg/ml) and normal rabbit serum (1:20) as primary antibody, and secondary antibody alone.
Detection of surface expression
Surface expression of ANCA antigens was detected by both fluorescence microscopy and flow cytometry after immunofluorescence staining. After immunofluorescence staining, cells were observed with a Nicon FXA microscope with FITC filter, excitation 450–490 nm and emission long pass 510 nm (Nikon, Garden City, NY), and the surface expression of ANCA antigens was identified by cell surface staining.
After immunofluorescence staining, cells were analysed by a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) linked to a Cicero/Cyclops system (Cytomation, Fort Collins, CO) for data acquisition and analysis, in which only neutrophils were gated and 20 000 events per sample were collected. The surface expression of ANCA antigens was confirmed by the increase of fluorescence intensity of cells.
Assessment of release of ANCA antigens
Release of ANCA antigens from neutrophils was assessed by measuring the increased concentration of ANCA antigens in plasma of incubated blood using anti-MPO and anti-lactoferrin ELISA. Microtitre plates (Costar, Cambridge, MA) were coated overnight with plasma (100 μl/well). After blocking, the plates were incubated with polyclonal rabbit anti-MPO antibody (1:3000) or rabbit anti-lactoferrin antibody (1:50) for 2 h at room temperature. Bound antibodies were detected with an alkaline phosphatase-conjugated affinity-purified donkey anti-rabbit IgG (H + L) (1:10 000) (Jackson) and alkaline phosphatase substrate kit (BioRad, Hercules, CA). Optical density (OD) was read at 405 nm and the results were calculated from MPO (Calbiochem) and lactoferrin (Calbiochem) standard curves [11].
Measurement of apoptosis
Cell apoptosis was measured by flow cytometry using both annexin V and propidium iodide (PI) detection. Unseparated leucocytes were obtained from incubated blood at different time points by lysis of erythrocytes with ammonium chloride lysing buffer [12]. Unfixed leucocytes were incubated with 100 μl Annexin-V-Fluos labelling solution containing 2 μl FITC-conjugated Annexin-V-Fluos labelling reagent (Boehringer, Mannheim, Germany) and 0·1 μg PI (Sigma) in incubation buffer (0·01 mol/l HEPES/NaOH pH 7·4, 0·14 mol/l NaCl, 0·005 mol/l CaCl2) per sample for 15 min in the dark, at room temperature and then another 0·4 ml incubation buffer was added. Cells were analysed by a FACScan flow cytometer linked to a Cicero/Cyclops system, in which only neutrophils were gated and 20 000 events per sample were collected. Apoptotic and non-apoptotic cells were differentiated by annexin V, which is a sensitive probe for phosphatidylserine exposed on the outer leaflet of the cell membrane in the early stages of apoptosis, and necrotic cells were excluded by PI+ staining.
For PI detection, unseparated leucocytes were fixed in ethanol overnight and stained with PI mixture [13]. DNA content was determined with a FACScan flow cytometer and 20 000 events per sample were collected. Ethanol fixation caused an increase of cell permeability allowing low molecular weight DNA fragments to leak out of cells. Thus, apoptotic cells were identified by a decrease in DNA content and formed the apoptotic sub-G0/G1 population peak to the left of the G0/G1 peak [13].
Analysis of relationship of surface expression and apoptosis
The relationship of surface expression of ANCA antigens and apoptosis of neutrophils was analysed by fluorescence microscopy and flow cytometry using concurrent staining for ANCA antigen expression and apoptosis. For fluorescence microscopy, cells were stained with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma), a DNA-specific dye, for 10 min after immunofluorescence staining using polyclonal anti-MPO antibody as primary antibody. The morphological features of neutrophils, including pyknotic nuclei, nuclear and cytoplasmic condensation, and the formation of apoptotic bodies, were considered as evidence of apoptosis [13–16]. The same cells were observed under microscopy by both a DAPI filter (excitation 330–380 nm and emission 420 nm) to detect nuclear morphology indicative of apoptosis, and a FITC filter for surface expression of ANCA antigens.
By flow cytometry, leucocytes were simultaneously incubated with 100 μl Annexin-V-Fluos labelling solution and 10 μl R-PE-conjugated anti-human MPO MoAb (Caltag Labs, Burlingame, CA) for 20 min, at room temperature. Cells were analysed by a FACScan flow cytometer, in which only neutrophils were gated. The surface expression of MPO was measured by the mean of fluorescence R-PE intensity in both living non-apoptotic and apoptotic cells, which were differentiated by annexin V and were excluded of necrosis by PI.
Statistical analysis
Analysis of variance was used to determine if any differences between group means were seen at specific time points within each experiment. When overall differences were found within the experiment, Dunnett's t-test was used to further compare means of each incubation and its associated time to the mean of the control group specific to the experiment [17]. This test evaluated the minimum significant difference between each group and control compared with the critical value of Dunnett's t at the P = 0·05 level, while controlling for multiple testing. PROC anova and PROC GLM in SAS® were used for calculating the statistical tests [18].
RESULTS
Surface expression of ANCA antigens detected by immunofluorescence microscopy
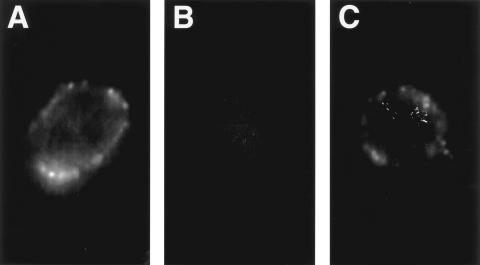
No immunofluorescence surface staining was observed on neutrophils in freshly isolated blood; however, surface staining for ANCA antigens, including MPO, PR3 and lactoferrin, was observed on almost all neutrophils cultured for 4, 8 or 24 h using polyclonal anti-MPO, monoclonal or polyclonal anti-PR3, or rabbit anti-lactoferrin as primary antibodies (Fig. 1). Surface staining was also observed on positive control neutrophils treated with 2 ng/ml of TNF for 30 min using anti-PR3 MoAb as primary antibody (Fig. 1), and no surface staining was observed on negative control neutrophils with secondary antibody alone.
Fig. 1.
Fluorescence photomicrographs of neutrophils after immunofluorescence staining using monoclonal anti-proteinase 3 (PR3) antibody as primary antibody (all three photographs had identical exposure time). (A) Positive control, neutrophil treated with 2 ng/ml of tumour necrosis factor for 30 min, shows surface staining. (B) Neutrophil from fresh whole blood shows no surface staining. (C) Neutrophil from blood cultured 8 h shows surface staining.
Surface expression of ANCA antigens detected by flow cytometry
By flow cytometry after immunofluorescence staining, neutrophils incubated for 4, 8 or 24 h showed positive staining with monoclonal anti-PR3, rabbit anti-PR3, human anti-PR3 IgG, polyclonal anti-MPO antibody, human anti-MPO IgG, rabbit anti-elastase antibody, rabbit anti-azurocidin serum, rabbit anti-cathepsin G antibody, sheep anti-lysozyme antibody, and rabbit anti-lactoferrin antibody as primary antibody (Figs 2 and 3). Compared with freshly cells, the means of fluorescence intensity increased with incubation for 4, 8, or 24 h (Fig. 4). Negative controls using normal human IgG or normal rabbit serum as primary antibody had means of fluorescence intensity that were the same as those of freshly isolated cells (Figs 2 and 3). Using monoclonal anti-MPO (Dako) as primary antibody, there were no differences in the fluorescence intensity of incubated cells and unincubated cells.
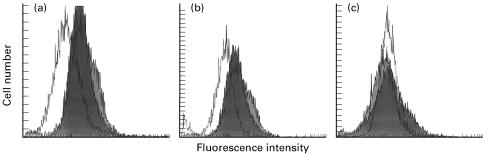
Fig. 2.
Fluorescence intensity of neutrophils by flow cytometry using human IgG antibodies as primary antibodies; myeloperoxidase (MPO)-ANCA IgG (a), proteinase 3 (PR3)-ANCA IgG (b), and normal human IgG (c). White peaks are neutrophils from uncultured fresh whole blood, dark peaks cultured 8 h, and grey peaks cultured 24 h. ANCA antigens, MPO and PR3, are present on the surface of cultured cells, but not on fresh cells. The three peaks do not separate in the control group using normal human IgG as primary antibody.
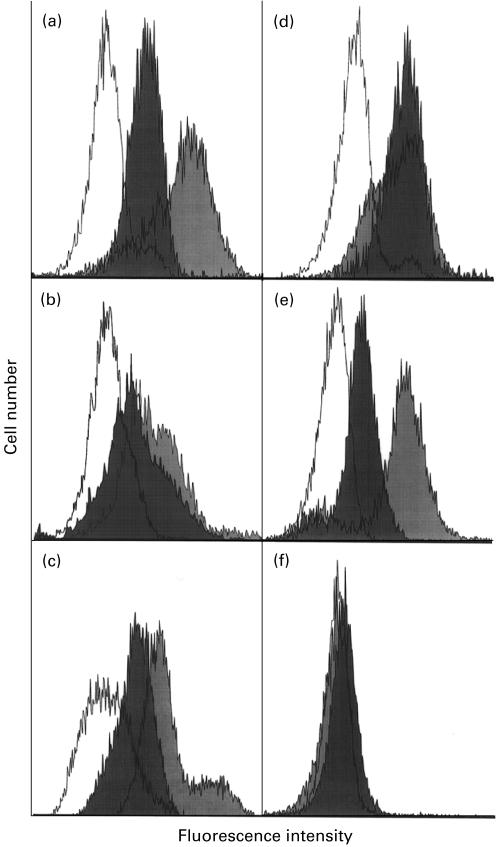
Fig. 3.
Fluorescence intensity of neutrophils by flow cytometry using rabbit IgG as primary antibodies; anti-myeloperoxidase (MPO) (a), anti-proteinase 3 (PR3) (b), anti-elastase (c), anti-cathepsin G (d), anti-lactoferrin (e), and normal rabbit serum (f). White peaks are neutrophils from uncultured fresh blood, dark peaks cultured 8 h, and grey peaks cultured 24 h. ANCA antigens, including MPO, PR3, elastase, cathepsin G, and lactoferrin, are present on the surface of cultured cells, but not on fresh cells. Three peaks do not separate in control group using normal rabbit serum as primary antibody.
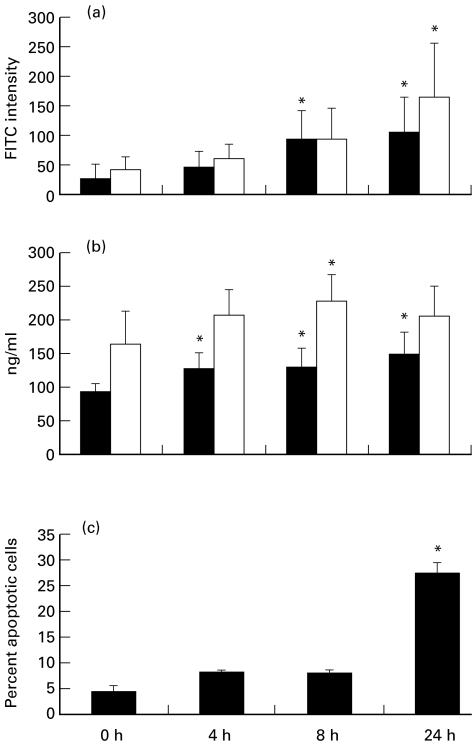
Fig. 4.
Time course of surface expression, release of granule antigens, and apoptosis in neutrophils from blood cultured for the indicated times. (a) Myeloperoxidase (MPO; ▪) and lactoferrin (□) surface expression by flow cytometry using rabbit antibodies to MPO and lactoferrin as primary antibodies and depicted as mean of FITC intensity. (b) The concentration of MPO and lactoferrin in plasma of cultured blood. (c) The percent of apoptotic neutrophils by flow cytometry using annexin V. *Statistically different from control (P < 0·05). Values are means ± s.e.m., n = 8 (a,c), n = 12 (b).
Our study indicated that ANCA antigens were not expressed on the surface of neutrophils in unseparated fresh whole blood, but ANCA antigens were on the surfaces of unincubated neutrophils which were isolated by dextran sedimentation and Hypaque–Ficoll gradient centrifugation [19] or ammonium chloride lysis buffer [12]. The mean fluorescence intensity of unincubated neutrophils from whole blood was 30·4 ± 8·2 using normal rabbit serum as primary antibody and was 27·1 ± 24·7 using rabbit anti-PR3 serum, 26·5 ± 24·1 using polyclonal rabbit anti-MPO antibody, and 41·3 ± 22·3 using rabbit anti-lactoferrin antibody as primary antibody. The mean increased to 358·2 ± 218·3, 320·6 ± 335·9, and 326·8 ± 347·2 in unincubated neutrophils isolated by dextran and Hypaque–Ficoll, and 101·5 ± 31·0, 104·4 ± 83·1, and 106·2 ± 47·5 by lysis buffer, respectively, using rabbit anti-PR3 serum, polyclonal rabbit anti-MPO antibody, or rabbit anti-lactoferrin antibody as primary antibody. The fluorescence of unincubated neutrophils revealed two intensity peaks in 11% of samples using whole blood and in 50% of samples using isolated neutrophils for immunofluorescence staining.
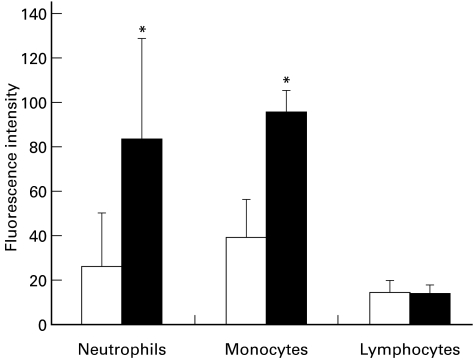
As would be expected, the surface expression of ANCA antigens also was detected on incubated monocytes, but not on incubated lymphocytes. Using polyclonal anti-MPO antibody as primary antibody, the means of fluorescence intensity were significantly increased in neutrophils and monocytes after incubation for 8 h, but not increased in lymphocytes (Fig. 5).
Fig. 5.
Myeloperoxidase (MPO) surface expression in neutrophils, monocytes and lymphocytes unincubated (□) or incubated for 8 h (▪) by flow cytometry using rabbit anti-MPO antibodies as primary antibodies and represented as mean of fluorescence intensity. MPO is on the surface of incubated neutrophils and monocytes at 8 h, but not on surface of incubated lymphocytes. *Statistically different from 0 h (P < 0·05). Values are means ± s.d.
Release of ANCA antigens from neutrophils
By ELISA, release of ANCA antigens from neutrophils incubated from 4 h to 24 h increased in a time-dependent manner. The primary granule protein, MPO, in plasma was 128·9 ± 39·7 ng/ml after 4 h, 131·0 ± 38·6 ng/ml after 8 h, and 166·7 ± 39·4 ng/ml after 24 h. The secondary granule protein, lactoferrin, in plasma was 207·0 ± 61·1 ng/ml after 4 h, 231·6 ± 62·7 ng/ml after 8 h, and 216·8 ± 61·2 ng/ml after 24 h. The increase of concentration of MPO after 4–24 h and lactoferrin after 8 h in plasma were statistically significant compared with unincubated neutrophils (86·9 ± 23·5 ng/ml for MPO and 162·2 ± 42·2 ng/ml for lactoferrin) (Fig. 4). The release of neutrophil granule proteins into the plasma is a reflection of full activation of neutrophils or neutrophil necrosis or both.
Apoptosis and necrosis of neutrophils
By flow cytometry, there was no significant increase in apoptosis until samples were incubated for 24 h using either annexin V or PI detection. The proportions of apoptotic neutrophils were 8·3 ± 1·1% at 4 h, 7·9 ± 2·7% at 8 h and 27·3 ± 7·2% at 24 h by annexin V detection, and the proportions of apoptotic unseparated leucocytes were 1·9 ± 2·6%, 2·3 ± 2·0% and 12·3 ± 8·3%, respectively, by PI. Compared with 4·7 ± 3·5% by annexin V and 1·5 ± 1·8% by PI at 0 h, the changes were only significant at 24 h by both annexin V and PI detection (Figs 4 and 6). The proportion of necrotic cells as determined by the annexin V flow cytometry profile was 2·9 ± 0·4% at 0 h and 11·5 ± 3·5% at 24 h.
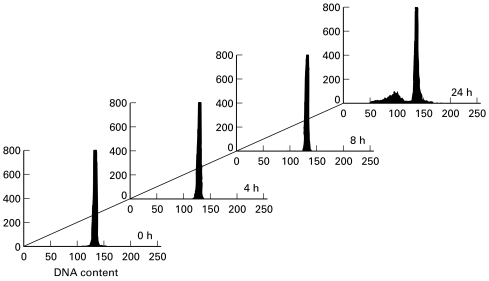
Fig. 6.
DNA content of leucocytes by flow cytometry using propidium iodide (PI) detection. DNA distributions were obtained following fixation of cells with ethanol and staining with PI. Cells containing degraded DNA representing apoptotic cells were located to the left of the G1 peak. Cells were cultured for 0, 4, 8, or 24 h and apoptotic peaks were observed only in cells cultured for 24 h.
Relationship of surface expression of ANCA antigens and apoptosis
By fluorescence microscopy, neutrophils in whole blood were analysed for concurrent MPO expression and apoptosis with both FITC immunofluorescence using polyclonal anti-MPO IgG and DNA dye, DAPI. With blood incubated 4 h or 8 h, the neutrophils showed MPO surface expression on non-apoptotic neutrophils with green cell surface staining and intact segmented blue nuclei. After incubation for 24 h, the cells demonstrated MPO surface expression either on apoptotic cells with pyknotic blue nuclei with chromatin condensation or on non-apoptotic cells with intact segmented blue nuclei (Fig. 7).
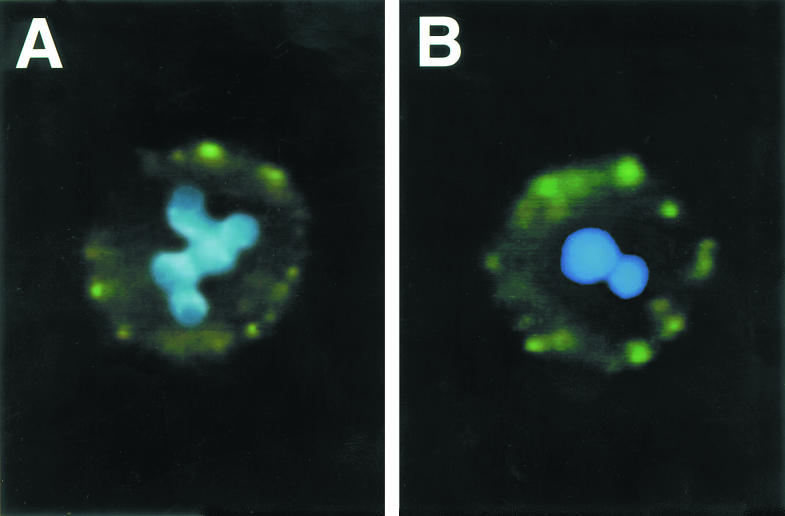
Fig. 7.
Double exposure fluorescence photomicrographs of neutrophils with double staining by both FITC immunofluorescence using polyclonal anti-myeloperoxidase (MPO) IgG as primary antibody (green staining) and DNA dye, 4′,6-diamidino-2-phenylindole (DAPI) (blue staining). (A) This neutrophil from blood cultured 8 h shows granular green cell membrane staining for MPO and a blue normally segmented nucleus with differences in brightness between heterochromatin and euchromatin. (B) This neutrophil from blood cultured 24 h shows cell surface MPO staining and a homogeneous pyknotic nucleus indicative of apoptosis.
By flow cytometry, neutrophils were analysed for the relationship of MPO surface expression and apoptosis by simultaneously staining cells with annexin V and PI for apoptosis and with R-PE-conjugated mouse anti-human MPO for MPO surface expression. In freshly isolated cells, the mean of PE fluorescence intensity was 39·1 ± 28·0. After cells incubated 24 h, about 30% of neutrophils became apoptotic. The mean surface expression was 76·0 ± 32·6 in apoptotic cells and 88·2 ± 15·4 in non-apoptotic cells. Thus, there was no significant difference between the mean MPO surface expression on apoptotic and non-apoptotic cells incubated for 24 h; however, both means were higher than the mean for freshly isolated cells. These data indicate that ANCA antigens were present on the surface of both apoptotic and non-apoptotic cells.
DISCUSSION
ANCA are found in the circulation of most patients with a distinctive category of small vessel vasculitis that includes Wegener's granulomatosis, microscopic polyangiitis, Churg–Strauss syndrome, and renal-limited disease with necrotizing and crescentic glomerulonephritis [1–4]. In vitro and in vivo evidence is mounting that ANCA are directly involved in the pathogenesis of this category of vascular inflammation [1–9,20–22]. For example, ANCA cause cytokine-primed neutrophils to degranulate and undergo a respiratory burst [5,6,9] that result in endothelial cell killing in vitro [20,21].
Most current pathogenic hypotheses require that ANCA antigens be expressed at the surface of neutrophils so that they can interact with ANCA to cause neutrophil activation [1–9,20–22]. Although a number of groups, including our own, have demonstrated that ANCA antigens, such as MPO and PR3, are expressed on the surface of primed neutrophils, there is controversy over whether or not ANCA antigens are expressed on normal quiescent (unstimulated) neutrophils [23–26], and whether or not neutrophils that are progressing toward apoptosis express ANCA antigens prior to undergoing apoptosis [10]. Our data indicate that unstimulated neutrophils in fresh blood from healthy donors do not express ANCA antigens on their surfaces. However, even the minor trauma caused by in vitro incubation for several hours or by neutrophil isolation procedures is enough stimulation to cause surface expression and slight release of ANCA antigens, as well as other neutrophil granule constituents, as has previously been proven by others [27–29]. This observation may explain the contention by some groups that ‘unstimulated’ neutrophils express ANCA antigens on their surfaces, because these groups have evaluated neutrophils after they had undergone the trauma of various isolation procedures [23–26]. The findings in our study indicate that isolation trauma is a crucial consideration when evaluating other published observations about ANCA antigen expression by ‘unstimulated’ neutrophils.
Our data confirm the observations of Gilligan et al. [10] that apoptotic neutrophils express ANCA antigens on their surfaces. However, in contradistinction to their contention that ANCA antigens appear on the surface of apoptotic neutrophils in the absence of prior neutrophil priming, our data indicate that neutrophils undergo priming and expression of ANCA antigens before there is any evidence of apoptosis. Given the mild degree of neutrophil perturbation that is required for priming, it would be surprising if they could sustain a stimulus intense enough to initiate apoptosis without inducing priming events. However, our data do not directly demonstrate that all neutrophils that become apoptotic have previously been primed.
The expression of ANCA antigens on the surface of apoptotic neutrophils may be important in both the afferent (immunogenic) and efferent (pathogenic) limbs of ANCA disease induction by enhancing the immunogenicity of ANCA antigens and by augmenting the inflammatory response at sites of vasculitis, respectively. Enhanced immunogenicity of self antigens in apoptotic bodies has been incriminated in the pathogenesis of a number of autoimmune diseases, especially systemic lupus erythematosus [30–32]. Opsonization of apoptotic cells enhances the immunogenicity of antigens in apoptotic bodies that are phagocytosed by macrophages [30–32]. Thus, the display of ANCA antigens in the context of neutrophil apoptotic bodies might be an important stimulus for initially breaking self tolerance to ANCA antigens. In addition, once an ANCA autoimmune response has been mounted, opsonization of apoptotic neutrophils by ANCA might accelerate and augment the ANCA autoimmune response.
There is substantial neutrophil apoptosis at sites of ANCA vasculitis, which is the major basis for the ‘leukocytoclastic’ appearance of the vascular inflammation at sites of ANCA vasculitis. Under physiologic conditions, apoptotic cells do not evoke significant inflammation; however, opsonization of apoptotic cells by antibodies causes greater activation of macrophages that phogocytose the apoptotic cells with increased secretion of proinflammatory cytokines, such as TNF-α [30]. Thus, opsonization of apoptotic neutrophils by ANCA could result in amplification of inflammation at sites of vasculitis.
In conclusion, we have demonstrated that (i) quiescent neutrophils have no ANCA antigens on their surface, (ii) there is expression of ANCA antigens and other granule constituents on neutrophil surfaces as the cells become primed and activated, and (iii) ANCA antigens are on surfaces of apoptotic neutrophils. ANCA on the surface of primed neutrophils is available to interact with ANCA to cause full-blown neutrophil activation that may be the basis for the inflammation of ANCA vasculitis. ANCA antigens on the surface of apoptotic neutrophils may be involved in the initiation of the ANCA autoimmune response, and might augment inflammation at sites of ANCA vasculitis.
REFERENCES
- 1.Kallenberg CGM, Brouwer E, Weening JJ, Cohen Tervaert JW. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ. Anti-neutrophil cytoplasmic autoantibodies: discovery, specificity, disease associations and pathogenic potential. Adv Pathol Lab Med. 1995;8:363–78. [Google Scholar]
- 3.Gross WL, Csernok E, Helmchen U. Antineutrophil cytoplasmic autoantibodies, autoantigens, and systemic vasculitis. APMIS. 1995;103:81–97. doi: 10.1111/j.1699-0463.1995.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ. Pathogenesis of the vascular and glomerular damage in ANCA-positive vasculitis. Nephrol Dial Transplant. 1998;13(Suppl. 1):16–20. doi: 10.1093/ndt/13.suppl_1.16. [DOI] [PubMed] [Google Scholar]
- 5.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leuk Biol. 1991;50:539–46. doi: 10.1002/jlb.50.6.539. [DOI] [PubMed] [Google Scholar]
- 7.Csernok E, Ernst M, Schmitt W, Bainton DF, Gross WL. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994;95:244–50. doi: 10.1111/j.1365-2249.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulder AH, Heeringa P, Brouwer E, Limburg PC, Kallenberg CG. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a Fc gamma RII-dependent process. Clin Exp Immunol. 1994;98:270–8. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kettritz R, Jennette JC, Falk RJ. Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol. 1997;8:386–94. doi: 10.1681/ASN.V83386. [DOI] [PubMed] [Google Scholar]
- 10.Gilligan HM, Bredy B, Brady HR, et al. Antineutrophil cytoplasmic autoantibodies interact with primary granule constituents on the surface of apoptotic neutrophils in the absence of neutrophil priming. J Exp Med. 1996;184:2231–41. doi: 10.1084/jem.184.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JJ, Tuttle RH, Falk RJ, Jennette JC. Frequency of anti-bactericidal/permeability-increasing protein (BPI) and anti-azurocidin in patients with renal disease. Clin Exp Immunol. 1996;105:125–31. doi: 10.1046/j.1365-2249.1996.d01-738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao MH, Jones SJ, Lockwood CM. Bactericidal/permeability-increasing protein (BPI) is an important antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in vasculitis. Clin Exp Immunol. 1995;99:49–56. doi: 10.1111/j.1365-2249.1995.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JJ, Kettritz R, Falk RJ, Jennette JC, Gaido ML. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol. 1996;149:1617–26. [PMC free article] [PubMed] [Google Scholar]
- 14.Darzynkiewicz Z, Bruno S, Bino GD, Gorczyca W, Hotz MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 15.Kettritz R, Falk RJ, Jennette JC, Gaido ML. Neutrophil superoxide release is required for spontaneous and FMLP-mediated but not for TNFα-mediated apoptosis. J Am Soc Nephrol. 1997;8:1091–100. doi: 10.1681/ASN.V871091. [DOI] [PubMed] [Google Scholar]
- 16.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunnett CW. A multiple comparisons procedure for comparing several treatments with a control. J AM Stat Assoc. 1955;50:1096–121. [Google Scholar]
- 18.North Carolina: SAS Institute Inc.; 1989. SAS/STAT® user's guide; pp. 209–24. 893–996. [Google Scholar]
- 19.Metcalf JA, Gallin JI, Nauseef WM, Root RK. New York: Raven Press; 1986. Laboratory manual of neutrophil function; pp. 2–6. [Google Scholar]
- 20.Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–83. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- 21.Savage CO, Pottinger BE, Gaskin G, Pusey C. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am J Pathol. 1992;141:335–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Porges AJ, Redecha PB, Kimberly WT, Csernok E, Gross WL, Kimberly RP. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol. 1994;153:1271–80. [PubMed] [Google Scholar]
- 23.Csernok E, Ludemann J, Gross WL, Bainton D. Ultrastructural localization of proteinase 3, the target antigen of ANCA circulating in Wegener's granulomatosis. Am J Pathol. 1990;137:1113–20. [PMC free article] [PubMed] [Google Scholar]
- 24.Csernok E, Schmitt WH, Ernst M, Bainton DF, Gross WL. Membrane surface proteinase 3 expression and intracytoplasmic immunoglobulin on neutrophils from patients with ANCA-associated vasculitides. AEMB. 1993;336:45–50. doi: 10.1007/978-1-4757-9182-2_5. [DOI] [PubMed] [Google Scholar]
- 25.Halbwachs-Mecarelli L, Bessou G, Lesavre P, Lopez S, Witko-Sarsat V. Bimodal distribution of proteinase 3 (PR3) surface expression reflects a constitutive heterogeneity in the polymorphonuclear neutrophil pool. FEBS Letters. 1995;374:29–33. doi: 10.1016/0014-5793(95)01073-n. [DOI] [PubMed] [Google Scholar]
- 26.Braun MG, Csernok E, Gross WL, Muller-Hermelink HK. Proteinase 3, the target antigen of anticytoplasmatic antibodies circulating in Wegener's granulomatosis: immunolocalization in normal and pathologic tissues. Am J Pathol. 1991;139:831–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Kuijpers TW, Tool ATJ, van der Schoot CE, Ginsel LA, Onderwater JJM, Roos D, Verhoeven AJ. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–11. [PubMed] [Google Scholar]
- 28.Fearon DT, Collins LA. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and the purification procedures. J Immunol. 1983;130:370–5. [PubMed] [Google Scholar]
- 29.Dransfield L, Buckle AM, Savill JS, McDowall AM, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (FcγRIII) expression. J Immunol. 1994;153:1254–63. [PubMed] [Google Scholar]
- 30.Manfredi AA, Rovere P, Galati G, et al. Apoptotic cell clearance in systemic lupus erythematosus. I. Opsonization by antiphospholipid antibodies. Arthritis Rheum. 1998;41:205–14. doi: 10.1002/1529-0131(199802)41:2<205::AID-ART4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Casciola-Rosen L, Rosen A, Petri M, Schlissel M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1996;93:1624–9. doi: 10.1073/pnas.93.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen A, Casciola-Rosen L, Ahearn J. Novel packages of viral and self-antigens are generated during apoptosis. J Exp Med. 1995;181:1557–61. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]