Abstract
The human IgA Fc receptor (FcαR, CD89) plays an important role in host defence against invading pathogens. To study the properties of the receptor, 12 MoAbs, namely, MIP7c, MIP8a, MIP9a, MIP10c, MIP11c, MIP14b, MIP15b, MIP38c, MIP59c, MIP65c, MIP68b and MIP71a, were generated. The inhibitory effects of the antibodies on FcαR functions were tested. Three of the antibodies, MIP7c, MIP8a and MIP59c, were able to block up to 90% of soluble FcαR binding to IgA-coated beads and 70–80% of neutrophil phagocytosis of IgA immune complexes (IC). MIP8a could also inhibit IgA IC-induced neutrophil lactoferrin release, while cross-linking of FcαR with MIP8a and anti-mouse IgG could elicit neutrophil lactoferrin release. However, IgA IC-induced lactoferrin release required both extracellular calcium and magnesium, whereas MIP8a-induced release did not require extracellular magnesium and only partially required extracellular calcium. In addition, the time course of IgA IC-induced lactoferrin release was slow. Lactoferrin was not detectable if the incubation time was less than 0·5 h. In contrast, MIP8a-induced lactoferrin release was fast. Lactoferrin could be detected within 5 min of incubation. Therefore, neutrophil lactoferrin release induced by IgA IC differed from that induced by cross-linking of FcαR with MIP8a.
Keywords: neutrophil, degranulation, divalent cation, FcR
INTRODUCTION
The human IgA Fc receptor (FcαR, CD89) plays an important role in host defence against invading pathogens. It mediates various cellular responses in myeloid cells, such as phagocytosis [1], respiratory burst [2,3] and release of inflammatory mediators and cytokines [3–5]. It can also promote antibody-dependent cell-mediated cytotoxicity (ADCC) [5,6].
The mechanisms of FcαR-mediated cellular responses have been studied by several groups. It has been reported that FcαR associates with the FcR γ-chain and transmembrane signal transduction by FcαR is mediated via the FcR γ-chain [7–9]. In the presence of the FcR γ-chain, cross-linking FcαR can induce Ca2+ flux and tyrosine phosphorylation. In the absence of the FcR γ-chain however, cells can not elicit the responses by cross-linking to the receptor [9,10]. Signalling through the FcR γ-chain involves at least two subfamilies of tyrosine kinases, syk and Btk [11]. We have reported that in the absence of complement, IgA immune complex (IC)-induced neutrophil lactoferrin release is a slow process and requires both extracellular calcium and magnesium [3,12].
FcαR belongs to the immunoglobulin superfamily. It has two immunoglobulin-like extracellular domains [13]. Although FcαR is functionally a Fc receptor, it shares only 20% homology with other Fc receptors. In comparison, FcαR shares more homology (35–40%) to the killer-inhibitory receptors and the leucocyte-inhibitory receptors [14]. Recently, Wines et al. [14] reported that residues 82–85 in the FG loop of the FcαR first domain are essential for IgA binding. Substitution of histidyl 85, arginyl 82 with other amino acids leads to loss of FcαR binding activity.
Despite much research on the subject, the properties of FcαR are still far from clear. In order to study the structure and functions of FcαR further, we have generated 12 MoAbs against the receptor and have examined their effect on FcαR function.
MATERIALS AND METHODS
Reagents
IgA2 anti-NIP antibody and 12.9NIP-BSA were prepared and purified as described [3]. Anti-FcαR MoAbs, MIP8a and MIP10c, were affinity-purified from mouse ascites through a protein A (Prosep-A) column (Bioprocessing Ltd, Consett, UK). The F(ab′)2 fragments of MIP8a and MIP10c were made by digestion with pepsin (Sigma Chemical Co., Poole, UK). The undigested antibody was removed by passing through the Prosep-A column. Anti-mouse IgG (Fab-specific) was raised in a New Zealand white rabbit, the antibody was affinity-purified and the F(ab′)2 fragments of the antibody were made by digestion with pepsin. Anti-lactoferrin antibody was raised in a New Zealand white rabbit and the antibody was affinity-purified through a lactoferrin (Calbiochem, Beeston, UK)-coupled CL-4B column. Alkaline phosphatase (Sigma) was conjugated to anti-lactoferrin antibody using the glutaraldehyde method as described [15].
Preparation of soluble FcαR
U937 cells grown in RPMI 1640 medium were treated with 5 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma) for 18 h at 37°C. The total RNA from the cells was then extracted using the guanidinium lysis method. Reverse transcription of poly (A)+ RNA to cDNA was carried out using a SUPER RT kit (HT Biotechnology Ltd, Cambridge, UK) following the manufacturer's instructions. FcαR cDNA was selected from the bulk cDNA by the polymerase chain reaction (PCR). The 5′ primer (s32) was 5′-TCAGCACGATGGACCCCAAA-3′ and the 3′ primer (n732stop) was 5′-CATTTAGATCAAGTTCTGCGTCGTG-3′ (Oligos-you-like, Cambridge, UK), which had a stop codon after nt726, the end of the FcαR extracellular region [16]. Thirty cycles of amplification (denaturation at 94°C for 1 min; annealing at 58°C for 1 min; extension at 72°C for 1·5 min) were carried out. The final extension was at 72°C for 5 min. FcαR cDNA from PCR was ligated on to pcDNA3 (Invitrogen, Leek, The Netherlands) at the EcoR V site. Transformation of competent NM522 Escherichia coli cells by FcαR-inserted pcDNA3 was carried out by heat shock at 42°C for 2 min. Colonies that were transformed with FcαR cDNA-inserted pcDNA3 were selected by colony hybridization using 32P-labelled FcαR cDNA as a probe. Orientation of FcαR cDNA in the plasmid was determined by restriction digestion using Hind III and Ssp I (New England Biolabs, Hitchin, UK).
FcαR cDNA-inserted pcDNA3 was introduced into Chinese hamster ovary (CHO)-K1 cells by CaCl2 precipitation. Transfected CHO cells were grown in Ham's F12 medium (Sigma) supplemented with 1·2 mg/ml Geneticin G418 sulphate (Life Technologies, Paisley, UK). Soluble FcαR expressed by CHO cells was affinity-purified using Sepharose CL-4B coupled with IgA2 anti-NIP antibody. The bound receptor was eluted using 0·1 m glycine pH 2·6.
Preparation of MoAbs against FcαR
Each of five female BALB/c mice (6 weeks old) initially received 20 μ g of soluble FcαR in 250 μ l of PBS mixed with 250 μ l of Freund's complete adjuvant (FCA; Sigma), intraperitoneally. Two further injections followed at 3-week intervals with the same amount of soluble FcαR in Freund's incomplete adjuvant (FIA). Nine weeks after the third injection, the mice received 500 μ l of U937 cells (1 × 107 cells treated with 5 ng/ml PMA) intraperitoneally, and 20 μg of soluble FcαR and 1 × 106 U937 cells in 150 μl PBS intravenously.
Three days after the last injection, the mice were killed. Their spleen cells were fused with NS0 cells using 50% polyethylene glycol solution (Sigma). Successfully fused cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 20% Fetal Clone I (HyClone, Cramlington, UK) and 1 × HAT media supplement (Sigma). Production of anti-FcαR antibodies from the cells was tested by flow cytometry and ELISA. In flow cytometry, the supernatant was incubated with U937 cells treated with 5 ng/ml PMA. Anti-FcαR antibody was detected by FITC-conjugated F(ab′)2 fragment of goat anti-mouse IgG (Sigma). In ELISA, 96-well plates were coated with purified soluble FcαR at 200 ng/well. Anti-FcαR antibody was detected by peroxidase-conjugated goat anti-mouse IgG (Sigma). Cells from positive wells were cloned by limited dilution.
Binding assay
NIP beads (100 μl; Cambridge Research Biochemicals, Northwich, UK) were incubated with 1·7 mg of IgA2 anti-NIP antibody at 4°C for 2 h. After washing three times with PBS, the beads were mixed with 2 ml of Sepharose CL-4B and suspended in PBS to 6 ml.
Supernatant (1 ml) from cells secreting MoAbs against FcαR was incubated with 7 μ l of 125I-labelled soluble FcαR (50 000 ct/min per μ l) at room temperature for 1 h. Fifty microlitres of the beads were then added and incubated at room temperature for 4 h with constant rotation. After washing three times with Hanks' balanced salt solution (HBSS) containing 2% bovine serum albumin (BSA), 1 mm calcium and 1·5 mm magnesium, the radioactivity of the beads was counted. All samples were tested in triplicate.
Isolation of neutrophils
Neutrophils were isolated from heparinized blood of healthy donors by Dextran T500 (Pharmacia, Uppsala, Sweden) sedimentation. EDTA (5 mm) was included at this stage to prevent complement activation. Neutrophils were then separated from mononuclear leucocytes by density centrifugation through discontinuous Percoll gradients (Pharmacia).
Phagocytosis assay
Anti-NIP IgA2 antibody (0·2 mg/ml) and 12.9NIP-BSA at equivalence in HBSS containing 1 mm calcium, 1·5 mm magnesium, 5% BSA and trace amounts of 125I-12.9NIP-BSA were incubated at 37°C for 3 h to form IC. Supernatant (37·5 μ l) from cells producing MoAbs against FcαR were incubated with 37·5 μ l of neutrophils (1 × 107 cells/ml) in 96-well plates at room temperature for 10 min, then 25 μ l of IgA2/NIP-BSA IC were added to each of the neutrophil wells and incubated at 37°C for 3 h in a CO2 incubator. The surface-bound IgA IC were solubilized with 50 μl of 2·4 mm NIP-CAP-OH (Cambridge Research Biochemicals)/15 mm EDTA in PBS at room temperature for 5 min. Then the cells were centrifuged through 200 μl oil (one volume of dinonyl phthalate was mixed with four volumes of dibutyl phthalate). The radioactivity at the top and bottom of the tubes was counted separately. All samples were tested in triplicate.
Lactoferrin release assay
Neutrophil degranulation was measured by lactoferrin release assays. When IgA IC were used to stimulate neutrophils, IgA (60 μ g/ml) and NIP-BSA at equivalence were mixed in HBSS containing 3% BSA at 37°C for 3 h. One hundred microlitres of a neutrophil suspension (4 × 106 cells/ml) containing 7·5 μ g/ml cytochalasin B (Sigma) were added to 96-well plates. Then 50 μl of IgA IC were added. When MIP8a was used to stimulate neutrophils, 4 × 105 neutrophils were first incubated with 25 μl of MIP8a F(ab′)2 fragments at room temperature for 10 min, then 25 μl of F(ab′)2 fragments of rabbit anti-mouse IgG (Fab-specific) (120 μ g/ml) were added. After incubation at 37°C, lactoferrin in the supernatant was measured by sandwich ELISA. Ninety-six-well plates were coated with anti-lactoferrin antibody. The bound lactoferrin was detected by alkaline phosphatase-conjugated anti-lactoferrin antibody. The standard curve was determined using lactoferrin (Calbiochem, Nottingham, UK). All samples were tested in triplicate.
RESULTS
Characterization of MoAbs against FcαR
Twelve clones (MIP7c, MIP8a, MIP9a, MIP10c, MIP11c, MIP14b, MIP15b, MIP38c, MIP59c, MIP65c, MIP68b and MIP71a) were found to produce antibodies against FcαR. They were all IgG1 except for MIP65c, which was IgG2b, and MIP68b, which was IgG2a.
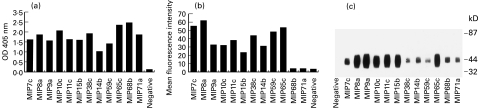
The antibodies were compared for their abilities to bind FcαR. The binding profiles varied when different methods were used. On ELISA, MIP65c and MIP68b had the highest absorbance (Fig. 1a). The signals for MIP8a, MIP10c, MIP38c and MIP71a were also high. The other antibodies were weaker. On flow cytometry, MIP7c and MIP8a were the strongest antibodies in detecting FcαR on neutrophils, while MIP68b and MIP71a, which had strong signals on ELISA, scarcely bound FcαR on neutrophils (Fig. 1b). On Western blotting, the bands for MIP8a, MIP9a, MIP10c, MIP11c, MIP15b and MIP65c were strong. In comparison, MIP38c, MIP68b and MIP71a, which had strong signals on ELISA, had much weaker bands on Western blotting (Fig. 1c).
Fig. 1.
Binding profiles of MoAbs to FcαR. (a) ELISA analysis of MoAb binding to soluble FcαR. Soluble FcαR (200 ng/well) was coated on 96-well plates. Tissue culture supernatant (25 μl) was tested. The negative control sample was 25 μl supernatant from cells which did not secrete antibodies. Peroxidase-conjugated anti-mouse IgG was used as the secondary antibody. Each data point was the mean of triplicate samples. (b) Flow cytometry analysis of MoAb binding to cell surface FcαR. Neutrophils (2 × 105) were first incubated with 50 μl of tissue culture supernatant in 500 μl of PBS containing 2 mm sodium azide and 1% bovine serum albumin. The negative control sample was 50 μ l supernatant from cells which did not secrete antibodies. The cells were stained with FITC-conjugated F(ab′)2 fragments of goat anti-mouse IgG. After fixing with 2% paraformaldehyde, 10 000 cells were analysed. (c) Western blotting of MoAbs. Soluble FcαR was run on non-reducing 7–17% SDS–PAGE and transferred on to nitrocellulose paper. The paper was then cut into strips and incubated separately with 1 ml of the antibody tissue culture supernatant. Anti-mouse IgG (Fc-specific) peroxidase conjugate was used as the secondary antibody. The bands were visualized using ECL system (Amersham Int. plc, Aylesbury, UK). The negative control sample was supernatant from cells that did not secrete antibodies.
Inhibition of FcαR functions by MoAbs
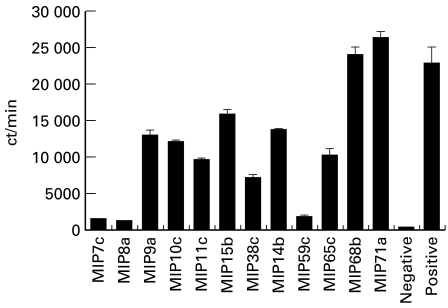
The antibodies were tested for blocking soluble FcαR binding to IgA-coated beads. The results are shown in Fig. 2. Three antibodies, MIP7c, MIP8a and MIP59c, were able to block 90% of FcαR binding. MIP68b and MIP71a had no effect on blocking FcαR binding to IgA beads. Other antibodies were less effective, blocking 30–70% of FcαR binding.
Fig. 2.
Blocking soluble FcαR binding to IgA beads by MoAbs. Tissue culture supernatant (1 ml) from cells secreting MoAb against FcαR was first incubated with 7 μl of 125I-FcαR. Then 50 μl of IgA beads were added. After washing, the remaining radioactivity from the beads was counted (see Materials and Methods). The positive sample was 1 ml of culture medium instead of cell supernatant. The negative control sample was NIP beads instead of IgA beads. The results are representative of three separate experiments.
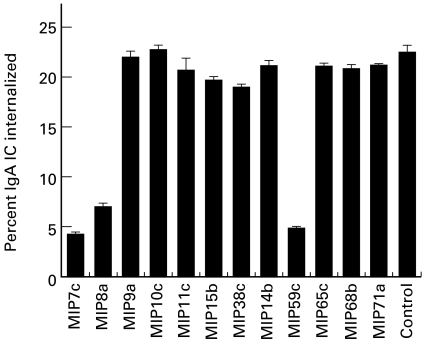
The antibodies were tested for inhibition of neutrophil phagocytosis of IgA IC. MIP7c, MIP8a and MIP59c, which effectively blocked FcαR binding to IgA beads, inhibited 70–80% of neutrophil phagocytosis of IgA IC (Fig. 3). Other antibodies had no effect at all.
Fig. 3.
Inhibition of neutrophil phagocytosis of IgA immune complexes (IC) by MoAbs. Cell supernatant (37·5 μl) was used to inhibit neutrophil (3·75 × 105 cells) phagocytosis of 5 μg IgA IC (see Materials and Methods). The control sample was culture medium instead of cell supernatant. The results are representative of three separate experiments.
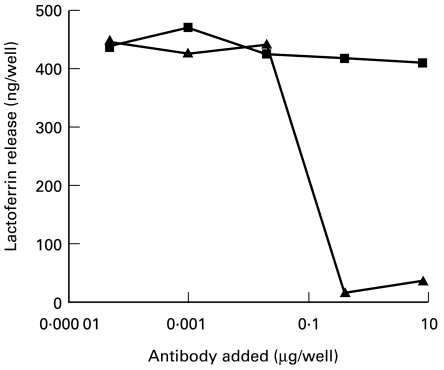
Two antibodies, MIP8a (active in inhibiting phagocytosis) and MIP10c (inactive in inhibiting phagocytosis), were tested for inhibition of neutrophil degranulation. MIP8a at a concentration of 2·7 μg/ml (0·4 μg/well) totally inhibited IgA IC-induced neutrophil lactoferrin release, whereas MIP10c did not inhibit lactoferrin release at a concentration of up to 67 μg/ml (10 μg/well) (Fig. 4).
Fig. 4.
Inhibition of neutrophil lactoferrin release by MIP8a and MIP10c. Neutrophils (100 μl) (4 × 106 cells/ml) were incubated with MIP8a F(ab′)2 fragments or MIP10c F(ab′)2 fragments in 96-well plates at room temperature for 10 min. Then 50 μl of IgA/NIP-BSA IC (60 μg/ml) were added to each well. After incubation at 37°C for 2 h, lactoferrin released from neutrophils was measured. ▪, MIP10c; ▴, MIP8a. Results are representative of three separate experiments.
Time course of MIP8a- and IgA IC-induced lactoferrin release
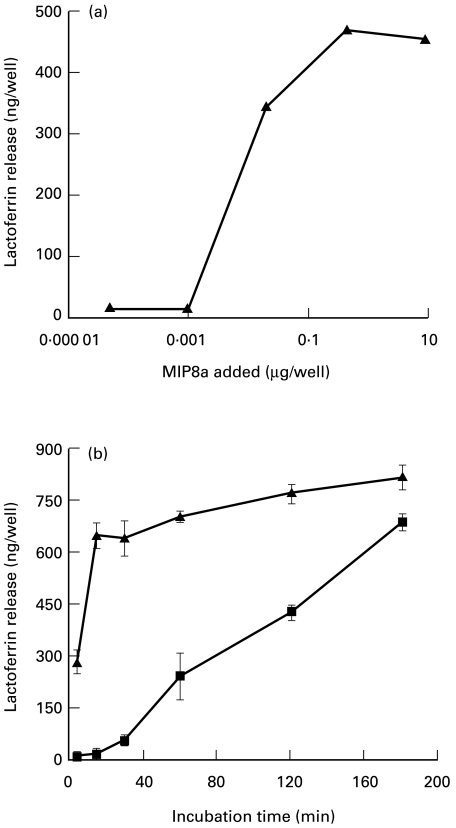
Since IgA IC-induced lactoferrin release is due to cross-linking of FcαR on neutrophils, cross-linking FcαR with MoAbs followed by F(ab′)2 anti-mouse IgG—which also cross-links the receptor aggregate—should also induce neutrophil lactoferrin release. This was shown to be the case at concentrations of MIP8a higher than 0·13 μg/ml (0·02 μg/well) (Fig. 5a).
Fig. 5.
Lactoferrin release induced by MIP8a and IgA immune complexes (IC). (a) Dose–response of MIP8a-induced lactoferrin release. Neutrophils (100 μl) (4 × 106 cells/ml) were incubated at room temperature for 10 min with 25 μl MIP8a F(ab′)2 fragments as indicated. Then 25 μl of F(ab′)2 fragments of rabbit anti-mouse IgG (120 μg/ml) were added to each well. After incubation at 37°C for 2 h, lactoferrin released from neutrophils was measured. (b) Time course of MIP8a- and IgA IC-induced lactoferrin release. For MIP8a-induced lactoferrin release, 100 μl of neutrophils (4 × 106 cells/ml) were incubated at room temperature for 10 min with 25 μl of MIP8a F(ab′)2 fragments (40 μg/ml). Then 25 μl of F(ab′)2 fragments of rabbit anti-mouse IgG (120 μg/ml) were added to each well. For IgA IC-induced lactoferrin release, 100 μ l of neutrophils (4 × 106 cells/ml) were incubated with 50 μl of IgA/NIP-BSA IC (60 μg IgA/ml). After incubation at 37°C for the times indicated, lactoferrin released from neutrophils was measured. ▪, IgA IC; ▴, MIP8a. Results are representative of three separate experiments.
Although cross-linking of FcαR with MIP8a followed by F(ab′)2 anti-mouse IgG could induce neutrophil lactoferrin release, the time course patterns of MIP8a- and IgA IC-induced release were different. MIP8a-induced release was fast, lactoferrin could be detected within 5 min of incubation. In comparison, the time course of IgA IC-induced lactoferrin release was slow. Lactoferrin was not detectable until the incubation time exceeded 0·5 h (Fig. 5b).
Requirement of divalent cations for MIP8a- and IgA IC-induced lactoferrin release
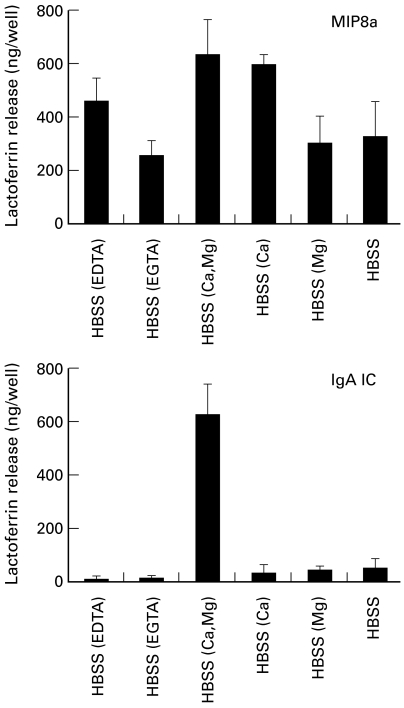
We previously reported that neutrophil lactoferrin release induced by IgA IC required extracellular calcium and magnesium [12]. To see the effects of divalent cations on MIP8a-induced lactoferrin release, samples were tested in the absence or presence of calcium and magnesium. EDTA was used to chelate both calcium and magnesium and EGTA was used to chelate calcium only. As expected, IgA IC-induced lactoferrin release occurred only when both calcium and magnesium were present (Fig. 6). In contrast, MIP8a-induced lactoferrin release was not affected when magnesium was absent and it was reduced but not abolished when calcium was absent.
Fig. 6.
Requirement of divalent cations for MIP8a- and IgA immune complex (IC)-induced neutrophil lactoferrin release. For MIP8a-induced lactoferrin release, 100 μl of neutrophils (4 × 106 cells/ml) were incubated with 25 μl of MIP8a F(ab′)2 fragments (40 μg/ml) at room temperature for 10 min. Then 25 μl of F(ab′)2 fragments of rabbit anti-mouse IgG (120 μ g/ml) were added to each well. For IgA IC-induced lactoferrin release, 100 μl of neutrophils (4 × 106 cells/ml) were incubated with 50 μl of IgA/NIP-BSA IC (60 μ g IgA/ml). After incubation at 37°C for 3 h, lactoferrin released from neutrophils was measured. Hanks' balanced salt solution (HBSS) (EDTA), medium containing 1 mm Ca2+, 1·5 mm Mg2+ and 6 mm EDTA; HBSS (EGTA), medium containing 1 mm Ca2+, 1·5 mm Mg2+ and 6 mm EGTA; HBSS (Ca, Mg), medium containing 1 mm Ca2+ and 1·5 mm Mg2+; HBSS (Ca), medium containing 1 mm Ca2+; HBSS (Mg), medium containing 1·5 mm Mg2+; HBSS, Ca2+-and Mg2+-free medium. Results are representative of three separate experiments.
DISCUSSION
As for other receptors, signalling through FcαR requires receptor cross-linking on the cell membrane. IgA IC cross-link FcαR by the receptor binding to the IgA in the complexes. MIP8a followed by anti-mouse IgG cross-links FcαR directly. Both methods should give the same result if efficient FcαR aggregation is produced [17]. However, our results showed that IgA IC- and MIP8a-induced neutrophil lactoferrin release were different, especially in their requirements for divalent cations. IgA IC-induced release depended on the presence of both calcium and magnesium extracellularly. Lactoferrin release was abolished if either of these cations was missing. In contrast, MIP8a-induced lactoferrin release was normal in the absence of magnesium and reduced but not abolished in the absence of calcium.
The role of divalent cations in IgA IC-induced neutrophil lactoferrin release is unknown. The simplest explanation is that FcαR requires divalent cations to bind IgA IC so that, when calcium and magnesium are absent, FcαR can not be cross-linked on the neutrophil membrane. However, it has been reported that FcαR does not require divalent cations to bind IgA [18]. We also tested soluble FcαR binding to IgA-coated beads. Our preliminary results showed that soluble FcαR bound IgA-coated beads equally well in calcium- and magnesium-free HBSS or HBSS containing both cations. Another explanation is that divalent cations are required not for FcαR binding to IgA IC but for enabling FcαR to maintain a proper conformation for transducing a signal.
Increase of intracellular calcium concentration leads to cell activation, including degranulation. There are two ways to increase intracellular calcium concentration: either by influx of calcium from the extracellular environment, or by the release of calcium from intracellular calcium pools. It is possible that calcium and magnesium play different functions for IgA IC-induced lactoferrin release. Magnesium might be necessary for the correct binding of FcαR to IgA, and calcium might act as a messenger for signal transduction. If this is the case, then cross-linking FcαR with IgA IC was unable to mobilize the intracellular calcium pools when extracellular calcium was absent.
It is not surprising that the binding profiles of the 12 MoAbs generated against FcαR differed when tested by different methods. In ELISA, soluble FcαR was coated on a plastic surface and the whole molecule was basically exposed in its natural form. In flow cytometry, FcαR was also in its natural form, but some parts of the receptor might have been buried among other membrane proteins. Therefore, not all of the extracellular region of the receptor could be accessed by the antibodies. In Western blotting, the whole soluble FcαR is exposed in denatured form and conformational epitopes may be destroyed and only linear epitopes detected. The variation in the antibody binding profiles shows that the antibodies recognize different epitopes. For example, MIP68b and MIP71a had high optical density values on ELISA, but they hardly recognized FcαR on neutrophils, indicating that these two antibodies recognized epitopes that were buried among other membrane proteins. Possibly, their epitopes were located close to the carboxyl terminal of the FcαR extracellular region. MIP38c, which had relatively strong signals on ELISA and flow cytometry, had a much weaker band on Western blotting, indicating that MIP38c may recognize conformational epitopes that were partially destroyed by denaturation. The precise positions of these epitopes are unknown at present and we are currently mapping them.
In conclusion, IgA IC-induced neutrophil lactoferrin release differed from MIP8a-induced release. IgA IC-induced release requiring divalent cations was slow, MIP8a-induced release did not require extracellular magnesium, and was fast. The MoAbs generated in this study recognized different epitopes on the FcαR and they will be useful tools for studying the structure and functions of the receptor.
Acknowledgments
We wish to thank Drs Tim Farries and Surinder Soond, Ms Caroline Blair and Mr David Parker for their help in the construction of the FcαR cDNA plasmid and in the expression of soluble FcαR. Ms Min-lu Shi purified MIP8a and MIP10c. We also thank Dr Sarah Coppendale for her help in preparation of the manuscript. W.Z. was supported in Cambridge by an award from the Raymond and Beverley Sackler Fund and a grant from the Royal Society for visiting scientists. This work was partially supported by grants from the National Natural Science Foundation of China (Grant Nos 39770691 and 39870728).
REFERENCES
- 1.Yeaman GR, Kerr MA. Opsonization of yeast by human IgA anti-mannan antibodies and phagocytosis by human polymorphonuclear leukocytes. Clin Exp Immunol. 1987;68:200–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Gorter A, Hiemstra PS, Leijh PCJ, van der Sluys ME, van den Barselaar MT, van Es LA, Daha MR. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology. 1987;61:303–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Voice J, Lachmann PJ. A systematic study of neutrophil degranulation and respiratory burst in vitro by defined immune complexes. Clin Exp Immunol. 1995;101:507–14. doi: 10.1111/j.1365-2249.1995.tb03142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Ghazaleh RI, Fujisawa T, Mestecky J, Kyte RA, Gleich GJ. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–400. [PubMed] [Google Scholar]
- 5.Morton HC, van Egmond M, van de Winkel JGJ. Structure and function of human IgA Fc receptors (FcαR) Crit Rev Immunol. 1996;16:423–40. [PubMed] [Google Scholar]
- 6.Deo YM, Sundarapandiyan K, Keler T, Wallace PK, Graziano RF. Bispecific molecules directed to the Fc receptor for IgA (FcαRI, CD89) and tumor antigens efficiently promote cell-mediated cytotoxicity of tumor targets in whole blood. J Immunol. 1998;160:1677–86. [PubMed] [Google Scholar]
- 7.Pfefferkorn LC, Yeaman GR. Association of IgA-Fc receptors (FcαR) with FcεRIγ2 subunits in U937 cells. J Immunol. 1994;153:3228–36. [PubMed] [Google Scholar]
- 8.Saito K, Suzuki K, Matsuda H, Okumura K, Ra C. Physical association of Fc receptor γ chain homodimer with IgA receptor. J Allergy Clin Immunol. 1995;96:1152–60. doi: 10.1016/s0091-6749(95)70200-8. [DOI] [PubMed] [Google Scholar]
- 9.Morton HC, van den Herik-Oudijk IE, Vossebeld P, Snijders A, Verhoeven AJ, Capel PJA, van de Winkel JGJ. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcR γ chain. J Biol Chem. 1995;270:29781–7. doi: 10.1074/jbc.270.50.29781. [DOI] [PubMed] [Google Scholar]
- 10.van Dijk TB, Bracke M, Caldenhoven E, Raaijmakers JAM, Lammers JJ, Koenderman L, de Groot RP. Cloning and characterization of FcαRb, a novel Fcα receptor (CD89) isoform expressed in eosinophils and neutrophils. Blood. 1996;88:4229–38. [PubMed] [Google Scholar]
- 11.Launay P, Lehuen A, Kawakami T, Blank U, Monteiro RC. IgA Fc receptor (CD89) activation enables coupling to syk and Btk tyrosine kinase pathways: differential signaling after IFN-γ or phorbol ester stimulation. J Leuk Biol. 1998;63:636–42. doi: 10.1002/jlb.63.5.636. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Lachmann PJ. Neutrophil lactoferrin release induced by IgA immune complexes can be mediated either by Fcα receptors or by complement receptors through different pathways. J Immunol. 1996;156:2599–606. [PubMed] [Google Scholar]
- 13.de Wit TPM, Morton HC, Capel PJA, van de Winkel JGJ. Structure of the gene for the human myeloid IgA Fc receptor (CD89) J Immunol. 1995;155:1203–9. [PubMed] [Google Scholar]
- 14.Wines BD, Hulett MD, Jamieson GP, Trist HM, Spratt JM, Hogarth PM. Identification of residues in the first domain of human Fcα receptor essential for interaction with IgA. J Immunol. 1999;162:2146–53. [PubMed] [Google Scholar]
- 15.Nakumura RM, Voller A, Bidwell DE. Enzyme immunoassays: heterogeneous and homogeneous systems. In: Weir DM, Herzenberg LA, Blackwell C, Herzenberg LA, editors. Handbook of experimental immunology. Vol. 1. Oxford: Blackwell Scientific Publications; 1986. p. 27.16. [Google Scholar]
- 16.Maliszewski CR, March CJ, Schoenborn MA, Gimpel S, Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990;172:1665–72. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzger H. Transmembrane signaling: the joy of aggregation. J Immunol. 1992;149:1477–87. [PubMed] [Google Scholar]
- 18.Chevailler A, Monteiro RC, Kubagawa H, Cooper MD. Immunofluorescence analysis of IgA binding by human mononuclear cells in blood and lymphoid tissue. J Immunol. 1989;142:2244–9. [PubMed] [Google Scholar]