Abstract
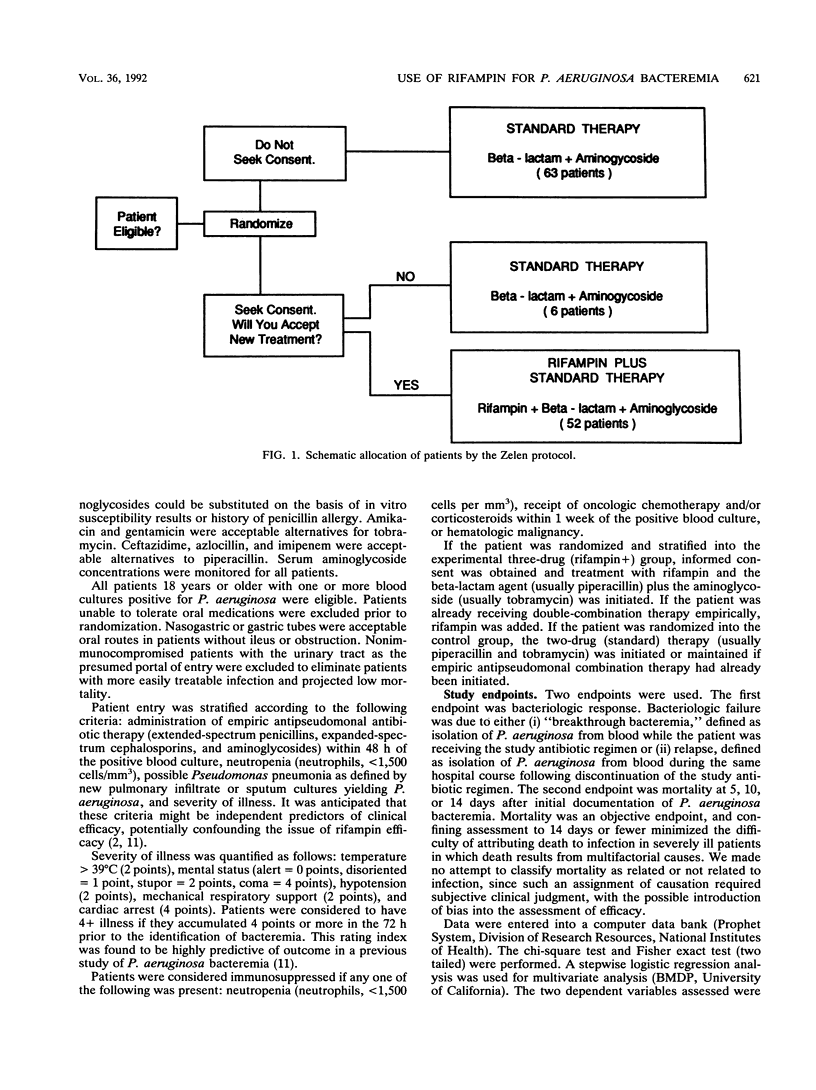
A multicenter, prospective randomized trial was conducted to determine if the addition of rifampin to a combination therapy of an antipseudomonal beta-lactam agent and aminoglycoside improves the outcome of patients with Pseudomonas aeruginosa bacteremia. The Zelen protocol for randomized-consent design was used. Consent was sought only from patients randomized to the experimental therapy (rifampin+). If the experimental therapy was refused, the patient would then receive the standard combination therapy (control); however, when outcome was evaluated, all patients randomized to the rifampin+ group, including those that declined rifampin, were compared with the control group. One hundred twenty-one consecutive hospitalized patients with positive blood cultures for P. aeruginosa were enrolled. Entry was stratified for prior use of empiric antipseudomonal antibiotics, neutropenia, severity of illness, and presence of pneumonia. Fifty-eight patients were randomized to receive rifampin (600 mg orally every 8 h for the first 72 h and then every 12 h for a total of 10 days) plus a beta-lactam agent plus an aminoglycoside. Sixty-three received the standard therapy of a beta-lactam plus an aminoglycoside agent (control). Bacteriologic cure occurred significantly more frequently in patients randomized to the rifampin+ regimen. Breakthrough or relapsing bacteremias occurred in 2% of the three-drug (rifampin+) group, compared with 14% for the two-drug (standard therapy) group. Despite this favorable trend in bacteriological response, no significant differences in survival were seen for the two treatment groups. Rifamycin derivatives warrant further clinical study as antipseudomonal agents. The Zelen protocol appears well suited for comparative trials of antimicrobial agents.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltch A. L., Smith R. P. Combinations of antibiotics against Pseudomonas aeruginosa. Am J Med. 1985 Jul 15;79(1A):8–16. doi: 10.1016/0002-9343(85)90185-8. [DOI] [PubMed] [Google Scholar]
- Bisbe J., Gatell J. M., Puig J., Mallolas J., Martinez J. A., Jimenez de Anta M. T., Soriano E. Pseudomonas aeruginosa bacteremia: univariate and multivariate analyses of factors influencing the prognosis in 133 episodes. Rev Infect Dis. 1988 May-Jun;10(3):629–635. doi: 10.1093/clinids/10.3.629. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Jadeja L., Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med. 1985 Sep;145(9):1621–1629. doi: 10.1001/archinte.145.9.1621. [DOI] [PubMed] [Google Scholar]
- Cross A., Allen J. R., Burke J., Ducel G., Harris A., John J., Johnson D., Lew M., MacMillan B., Meers P. Nosocomial infections due to Pseudomonas aeruginosa: review of recent trends. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S837–S845. doi: 10.1093/clinids/5.supplement_5.s837. [DOI] [PubMed] [Google Scholar]
- Curran W. J. Sounding Board. Reasonableness and randomization in clinical trial: fundamental law and government regulation. N Engl J Med. 1979 May 31;300(22):1273–1275. doi: 10.1056/NEJM197905313002213. [DOI] [PubMed] [Google Scholar]
- Ellenberg S. S. Randomization designs in comparative clinical trials. N Engl J Med. 1984 May 24;310(21):1404–1408. doi: 10.1056/NEJM198405243102141. [DOI] [PubMed] [Google Scholar]
- Flick M. R., Cluff L. E. Pseudomonas bacteremia. Review of 108 cases. Am J Med. 1976 Apr;60(4):501–508. doi: 10.1016/0002-9343(76)90716-6. [DOI] [PubMed] [Google Scholar]
- Fu K. P., Lasinski E. R., Zoganas H. C., Kimble E. F., Konopka E. A. Efficacy of rifampicin in experimental Bacteroides fragilis and Pseudomonas aeruginosa mixed infections. J Antimicrob Chemother. 1985 May;15(5):579–585. doi: 10.1093/jac/15.5.579. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Bryan L. E. Resistance of Pseudomonas aeruginosa to new beta-lactamase-resistant beta-lactams. Antimicrob Agents Chemother. 1984 Oct;26(4):485–488. doi: 10.1128/aac.26.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf M., Yu V. L., Sharp J., Zuravleff J. J., Korvick J. A., Muder R. R. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989 Nov;87(5):540–546. doi: 10.1016/s0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- Horwitz R. I., Feinstein A. R. Advantages and drawbacks of the Zelen design for randomized clinical trials. J Clin Pharmacol. 1980 Jul;20(7):425–427. [PubMed] [Google Scholar]
- Korvick J. A., Yu V. L. Antimicrobial agent therapy for Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Nov;35(11):2167–2172. doi: 10.1128/aac.35.11.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvick J., Yu V. L., Hilf M. Susceptibility of 100 blood isolates of Pseudomonas aeruginosa to 19 antipseudomonal antibiotics: old and new. Diagn Microbiol Infect Dis. 1987 Jun;7(2):107–111. doi: 10.1016/0732-8893(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Lynch M. J., Drusano G. L., Mobley H. L. Emergence of resistance to imipenem in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Dec;31(12):1892–1896. doi: 10.1128/aac.31.12.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Rosner F. The ethics of randomized clinical trials. Am J Med. 1987 Feb;82(2):283–290. doi: 10.1016/0002-9343(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Rubin J., Stoehr G., Yu V. L., Muder R. R., Matador A., Kamerer D. B. Efficacy of oral ciprofloxacin plus rifampin for treatment of malignant external otitis. Arch Otolaryngol Head Neck Surg. 1989 Sep;115(9):1063–1069. doi: 10.1001/archotol.1989.01860330053016. [DOI] [PubMed] [Google Scholar]
- Schimpff S., Satterlee W., Young V. M., Serpick A. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med. 1971 May 13;284(19):1061–1065. doi: 10.1056/NEJM197105132841904. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Spohr M., Bauer D. Pseudomonas aeruginosa: in vitro susceptibility to antimicrobial drugs, single and combined, with and without defibrinated human blood. Chemotherapy. 1988;34(4):284–297. doi: 10.1159/000238582. [DOI] [PubMed] [Google Scholar]
- Valdes J. M., Baltch A. L., Smith R. P., Hammer M. C., Ritz W. J. The effect of rifampicin on the in-vitro activity of cefpirome or ceftazidime in combination with aminoglycosides against Pseudomonas aeruginosa. J Antimicrob Chemother. 1990 Apr;25(4):575–584. doi: 10.1093/jac/25.4.575. [DOI] [PubMed] [Google Scholar]
- Yu V. L., Zuravleff J. J., Peacock J. E., DeHertogh D., Tashjian L. Addition of rifampin to carboxypenicillin-aminoglycoside combination for the treatment of Pseudomonas aeruginosa infection: clinical experience with four patients. Antimicrob Agents Chemother. 1984 Oct;26(4):575–577. doi: 10.1128/aac.26.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelen M. A new design for randomized clinical trials. N Engl J Med. 1979 May 31;300(22):1242–1245. doi: 10.1056/NEJM197905313002203. [DOI] [PubMed] [Google Scholar]
- Zuravleff J. J., Chervenick P., Yu V. L., Muder R. R., Diven W. F. Addition of rifampin to ticarcillin-tobramycin combination for the treatment of Pseudomonas aeruginosa infections: assessment in a neutropenic mouse model. J Lab Clin Med. 1984 Jun;103(6):878–885. [PubMed] [Google Scholar]
- Zuravleff J. J., Yu V. L., Yee R. B. Ticarcillin-tobramycin-rifampin: in vitro synergy of the triplet combination against Pseudomonas aeruginosa. J Lab Clin Med. 1983 Jun;101(6):896–902. [PubMed] [Google Scholar]


