Abstract
Administration of rabbit anti-rat lung serum (PNTS) to rats produces a fulminant haemorrhagic pneumonitis sensitive to the availability of complement. The present experiments were undertaken to assess whether a high dose of IVIG can affect the development of this kind of cytotoxic reaction. The experimental design included groups of Wistar rats pretreated intravenously with physiologic saline, IVIG or a preparation of human F(ab′)2 fragments. One hour later the animals were challenged with either saline or PNTS. At 30 min after challenge, blood was collected and the lungs were removed. Pulmonary damage was evaluated by light microscopy; C3 deposits and the binding of immunoglobulins to the alveolar septa were assayed by immunofluorescence. The serum complement activity of the classical and alternative pathways was estimated by a kinetic technique. Pretreatment with IVIG decreased binding of rabbit anti-lung antibodies to alveolar septa and prevented the deposition of C3. These results indicate that pretreatment with IVIG inhibits the binding of the pathogenic antibody to lung tissue. Human IgG binding was not detected in any animal. The protection against lung injury afforded by pretreatment with IVIG, in contrast to the pneumotoxic effect of PNTS observed in control animals, was evident despite the administration of F(ab′)2 to the rats. Since pretreatment with F(ab′)2 failed to prevent the acute lung lesion, our results indicate that the attenuation afforded by IVIG in this model of complement-dependent tissue injury seems to be related to the integrity of the IgG molecule.
Keywords: IVIG, antibody binding, immunopneumonitis, anti-lung serum, complement system
INTRODUCTION
Starting from the initial report by Imbach et al. [1] which demonstrated the efficiency of high-dose IVIG in the control of idiopathic thrombocytopenic purpura, this preparation has been successfully used in a series of autoimmune or inflammatory diseases [2,3]. Its mechanisms of action, which have not been fully elucidated [4], include anti-idiotypic neutralization of autoantibodies, modulation of the action of cytokines and of the function of lymphocytes and Fc receptors, and interference with the complement system [4,5].
In vivo, the IVIG preparations showed the ability to attenuate the lesion induced by the activation of the complement system both in experimental animal models [6] and in humans [7,8]. IVIG inhibited C3 uptake by acting as the preferential acceptor of activated C3 and C4 fragments, with subsequent reduction of the supply of C3b and C4b fragments for in situ deposition [6,8–10]. These data were further supported by the demonstration that the reduction of C3 and C4 was due to scavenging by IVIG and not to activation or consumption, with IgM-enriched IVIG being more efficient in this function than IVIG with pure IgG [11]. Another mechanism was related to the property of immunoglobulin molecules to bind to the C1q fragment, thus preventing this fragment from being deposited on its target [12–14]. IVIG can also reduce the activation of complement by increasing the physiological cleavage of C3b in C3b(n)–IgG complexes [15]. In contrast, IVIG can induce complement activation, as demonstrated by the rise of the circulating complement activation products BbC3bc, C5a and terminal SC5b-9 complement complex without changes in antigenic concentrations of complement components or in haemolytic activity of the classical and alternative pathways [16].
In the present study we investigated the effect of administration of high doses of IVIG on antibody binding and on C3 deposition in the lungs of rats submitted to the experimental model of acute immunopneumonitis induced by pneumotoxic (anti-lung) serum, in which the lesion depends on the complement system [17].
MATERIALS AND METHODS
Female rats of the Wistar strain weighing 85–105 g were used throughout the study. The animals were handled according to the 1991 Ethical Guidelines of the Brazilian College of Animal Experimentation (COBEA).
Pneumotoxic serum
Rabbit anti-rat lung serum (PNTS) was prepared from rabbits hyperimmunized with rat lung homogenates. The anti-lung sera were pooled and the pool was assayed to determine the lethal dose (dose required to cause death of at least 90% of the animals within 30 min of i.v. injection) and the sublethal dose (the highest PNTS dose that would permit the survival of at least 70% of the animals within 30 min of i.v. injection). In the experiments reported here the lethal dose and the sublethal dose were 0·6 ml/rat and 0·4 ml/rat, respectively.
Reagents
IVIG (Sandoglobulin) and human F(ab′)2 fragment for i.v. use (Gammavenin) were used at 15% concentration. F(ab′)2 fragment for i.v. use was previously dialysed against physiological saline (PS) for 24 h.
Experimental design
The experimental model consisted of pretreatment and challenge of the animals with i.v. injections as indicated in the scheme in Table 1.
Table 1.
| ↓ Pretreatment | ↓ Challenge | ↓ Outcome | |
|---|---|---|---|
| Rat | PS, IVIG or F(ab′)2 | PS or PNTS | Lethal effect or sacrifice of surviving animals; blood collection; lung removal |
PS, Physiological saline; IVIG, IVIG preparation; F(ab′)2, F(ab′)2 preparation.
To define the IVIG dose, the rats were divided into three groups of eight animals each according to the amount of IVIG administered during pretreatment: 100 mg, 200 mg or 300 mg (groups G100, G200 and G300, respectively). Another group of 14 animals was pretreated with PS (group GPNTS). A lethal dose of PNTS was administered to all animals 1 h later. Since the animals in group G300 presented lower pulmonary damage, the dose of 300 mg was used both for IVIG and for F(ab′)2. Finally, a group of 13 rats (Gcontrol) was pretreated with PS and challenged with PS.
Blood collection and lung preparation
Blood was collected by cardiac puncture after sodium thiopental (Thionembutal) anaesthesia and chest opening. The chest was opened, the lungs were removed and their weight was reported as lung weight/100 g body weight (LW/BW) index. Fragments of the right lung were obtained for light and immunofluorescence microscopy studies. Frozen lung samples embedded in Tissue-Tek medium (Miles Labs, Elkhart, IN) were stained with FITC-conjugated goat anti-rabbit IgG at 1:80 dilution, goat anti-rat C3 (Organon Teknika Corp., Durham, NC) at 1:15 dilution, or rabbit anti-human IgG (Behring, Marburg, Germany) at 1:20 dilution. The fluorescence intensity (mean density) of the binding of rabbit antibodies to lung parenchyma was quantified using the Scion Image software (National Institutes of Health, Bethesda, MD). The images were acquired by an image acquisition system connected to an Axiophot microscope (Zeiss, Obercochen, Germany). The total intensity of each field was measured and the background mean density was subtracted from it in order to obtain the actual mean density of the field. Five fields per slide (per rat) were analysed.
Determination of the haemolytic activity of the complement system
Buffers
Triethanolamine-buffered saline with 0·1% gelatin (TBS) prepared to contain 0·005 m Mg2+ and 0·0001 m Ca2+ (TBS Mg2+ Ca2+) was used as medium for the determination of the classical pathway (CP). TBS containing 0·002 m Mg2+ and 0·008 m ethylene-glycol-bis (β-amminoethyl ether) N,N,N′,N′-tetraacetic acid (TBS-Mg2+ EGTA) was used for the determination of alternative pathway (AP) activity.
Cells
Suspensions of sensitized sheep erythrocytes in TBS Mg2+ Ca2+ and uncoated rabbit erythrocytes in TBS-Mg2+ EGTA were standardized at concentrations sufficient to reach an optical density (OD) of 0·8 at 700 nm (Beckman DU640; Fullerton, CA) when mixed with the diluted serum sample.
Haemolytic assay
The complement activity of CP and AP was determined by a kinetic technique [18], adapted [19]. Briefly, 1·0 ml of a prewarmed preparation of serum diluted in TBS Mg2+ Ca2+ for CP and in TBS-Mg2+ EGTA for AP was added to 0·2 ml of sensitized sheep erythrocytes for CP or of rabbit erythrocytes for AP and preincubated in the thermoregulated (37°C) cuvette holder of the spectrophotometer, and the graphic recorder was activated to register the change in OD. The time elapsed for the OD to be reduced from 0·8 to 0·4 (t½) is the time needed for lysis of 50% of the cells in the reaction medium.
Determination of human serum IgG concentration
The procedure was carried out by nephelometry using an automatic ICS analyser II (Beckman).
Statistical analysis
LW/BW index data were analysed by the Kruskal–Wallis test and the Fisher least significant difference test was applied to the complement results. The fluorescence intensity data, reported as medians, were analysed by the Mann–Whitney U-test. The Tukey–Kramer multiple comparisons test was applied to the complement results and the data are reported as means ± s.d. The level of significance was set at P < 0·05.
RESULTS
Determination of the protective IVIG dose in animals submitted to challenge with a lethal PNTS dose
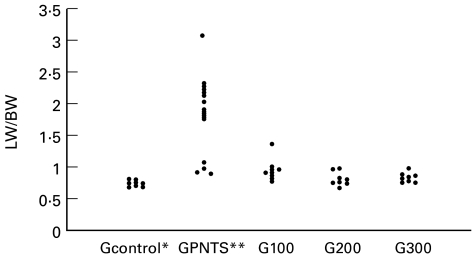
Figure 1 presents the distribution of the LW/BW indexes for the animals used for the determination of the protective IVIG dose. The LW/BW index for the group treated only with a lethal dose of PNTS (GPNTS) (1·78 ± 0·63) differed significantly from those for the Gcontrol (0·71 ± 0·04), G200 (0·79 ± 0·10) and G300 (0·82 ± 0·06) groups. Group G100 (0·95 ± 0·17) differed significantly from the Gcontrol group but not from groups GPNTS, G200 or G300.
Fig. 1.
Determination of the protective IVIG dose: lung weight/100 g body weight index (LW/BW) in animals treated with a lethal dose of pneumotoxic serum (PNTS); *P < 0·05 compared with group G100; **P < 0·05 compared with groups Gcontrol, G200 and G300.
With respect to mortality rate, 10 of 14 animals from the GPNTS group (71%) died during the observation period (nine died up to 5 min after the injection) and four survived up to 30 min, when they were killed. In contrast, all animals in groups G100, G200 and G300 survived throughout the 30-min period of observation after i.v. administration of the lethal PNTS dose. Thus, the three IVIG doses used were efficient in preventing the death of animals treated with a lethal PNTS dose.
Determination of human IgG concentration in the serum of animals treated with IVIG or F(ab′)2
Serum IgG concentration was determined in three groups of animals treated intravenously with PS, IVIG or F(ab′)2. Mean serum concentration of human IVIG was 0·027 ± 0·008 mg/ml in animals treated with PS, as opposed to 42·1 ± 14·2 mg/ml in animals treated with IVIG and 1·92 ± 0·53 mg/ml in animals treated with F(ab′)2. The F(ab′)2 preparation contained small amounts of the Fc portion, a fact possibly explaining these findings, since the reference antibody of the method used for determination is specific for γ-chains. Traces of human IgG observed in animals treated only with PS were probably due to non-specific cross-reaction.
Effect of treatment with IVIG and with F(ab′)2 on animals challenged with a sublethal PNTS dose
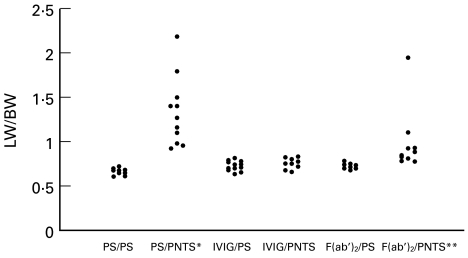
Since the administration of lethal PNTS doses causes rapid animal death it was decided to use sublethal doses of this serum (0·4 ml/100 g BW) in order to obtain a longer survival. The distribution of LW/BW indexes is presented in Fig. 2. Animals in the PS/PNTS group presented higher LW/BW indexes (1·32 ± 0·38) than those of all other groups and these differences were highly significant compared with groups PS/PS (0·66 ± 0·03), IVIG/PS (0·71 ± 0·05), IVIG/PNTS (0·74 ± 0·06) and F(ab′)2/PS (0·71 ± 0·02). The LW/BW indexes for the animals in the F(ab′)2/PNTS groups (0·94 ± 0·33) were higher than those for the remaining groups, except for the PS/PNTS group, although the difference was significant only compared with the PS/PS group. Groups PS/PS, IVIG/PS, IVIG/PNTS and F(ab′)2/PS did not differ significantly from one another.
Fig. 2.
Lung weight/100 g body weight index (LW/BW) of animals treated with IVIG or F(ab′)2 and challenged with a sublethal dose of pneumotoxic serum (PNTS). *P < 0·05 compared with groups PS/PS, IVIG/PS, IVIG/PNTS and F(ab′)2/PS; **P < 0·05 compared with group PS/PS.
Histopathologic analysis of the lungs
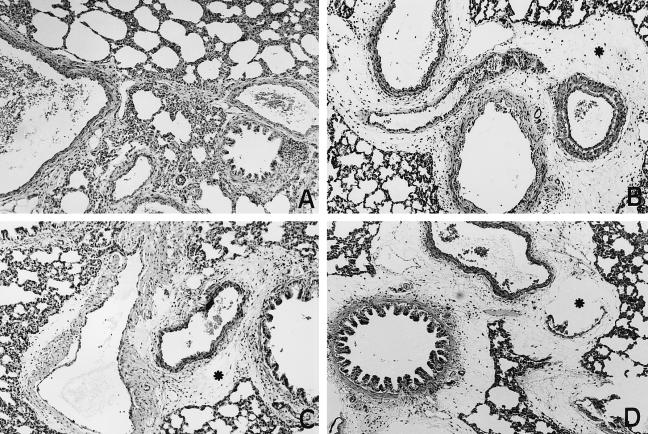
All the animals in groups PS/PS, IVIG/PS and F(ab′)2/PS presented histologically normal lung parenchyma (Fig. 3A). In contrast, light microscopy analysis of lung sections from all animals in groups PS/PNTS and F(ab′)2/PNTS showed intra-alveolar oedema, haemorrhage and congestion of septal capillaries, always of a focal nature, and marked oedema of the tunica adventitia of the pulmonary arteries (Fig. 3B,D). All animals in the IVIG/PNTS group showed discrete oedema of the tunica adventia of the pulmonary artery and congestion of septal capillaries (Fig. 3C), always of a focal nature and considerably less intense than in the animals of the PS/PNTS and F(ab′)2/PNTS groups.
Fig. 3.
Histological sections of rat lungs. (A) Normal aspect in an animal from group PS/PS. (B,D) Marked oedema of the adventitia of the pulmonary arteries (*) in animals from groups PS/PNTS and F(ab′)2/PNTS, respectively. (C) Slight oedema of the adventitia of the pulmonary arteries (*) of an animal from group IVIG/PNTS. (Haematoxylin–eosin, × 300.)
Deposition of rabbit antibodies in the lungs
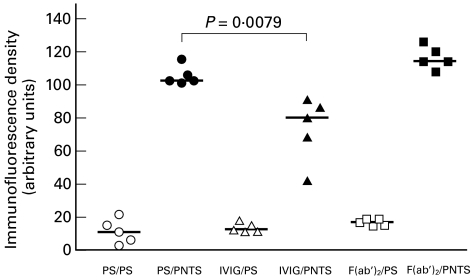
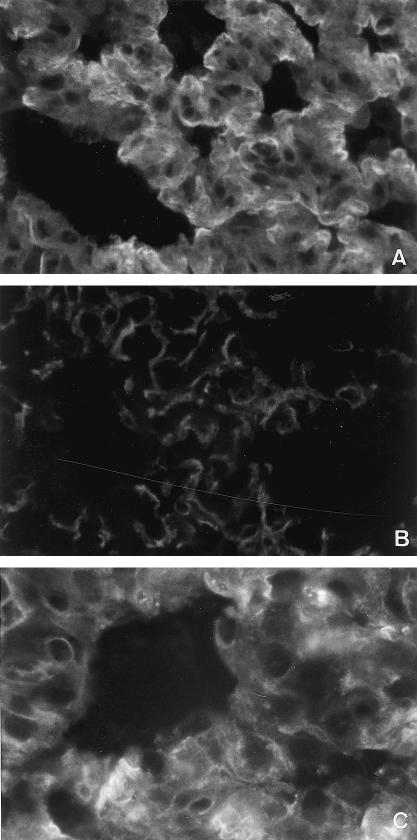
The deposition of rabbit antibodies in lung tissue evaluated by fluorescence intensity is shown in Fig. 5. Fluorescence intensity was very low in animals from groups PS/PS (10·4), IVIG/PS (11·7) and F(ab′)2/PS (16·1). In contrast, fluorescence intensity was higher in the lungs from animals in groups PS/PNTS (105·9) and F(ab′)2/PNTS (113·7), with a linear pattern and continuous and diffuse distribution in the alveolar septa (Fig. 4A,C). In the animals from the IVIG//PNTS group (79·85), the fluorescence intensity was lower than that observed in groups PS/PNTS (P < 0·05) and F(ab′)2/PNTS (Fig. 5) with the same patterns (Fig. 4B).
Fig. 5.
Effect of the pretreatment with IVIG and F(ab′)2 on the binding of PNTS on rat lung. Each group consisted of five rats. Symbols represent means of five determinations of immunofluorescence intensity in lung sections and horizontal bars indicate median values. The difference between the PS/PNTS and IVIG/PNTS groups was determined by the Mann–Whitney U-test; P = 0·0079 is considered highly significant.
Fig. 4.
Immunofluorescence microscopy of rat lung sections showing deposition of rabbit antibody in a linear pattern with continuous and diffuse distribution in the pulmonary septa. (A) PS/PNTS group with strong intensity. (B) IVIG/PNTS group with weak intensity. (C) F(ab′)2/PNTS group with strong intensity. (× 600.)
Deposition of human IVIG in the lungs
No human IgG was detected in the lungs of any animal.
Deposition of the C3 component of the complement system in the lungs
The C3 component was not detected in the lungs from groups PS/PS, IVIG/PS, IVIG/PNTS or F(ab′)2/PS as assessed by immunofluorescence microscopy. In the lungs from group PS/PNTS, fluorescence was of moderate intensity with a granular pattern and was distributed in a discontinuous and focal manner in the pulmonary alveolar septa. Fluorescence intensity was weak in most animals in group F(ab′)2/PNTS (moderate in one animal), always of a granular pattern and with a distribution similar to that described for the lungs of the animals in group PS/PNTS.
Determination of the lytic activity of serum complement (classical pathway) in animals pretreated with IVIG or F(ab′)2 and challenged with a sublethal dose of PNTS
The serum lytic activity did not differ significantly among groups PS/PS (254·1 ± 39·7 s), IVIG/PS (331·1 ± 68·9 s) and F(ab′)2/PS (331·4 ± 68·6 s). Complement lytic activity was lower in the IVIG/PNTS group, as determined by the higher t½ (748·7 ± 68·3 s), and significantly different from groups PS/PS, IVIG/PS and F(ab′)2/PS. In contrast, all measurements were undetermined in groups PS/PNTS and F(ab′)2/PNTS (t½ > 1200 s).
Determination of the lytic activity of serum complement (alternative pathway) of animals pretreated with IVIG or F(ab′)2 and challenged with a sublethal dose of PNTS
Only in the serum of group PS/PS animals was it possible to determine the lytic activity of complement (196·1 ± 39·4 s), whereas in all the animals of the other groups this activity was undetermined (t½ > 1200 s).
DISCUSSION
In the present study we investigated the protective effect of an IVIG preparation for clinical use in an experimental model of complement-dependent acute haemorrhagic immunopneumonitis [17] in order to assess some of the possible immunological mechanisms involved in this process.
None of the animals previously treated with IVIG died after being injected with a lethal PNTS dose, and all presented a clear reduction of pulmonary damage that was correlated with the IVIG dose used. Improved survival time was also observed in another experimental model of acute immunological tissue injury in guinea pigs treated with IVIG and submitted to Forssman shock [6].
The evaluation of pulmonary injury by the LW/BW index showed that animals from the IVIG/PNTS group presented pulmonary lesions of lower intensity compared with the animals in the other groups which also received a sublethal dose of PNTS. The F(ab′)2 preparation also conferred some protection, since the pulmonary lesions of the animals in the F(ab′)2/PNTS group, although present, were less severe than those of the animals in the PS/PNTS group. These results indicate that the biological property of attenuating tissue injury exhibited by the IVIG preparation depends on the integrity of the molecule (therefore with the presence of the heavy chain). This assumption is supported by studies in which the human Fc fragment of IgG incubated in vitro with frozen kidney sections from patients with glomerular disease induced dissociation of IgG deposits from the glomerular capillary wall [20].
In this immunopneumopathy model, we observed the binding of PNTS on the alveolar basement membrane, as previously described by other authors [21,22]. Quantification of anti-lung deposits demonstrated that, while the animals in group IVIG/PNTS showed significantly lesser tissue deposition of this antibody, the animals in groups PS/PNTS and F(ab′)2/PNTS showed intense deposition. These results suggest that IVIG reduced the antibody binding, an effect that was not observed with the use of the F(ab′)2 preparation. Concerning these findings, in the model of autoimmune nephritis induced by mercury chloride in Brown-Norway rats, IVIG did not change the deposition of rat autoantibody (IgG) on the glomerular capillary wall, but F(ab′)2 fragments obtained from the IVIG preparation were effective in inhibiting anti-laminin activity in vitro [23]. To our knowledge, the in vivo interference of IVIG with antibody binding to the tissue target antigen observed here has not been previously demonstrated. However, in contrast to our results, no attenuation of rabbit anti-Forssman antibody binding was observed in guinea pigs submitted to Forssman shock and treated with IVIG [6]. Also, administration of goat IgG in Heymann experimental nephritis did not reduce the deposition of the pathogenic antibody [24]. Furthermore, IVIG (pure IgG or IgM-enriched) also did not interfere with the renal deposition of mouse IgG2a MoAb against the rat Thy1.1 antigen (anti-Thy1 nephritis model) [11]. These discrepancies may be caused by the different structural locations of the target antigen within the different animals models [25–27]. Furthermore, even though complement depletion results in protection of both PNTS-treated rats [17] and of guinea pigs submitted to Forssman shock [28,29], guinea pigs seem to be less sensitive to decomplementation induced by aggregated IgG than to the acute haemorrhagic immunopneumonitis provoked by pneumotoxic serum [30]. It should be pointed out however that in the present study neither IVIG nor F(ab′)2 presented binding to the rat lung, a result similar to those reported by others for IVIG [6]. On the other hand, there are reports of in vivo impairment of glomerular deposits induced by IVIG in patients with different diseases [20, 31, 32] which was attributed to dissociation of the immunocomplexes in situ.
The experimental immunopathologic model used here is characterized by C3 deposition in pulmonary tissue, as previously demonstrated by other authors [21]. In our experiments no C3 deposition was observed in the lungs of IVIG/PNTS animals, and the deposition observed in F(ab′)2/PNTS animals was of lower intensity than that observed in PS/PNTS animals. These results may be interpreted on the basis of the absence of in situ C3 deposition consequent to the lack of formation of the anti-basement membrane antibody immunocomplex in animals pretreated with IVIG. In contrast, in other experimental models IVIG reduced C3 and C5b-9 deposition in the glomeruli in the rat anti-Thy1 nephritis model, and no C3 or C5b-9 was detected with IgM-enriched IVIG. Considering that in this same experiment the pathogenic antibody was not affected in terms of binding to the target antigen, the effect of IVIG must be related to the complement system. Indeed, C4 binding was demonstrated both in IgM and IgG molecules, supporting the idea of a scavenging action of these immunoglobulins on the complement components [6, 33, 34]. This hypothesis is more probable than that of activation and consumption to explain inhibition. The reduction of C3 deposition induced by IVIG was also reported in the hyperacute xenograft rejection model (guinea pig to rat cardiac xenografting) through F(ab′)2-mediated anti-complement activity [35].
A question that should be further explored in future studies concerns the effects of IVIG on the lytic activity of serum complement: whether they are related to the lack of activation or inhibition of the system (there was no C3 deposition in IVIG/PNTS animals) or whether they interfered directly with the initial activation reactions. Indeed, a reduction of C3 deposition in the target tissue induced by IVIG treatment was reported [24], as also was the inhibition of C3 uptake onto IgG-sensitized erythrocytes [6]. Conversely, IVIG induces an increase in the products of complement activation (Bb, C3bc and terminal SC5b-9 complement complex) [12], as well as an increase in the formation of Clrs–Clinh complexes and C4bc [14]. In addition, it was demonstrated that, in vitro, IVIG produces competitive binding of activated C1q, C4 and C3 to IgG [12] and can also neutralize excessive C1 activation and the consequent C4 consumption in the serum of a patient with paraproteinaemia and hypocomplementaemia [13]. This effect was due to the interaction of the Fc fragment of immunoglobulin with products of activation of the classical pathway [12,36]. Our results show that animals of the IVIG/PNTS group presented a clear-cut reduced haemolytic activity compared with the control groups. However, no detectable haemolytic activity occurred in PS/PNTS animals or in F(ab′)2/PNTS animals. The finding that the lytic activity of CP was preserved in animals treated with IVIG or F(ab′)2 suggests that neither preparation interferes per se with the complement system, as also observed for IVIG under similar conditions in guinea pigs by others [33]. Also in humans, no decrease in complement haemolytic activity (classical and alternative CH50) was observed in vivo[16] even though in another study IVIG inhibited the classical pathway in vitro [12]. Indeed, there is evidence that IVIG may interfere with the classical pathway of complement at some stages [6, 24, 33, 34], but not with the phase of recognition [37]. Concerning the haemolytic activity of the alternative pathway, it was impossible to determine t½ in the animals that were pretreated with IVIG or F(ab′)2. However, this finding deserves to be further examined, since it was reported that a different preparation of IgG molecules (obtained by reduction and alkylation) has the ability to enhance AP activity in vitro [38].
It should be pointed out that the soluble complex (SC5b-9) of the complement system increases hydraulic conductivity in the pulmonary venules, reducing the capacity of the endothelial barrier through a mechanism apparently dependent on integrins and not dependent on the presence of other anaphylatoxins, on the membrane attack complex or on leucocytes [39]. This activity would explain the severe pulmonary oedema and oedema of the tunica adventitia of the pulmonary arteries that was observed within a very short time in the experimental model examined here.
We conclude that an infusion of a high dose of IVIG affects the binding of pathogenic antibody to the tissue target and attenuates the inflammatory reaction as observed in this experimental model of complement-dependent immunopathological lesion.
Acknowledgments
The authors wish to thank Dr João Santana da Silva for kindly providing FITC-conjugated anti-rat IgG rabbit serum, Dr Uilho Antônio Gomes for support with the statistical analysis, Adalberto Valladas Verceze, Erika Pontin Delloiagono Gual and Denize Brufato Ferraz for technical assistance, and Alex Adriano da Silva for the computation work. This work was partially supported by FAEPA.
REFERENCES
- 1.Imbach P, Barandum S, d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–31. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 2.Keller T, McGrath K, Newland A, et al. Consensus statement. Indications for use of intravenous immunoglobulin. Recommendations of the Australasian society of blood transfusion consensus symposium. Med J Aust. 1993;159:204–6. doi: 10.5694/j.1326-5377.1993.tb137790.x. [DOI] [PubMed] [Google Scholar]
- 3.NIH Consensus Conference. Intravenous immunoglobulin. Prevention and treatment of disease. JAMA. 1990;264:3189–93. [PubMed] [Google Scholar]
- 4.Kazatchkine MD. Mechanisms of action of intravenous immunoglobulin therapy in autoimmune and inflammatory disorders. In: Kazatchkine MD, Louwagie A, editors. Immunoglobulins: extending the horizon The Proceedings of a satellite symposium held at the first meeting of the European Haematology Association. New York: The Parthenon Publishing Group Ltd; 1994. pp. 11–22. [Google Scholar]
- 5.Mouthon L, Kaveri SV, Spalter SH, et al. Mechanisms of action of intravenous immunoglobulin in immune-mediated diseases. Clin Exp Immunol. 1996;104:3–9. [PubMed] [Google Scholar]
- 6.Basta M, Kirshbom P, Frank MM, et al. Mechanism of therapeutic effect of high-dose intravenous immunoglobulin. Attenuation of acute, complement-dependent immune damage in a guinea pig model. J Clin Invest. 1989;84:1974–81. doi: 10.1172/JCI114387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329:1993–2000. doi: 10.1056/NEJM199312303292704. [DOI] [PubMed] [Google Scholar]
- 8.Basta M, Dalakas MC. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest. pp. 1729–35. [DOI] [PMC free article] [PubMed]
- 9.Berger M, Rosenkranz P, Brown CY. Intravenous and standard immune serum globulin preparations interfere with uptake of 125-I-C3 onto sensitized erythrocytes and inhibit hemolytic complement activity. Clin Immunol Immunopathol. 1985;34:227–36. doi: 10.1016/0090-1229(85)90027-3. [DOI] [PubMed] [Google Scholar]
- 10.Basta M. Modulations of complement. Mediated immune damage by intravenous immune globulin. Clin Exp Immunol. 1996;104:21–25. [PubMed] [Google Scholar]
- 11.Rieben R, Roos A, Muizert Y, et al. Immunoglobulin Mm-enriched human intravenous immunoglobulin prevents complement-activation in vitro and in vivo in a rat model of acute inflammation. Blood. 1999;93:942–51. [PubMed] [Google Scholar]
- 12.Mollnes TE, Hogasen K, Hoaas BFF, et al. Inhibition of complement-mediated red cell lysis by immunoglobulins is dependent on the IG isotype and its C1 binding properties. Scand J Immunol. 1995;41:449–56. doi: 10.1111/j.1365-3083.1995.tb03591.x. [DOI] [PubMed] [Google Scholar]
- 13.Qi M, Schifferli JA. Inhibition of complement activation by intravenous immunoglobulins. Arthritis Rheum. 1995;38:146. doi: 10.1002/art.1780380123. [DOI] [PubMed] [Google Scholar]
- 14.Mollnes TE, Andreassen IH, Hogasen K, et al. Effect of whole and fractionated intravenous immunoglobulin on complement in vitro. Mol Immunol. 1997;34:719–29. doi: 10.1016/s0161-5890(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 15.Lutz HU, Stammler P, Jelezarova E, et al. High doses of immunoglobulin G attenuate immune aggregate-mediated complement activation by enhancing physiologic cleavage of C3b in C3b(n)–IgG complexes. Blood. 1996;88:184–93. [PubMed] [Google Scholar]
- 16.Mollnes TE, Hogasen K, De Carolis C, et al. High-dose intravenous immunoglobulin treatment activates complement in vivo. Scand J Immunol. 1998;48:312–7. doi: 10.1046/j.1365-3083.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho IF, Oliveira HL, Laus-Filho JA, et al. Prevention of acute immunological lung lesion in rats by decomplementing treatment. Immunology. 1969;16:633–41. [PMC free article] [PubMed] [Google Scholar]
- 18.Polhill RB, Pruitt KM, Jr, Johnston RB., Jr Kinetic assessment of alternative complement pathway activity in a hemolytic system. I. Experimental and mathematical analysis. J Immunol. 1978;121:363–70. [PubMed] [Google Scholar]
- 19.Ferriani VPL, Barbosa JE, Carvalho IF. Serum haemolytic classical and alternative pathways of complement in infancy: age-related changes. Acta Pediatr Scand. 1990;79:3122–7. doi: 10.1111/j.1651-2227.1990.tb11464.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin C-Y, Hsu H-C, Chiang H. Improvement of histological and immunological change in steroid and immunosuppressive drug-resistant lupus nephritis by high-dose intravenous gamma globulin. Nephron. 1989;53:303–10. doi: 10.1159/000185772. [DOI] [PubMed] [Google Scholar]
- 21.Hagadorn JE, Vazquez JJ, Kinney TR. Immunopathologic studies of an experimental model resembling Goodpasture's syndrome. Am J Pathol. 1969;57:17–25. [PMC free article] [PubMed] [Google Scholar]
- 22.Willoughby WF, Dixon FJ. Experimental hemorrhagic pneumonitis produced by heterologous anti-lung antibody. Immunol. 1970;104:28–37. [PubMed] [Google Scholar]
- 23.Rossi F, Bellon B, Vial MC, et al. Beneficial effect of human therapeutic intravenous immunoglobulins (IVIG) in mercury-chloride-induced autoimmune disease of Brown-Norway rats. Clin Exp Immunol. 1991;84:129–33. doi: 10.1111/j.1365-2249.1991.tb08135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nangaku M, Pippin J, Richardson CA, et al. Beneficial effects of systemic immunoglobulin in experimental membranous nephropathy. Kidney Int. 1996;50:2054–62. doi: 10.1038/ki.1996.529. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka N, Leduc EH. A study of the cellular distribution of Forssman antigen in various species. J Immunol. 1956;77:198–212. [PubMed] [Google Scholar]
- 26.Spear GS. Forssman antigen in the guinea pig: a histologic study. Bull Johns Hopkins Hosp. 1962;111:252–65. [PubMed] [Google Scholar]
- 27.Bagchus WM, Hoedemmaeker PJ, Rozing J, et al. Glomerulonephritis induced by monoclonal anti-Thy1.1 antibodies. A sequential histological and ultrastructural study in the rat. Lab Invest. 1986;55:680–7. [PubMed] [Google Scholar]
- 28.Jensen JÁ. A specific inactivator of mammalian C′4 isolated from nurse shark (Ginglymostoma cirratum) serum. J Exp Med. 1969;130:217–41. doi: 10.1084/jem.130.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spear GS, Kiara I. Complement and heterophile shock. Johns Hopkins Med J. 1970;126:210–6. [PubMed] [Google Scholar]
- 30.Carvalho IF, Sarti W. Experimental immunological lung lesion in rats and guinea pigs studies on the mechanism of the pulmonary damage. In: Miescher PA, editor. Immunopathology VI International Symposium. Basel: Schwabe Publishers; 1970. pp. 366–76. [Google Scholar]
- 31.Palla R, Cirami C, Panichi V, et al. Intravenous immunoglobulin therapy of membranous nephropathy: efficacy and safety. Clin Nephrol. 1991;35:P98–104. [PubMed] [Google Scholar]
- 32.Rostoker G, Desvaux-Belghit D, Pilatte Y, et al. High-dose immunoglobulin therapy for severe IgA nephropathy and Henoch-Schönlein purpura. Ann Intern Med. 1994;120:476–84. doi: 10.7326/0003-4819-120-6-199403150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Basta M, Langlois PF, Marques M, et al. High-dose intravenous immunoglobulin modifies complement-mediated in vivo clearance. Blood. 1989;74:326–33. [PubMed] [Google Scholar]
- 34.Basta M, Fries LF, Frank MM. High doses of intravenous Ig inhibit in vitro uptake of C4 fragments onto sensitized erythrocytes. Blood. 1991;77:376–80. [PubMed] [Google Scholar]
- 35.Latremouille CH, Genevaz D, Hu MC, et al. Normal human immunoglobulins for intravenous use (IVIG) delay hyperacute xenograft rejection through F(ab′)2-mediated anti-complement activity. Clin Exp Immunol. 1997;110:122–6. doi: 10.1046/j.1365-2249.1997.4591358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomura S, Miyasaki Y, Miyake T, et al. IgG inhibits the increase of platelet-associated C3 stimulated by anti-platelet antibodies. Clin Exp Immunol. 1993;93:452–5. doi: 10.1111/j.1365-2249.1993.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basta M, Fries LF, Frank MM. High doses of intravenous immunoglobulin do not affect the recognition phase of the classical complement pathway. Blood. 1991;78:700–2. [PubMed] [Google Scholar]
- 38.Bing DH. The interaction of immune serum globulin and immune globulin intravenous with complement. Mol Immunol. 1983;20:892–900. doi: 10.1016/0161-5890(83)90087-1. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa S, Tsukada H, Bhattacharya J. Soluble complex of complement increases hydraulic conductivity in single microvessels of rat lung. J Clin Invest. 1993;91:103–9. doi: 10.1172/JCI116157. [DOI] [PMC free article] [PubMed] [Google Scholar]