Abstract
Inhalation of dust from swine confinement buildings induces airway inflammation with an increase in both inflammatory cell numbers and secretion of proinflammatory cytokines in the lungs. It is not known whether anti-asthma drugs, which influence airway inflammation in asthma, also influence the airway reaction to inhaled organic dust. In the present study we examined the effects of a ß2-agonist (salmeterol) and an inhaled steroid (fluticasone) on the swine dust-induced cell and cytokine content of the lower airways, and cytokine release in cultured alveolar macrophages. Healthy volunteers were pretreated with inhaled salmeterol (n = 8), fluticasone propionate (n = 8) or placebo (n = 8) for about 2 weeks and exposed to dust in a pig house. Bronchoalveolar lavage was performed both before medication and after dust exposure. Cell diferential counts and cytokine analyses in bronchoalveolar lavage fluid (BALF) were examined. Alveolar macrophages were cultured and cytokine release was studied, both in unstimulated cells and after lipopolysaccharide (LPS) stimulation. Unstimulated alveolar macrophages from swine dust-exposed individuals released less IL-6, IL-8 and tumour necrosis factor-alpha (TNF-α) after, than before, exposure (P < 0·01). Medication did not influence basal cytokine production. Fluticasone inhibited LPS-induced IL-6 and IL-8 release (P < 0·05). There was no significant difference between the groups. There was a large and significant increase (P < 0·05) in alveolar macrophage, granulocyte, lymphocyte numbers, and IL-6 and TNF-α content in BALF in all three groups following dust exposure, with no significant difference between the groups. These findings suggest that drugs which are known to influence and control airway inflammation in asthma do not have major effects on airway inflammation induced by the inhalation of organic dust.
Keywords: organic dust, cells, cytokines, asthma medication, airway inflammation
INTRODUCTION
Inhalation of organic dust from a swine confinement building induces airway inflammation in healthy human volunteers. Three hours of exposure causes more than a doubling of alveolar macrophage numbers, a 75-fold increase in neutrophil numbers and a three-fold increase in lymphocyte numbers in bronchoalveolar lavage fluid (BALF) [1]. The exposure may also induce systemic effects such as increased body temperature and increased levels of acute-phase proteins in peripheral blood [1]. In addition, the concentrations of the proinflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), IL-6, IL-1 and IL-8 are elevated in nasal and bronchoalveolar lavage fluids, and TNF-α and IL-6 concentrations increase in peripheral blood following exposure to swine dust [2,3]. These findings indicate that IL-6, IL-8 and TNF-α actively participate in the initiation and maintenance of airway inflammation following inhalation of organic dust from a swine house.
Alveolar macrophages play an important regulatory role in the development of inflammatory reactions, by releasing an array of inflammatory cytokines including TNF-α, IL-1ß, IL-6 and IL-8. Previous studies have shown that cultured alveolar macrophages from healthy subjects secrete TNF-α, IL-6 and IL-8 upon swine dust and lipopolysaccharide (LPS) stimulation [4,5]. Fluticasone propionate almost totally inhibits LPS-induced TNF-α and IL-6 secretion and inhibits partially the IL-8 secretion of alveolar macrophages at a concentration of 10−8 min vitro[6]. There are no clear data whether salmeterol influences cytokine release and whether this exerts clinically significant effects in man.
In the present study we collected BALF and cultured alveolar macrophages from healthy individuals treated with inhaled fluticasone, salmeterol or placebo, before and after exposure to swine dust. The aim of the study was to find out whether the most commonly used asthma medications, which interfere with inflammatory responses in asthma, also influence the airway inflammatory response to organic dust inhalation. Particularly, we were interested in the capability of the alveolar macrophages, obtained before and after dust exposure and medication, to release cytokines in response to LPS in vitro, in order to find out whether treatment had influenced alveolar macrophage function.
SUBJECTS AND METHODS
Stimuli and drugs
LPS (Lipopolysaccharide B Escherichia coli 0111:B4; Difco, Detroit, MI) was dissolved in RPMI 1640 media (Seromed Biochrom KG, Berlin, Germany). Fluticasone propionate (Flutide Diskus) and salmeterol (Serevent Diskus) were generously provided by Glaxo Wellcome (Stevenage, UK).
Subjects
The study involved 24 healthy, non-smoking subjects (19 men) with a mean age of 27 years (range 21–46 years). To be considered eligible for participation, all subjects were required to have normal results of physical examination, chest x-ray and spirometry. They should have no history of asthma or allergic diseases, as evaluated by a questionnaire. The study was performed with the informed consent of all subjects and approved by the ethics committee at the Karolinska Institute.
Study design
Bronchoalveolar lavage was performed on all subjects. The subjects were then randomized into three groups, eight individuals in each group. The subjects of one group received fluticasone propionate (500 μ g bid for inhalation); in the second group, the subjects received salmeterol (50 μ g bid for inhalation) and the third group inhaled placebo, in a single blind manner. The medication lasted for 10–14 days, and about 1 h after the last dosing all subjects were exposed to swine dust while weighing pigs for 3 h in a swine confinement building containing 700–900 pigs. Two subjects, from different groups, were exposed on each occasion. Personal samplers were used for exposure measurements. Finally, the second bronchoalveolar lavage was performed 24 h after the start of the exposure.
Measurements of exposure
Each subject carried one IOM inhalable dust sampler (SKC Ltd, Dorset, UK) and two plastic cyclone samplers designed to separate the respirable dust particulates from the non-respirable fraction (Casella London Ltd, Bedford, UK) in the breathing zone, together with Airchek 50 personal sample pumps (SKC). The IOM sampler and one of the two cyclone samplers on each person were equipped with 25-mm Nuclepore filters with a pore size of 0·4 μ m. The other cyclone sampler on each person was equipped with 25-mm Millipore filter SCWP with a pore size of 8 μ m. The sampling was performed at an airflow of 2·0 l/min for the IOM samplers and 1·9 l/min for the cyclone samplers. Inhalable and respirable dust were measured by weighing after 24 h of conditioning, using a Mettler ME22 balance (Mettler, Greisensee, Switzerland) and reference filters.
The Nuclepore filters were analysed for endotoxin by extracting the material from the filters into 10 ml endotoxin-free water for 1 h while rotating. The extracts were centrifuged for 10 min at 1000 g and the supernatants were frozen at −70°C for later analysis using the chromogen version of Limulus amebocyte assay (QCL-1000, Endotoxin; BioWhittaker, Walkersville, MD; with E. coli 0111:B4 as standard).
Bronchoalveolar lavage
Bronchoscopy was performed through the nose with a flexible fibreoptic bronchoscope (Olympus Type 4B2) under local anaesthesia with 2% lidocain (Xylocaine; Astra, Södertälje, Sweden) after premedication with morphine–scopolamine. The bronchoscope was wedged into a subsegment middle lobe bronchus and sterile saline solution, at 37°C, was instilled in 5 × 50 ml aliquots. The fluid was gently aspirated and collected in a siliconized plastic bottle kept on ice. The BALF was filtered through a Dacron prefilter (Type AP 32 Millipore) and centrifuged (200 g, 10 min, 4°C). The pellet was resuspended in RPMI 1640 culture medium. Cell viability was determined by the exclusion of trypan blue solution (0·5% in normal saline; Amimed, Allschwil, Switzerland) before seeding onto plates and after the experiment. The total number of cells was enumerated using a haemocytometer. Differential cell counts were performed by flow cytometry (Epics Profile II; Coulter Electronics Inc., Hialeah, FL). The samples were prepared in a Coulter Q-prep (Coulter) and incubated for 10 min with CD14-CD45 MoAbs (Mo2-RD1/Kc56-FITC; Cytostat/Coulter Corp.)
Alveolar macrophages
About 1·5–3 million bronchoalveolar lavage cells per well were added to sterile six-well culture plates (Nunc, Roskilde, Denmark). The cells were then allowed to adhere for 2 h at 37°C, 5% CO2 in RPMI medium containing 5% heat-inactivated fetal calf serum (FCS), 1% penicillin/streptomycin and 0·5% gentamycin (Seromed Biochrom). The non-adherent cells were then removed by washing twice with serum-free medium. The actual number of alveolar macrophages was calculated by multiplying the number of seeded cells by the proportion of macrophages obtained by flow cytometry.
The alveolar macrophages from each person were, in duplicates, either unstimulated or LPS-stimulated. The unstimulated cells were incubated in serum-free RPMI medium (1% penicillin/streptomycin, 0·5% gentamycin) for 24 h; and the other cells were stimulated with LPS (100 μ g/ml) in serum-free RPMI medium (1% penicillin/streptomycin, 0·5% gentamycin) for 24 h.
Cytokine assays
TNF-α in BALF was analysed using commercial high sensitivity sandwich enzyme immunoassay kits (Quantikine; R&D Systems, Europe Ltd, Abingdon, UK). The detection range was 0·5–32 pg/ml. IL-6 levels in macrophage cell culture supernatants were measured with an ELISA developed in our laboratory; the procedure is described in detail by Larsson et al. [7], using commercial available antibodies. Briefly, IL-6 in BALF was measured with ELISA together with an enzyme amplified detection system. The anti-human capture MoAb was IL-6: MAB206, the detection biotinylated anti-human polyclonal antibody was IL-6: BAF206, and the recombinant human standard was IL-6: 206-IL-010 (R&D Systems Europe). An enzyme cycling system AMPAK (Dako Diagnostics Ltd, Ely, UK) was used to amplify the colourimetric signal generated by alkaline phosphatase. In the amplified system, the lowest standard was 0·125 pg/ml and in the unamplified system the detection range was 3–375 pg/ml. IL-8 in BALF and in macrophage cell culture supernatants and TNF-α in the macrophage cell culture supernatants were also measured using ELISA methods developed in our laboratory. The anti-human capture MoAbs were, IL-8: MAB208, and TNF-α: MAB610 (R&D Systems Europe). The detection biotinylated anti-human polyclonal antibodies were IL-8: BAF208, and TNF-α: BAF210. The recombinant human standards were IL-8: 208-IL-010, and TNF-α: 210-TA-010 (R&D Systems Europe). Quantikine serum controls at three different concentrations were used as calibrators in all three methods. The detection ranges in the IL-8 and TNF-α assays were 25–3200 pg/ml and 15·6–1000 pg/ml, respectively.
Absorbance was read at 450 nm and 650 nm using a Thermomax 250 reader (Molecular Devices, Sunnyvale, CA). The cytokines were measured and production was expressed as pg/ml or ng/106 cells. An intra-assay coefficient of variation of < 10% and an interassay coefficient of variation < 20% was accepted.
Statistical analysis
Exposure measurements, concentrations of cells and cytokines in BALF are given as median values (25th to 75th percentiles). Comparisons before and after exposure were performed by means of Wilcoxon's signed rank test. The difference between groups was tested by the Kruskal–Wallis and Mann–Whitney U-tests. For calculations, concentration values below the detection limit were assigned the value of the lowest detection limit. Cytokine concentration in cell culture supernatants is presented as mean (± s.e.m.). Comparisons of cell culture results were assessed using analysis of variance (anova) with the Fisher's PLSD test, when appropriate, and Student's t-test. P < 0·05 was considered significant. Statistical analysis was performed using the StatView program, version 4·02 for Macintosh (Abacus Concepts, Inc, Berkeley, CA).
RESULTS
Exposure measurements
The concentration of inhalable dust was 25·4 mg/m3 (21·2–34·7 mg/m3), of which the endotoxin concentration was 733 ng/m3 (402–1068 ng/m3) (n = 22). The concentration of respirable dust was 1·02 mg/m3 (0·76–1·27 mg/m3) (n = 22), of which the endotoxin concentration was 32 ng/m3 (13–56 ng/m3) (n = 22). There was no significant difference in exposure between the groups (Table 1).
Table 1.
The concentration of inhalable and respirable dust and the concentration of endotoxin in inhalable and respirable dust in the placebo, fluticasone and salmeterol groups. There was no significant difference of exposure between the groups.
| Placebo | Fluticasone | Salmeterol | |
|---|---|---|---|
| Inhalable dust (mg/m3) | |||
| Median | 27 | 27 | 23 |
| 25th to 75th percentile | (18–39) | (21–34) | (22–27) |
| Inhalable endotoxin (ng/m3) | |||
| Median | 835 | 842 | 577 |
| 25th to 75th percentile | (371–1230) | (511–1157) | (402–903) |
| Respirable dust (mg/m3) | |||
| Median | 0·96 | 1·04 | 1·06 |
| 25th to 75th percentile | (0·70–1·21) | (0·76–1·34) | (0·82–1·27) |
| Respirable endotoxin (ng/m3) | |||
| Median | 32 | 35 | 27 |
| 25th to 75th percentile | (17–51) | (15–48) | (15–51) |
Cells in BALF
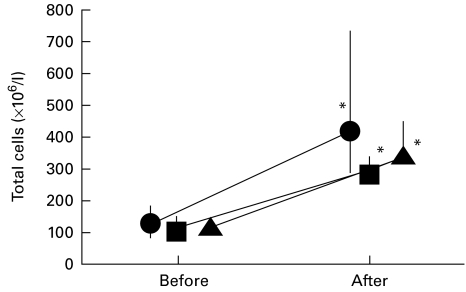
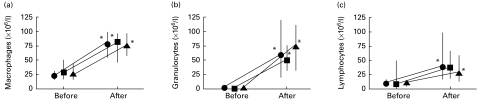
The total number of cells in BALF was 116 (83–148) × 106 cells/l before exposure and 324 (286–468) × 106 cells/l after exposure (P < 0·05), the increase being similar in all three groups (Fig. 1). There was no significant difference in the increase of the cellular concentration in BALF between the three groups. The amount of alveolar macrophages, granulocytes and lymphocytes present in the lavage was 67% (59–76%), 3·7% (2·7–5·5%) and 29% (21–37%), respectively, of the total cell count before exposure, and 46% (37–50%), 34%(28–39) and 20% (14–32%), respectively, of the total cell count after exposure. The granulocyte concentration increased about 50-fold in the placebo and salmeterol group (from 1·2 (0·8 to 1·6) and 1·4 (0·8–3·4) to 60 (19–121) and 75 (31–111) × 106 granulocytes/l, respectively; P < 0·05) and 30-fold in the fluticasone group (from 1·6 (1·0 to 5·0) to 49 (31–74) × 106 granulocytes/l, respectively; P < 0·05) (Fig. 2). The number of alveolar macrophages increased about three-fold in all groups (P < 0·05); in the placebo group the increase was from 23 (15 to 31) to 80 (55–100) × 106 macrophages/l. The number of lymphocytes increased four-fold in the placebo group (from 10 (6 to 16) to 40 (17–99) × 106 lymphocytes/l, respectively; P < 0·05), increased 3·4-fold in the fluticasone group (from 11 (5 to 49) to 36 (19–67) × 106 lymphocytes/l), and increased 2·5-fold in the salmeterol group (from 12 (5 to 15) to 30 (14–58) × 106 lymphocytes/l (P < 0·05). The increase in the number of alveolar macrophages, granulocytes and lymphocytes did not differ significantly between the three groups.
Fig. 1.
Total cell concentration × 106/l in bronchoalveolar lavage before medication and exposure and 24 h after exposure in the placebo (•), fluticasone (▪) and salmeterol (▴) groups. The median and 25th to 75th percentiles are shown. *P < 0·05 compared with pre-exposure values. The increase in cells did not significantly differ between the groups (n = 8 in each group).
Fig. 2.
Alveolar macrophage (a), granulocyte (b) and lymphocyte (c) content in bronchoalveolar lavage fluid before and after exposure to swine dust. Results are presented as median and 25th to 75th percentiles. *P < 0·05 compared with pre-exposure values. There were no significant differences between the groups (n = 8 in each group). •, Placebo; ▪, fluticasone; ▴, salmeterol.
Cytokines in BALF
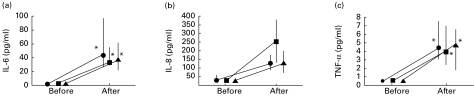
The concentration of IL-6, IL-8 and TNF-α in BALF was 1·8 (1·3–2·4), <25 (< 25–39) and <0·5 (< 0·5–0·5) pg/ml, respectively, before exposure (n = 24). There were no significant differences in the increases of cytokine concentration before, compared with after, exposure between the three groups (Fig. 3).
Fig. 3.
IL-6 (a), IL-8 (b) and tumour necrosis factor-alpha (TNF-α) (c) concentrations in bronchoalveolar lavage before medication and exposure and 24 h after exposure in the placebo (•), fluticasone (▪) and salmeterol (▴) groups. The median and 25th to 75th percentiles are shown. *P < 0·05 compared with pre-exposure values. The increase in IL-6, IL-8 and TNF-α did not significantly differ between the groups (n = 8 in each group).
Cultured alveolar macrophages
Cell viability before seeding onto plates was >89% in all 48 BAL samples, and the viability of the adherent cells after 24 h incubation was measured in 54 cases and was > 79%, except in two cases where it was slightly lower.
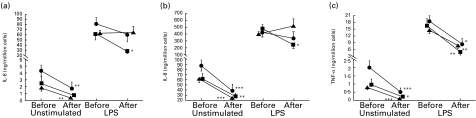
Basal production of IL-6, IL-8 and TNF-α by alveolar macrophages after 24 h in culture, from subjects before exposure in the swine house, are shown in Table 2. The basal (pre-exposure) production of TNF-α in macrophages was significantly higher in the placebo group compared with the fluticasone and salmeterol groups (P < 0·05; Fig. 4). Basal IL-6, IL-8 and TNF-α production was significantly lower after, than before, exposure in the placebo and salmeterol groups (P < 0·01 for all three cytokines). Similarly, in the fluticasone group, IL-8 and TNF-α production was lower after, than before, exposure (P < 0·01 and P < 0·05, respectively). There was no significant difference between the groups in basal cytokine production from macrophages obtained after dust exposure.
Table 2.
Unstimulated (24 h in RPMI medium) and stimulated (24 h in 100 μ g/ml lipopolysaccharide (LPS) in RPMI medium) cytokine release of alveolar macrophages from healthy individuals before exposure to swine dust
| Group | Incubation 24 h | IL-6 (ng/million cells) | IL-8 (ng/million cells) | TNF-α (ng/million cells) |
|---|---|---|---|---|
| Placebo | Unstimulated | 4·31 ± 0·81 | 87·2 ± 10·7 | 2·01 ± 0·41 |
| Fluticasone | Unstimulated | 2·46 ± 0·98 | 61·8 ± 10·5 | 0·97 ± 0·33 |
| Salmeterol | Unstimulated | 1·82 ± 0·38 | 61·2 ± 6·5 | 0·80 ± 0·15 |
| Placebo | LPS-stimulated | 77·9 ± 12·6 | 437 ± 66 | 18·4 ± 2·2 |
| Fluticasone | LPS-stimulated | 58·5 ± 12·1 | 491 ± 59 | 16·3 ± 3·1 |
| Salmeterol | LPS-stimulated | 59·7 ± 5·1 | 415 ± 33 | 14·6 ± 1·5 |
Results are presented as mean ±s.e.m.
Fig. 4.
Unstimulated (24 h in RPMI medium) and lipopolysaccharide (LPS; 100 μg/ml for 24 h)-induced IL-6 (a), IL-8 (b) and tumour necrosis factor-alpha (TNF-α) (c) release in cultured alveolar macrophages from 24 healthy subjects, before and after the participants had been exposed to swine dust. Prior to exposure, eight subjects inhaled fluticasone propionate (▪), eight inhaled salmeterol (▴) and eight were given a placebo (•) for 10–14 days. The cells from each subject (before and after medication and exposure) were in duplicates either unstimulated or LPS-stimulated. The results are thus based on 16 (8 × 2) observations in each group and are presented as mean ± s.e.m. *P < 0·05; **P < 0·01; ***P < 0·001 post-exposure values compared with values obtained before the start of medication. The difference between the groups did not reach statistical significance (F = 3·1; P = 0·057 for IL-6 release in LPS-stimulated cells after exposure; F = 4·3; P < 0·05 for TNF-α release in unstimulated cells before exposure, where placebo was higher than the other groups).
Cytokine levels in culture supernatants from 24 h LPS-treated alveolar macrophages before exposure are shown in Table 2. There was no significant difference between the groups. LPS-induced cytokine release, compared with unstimulated cells, increased significantly (P < 0·01) in all three groups before and after exposure (Fig. 4). LPS-induced TNF-α release from macrophages was lower after, than before, dust exposure in all groups (P < 0·05) and there was no significant difference between the groups. In the placebo and salmeterol groups, LPS-induced IL-6 and IL-8 release was not significantly different after, from before, dust exposure. LPS-induced IL-6 and IL-8 release in the fluticasone group was significantly reduced after exposure, compared with pre-exposure values (P < 0·05), but the difference between the groups did not reach significance (F = 3·1; P = 0·057 for IL-6, and F = 2·5; P = 0·089 for IL-8).
DISCUSSION
In this study we focused on the cell and cytokine responses in the lower airways to organic dust exposure following corticosteroid or ß2-agonist treatment in normal healthy individuals. The striking finding was that treatment had no significant influence on the large increase in the cell and cytokine content in BALF observed following dust exposure, and that treatment did not dramatically affect the cytokine response by cultured alveolar macrophages.
Regardless of the treatment, however, we have shown that unstimulated cultured alveolar macrophages from healthy subjects exposed to organic dust in a swine house had significantly reduced IL-6, IL-8 and TNF-α release after, compared with before, exposure. A similar down-regulation of alveolar macrophages to a ‘low heat’ status has been shown by others. Thus, Helleday et al. [8] found that alveolar macrophages from healthy individuals exposed to 3·5 p.p.m. NO2 for 20 min showed a tendency to reduced phagocytosis following exposure. It has also been demonstrated that unstimulated alveolar macrophages from asthmatic subjects, cultured for 24 h, produce significantly lower levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) and TNF-α than those from non-asthmatic individuals [9]. It has been suggested that a decreased activating state in alveolar macrophages may lead to an enhanced susceptibility to infections [10]. The clinical relevance of this finding is unclear. There are however epidemiological data supporting a high prevalence of chronic bronchitis (and presumably airway infections) in pig farmers [11].
Interestingly, cultured alveolar macrophages behaved differently when stimulated with LPS after, compared with before, dust exposure. The cells reacted with a decreased ability to produce TNF-α upon LPS stimulation, produced similar amounts of IL-8 and almost similar amounts of IL-6, compared with macrophages obtained from the same subjects prior to exposure. Maus et al. [12] found that LPS-stimulated alveolar macrophages in vitro from patients with severe community-acquired pneumonia released lower levels of TNF-α and IL-6, but not IL-8, compared with healthy individuals. In the presence of LPS however, the macrophages of asthmatic individuals seemed to produce significantly more GM-CSF, TNF-α and IL-8 than those from non-asthmatic subjects [9]. The explanation for the reduced TNF-α release in response to LPS may be endotoxin tolerance [13,14], since respirable swine dust contained endotoxin. Mengozzi et al. [15] found that a 4-h pre-exposure with LPS down-regulated TNF, but not IL-6 and IL-8, production from human monocytes. Our study shows that macrophages recovered from inflamed airways have a reduced basal release of IL-6, IL-8 and TNF-α and react normally to LPS stimulation with regard to IL-6 and IL-8, but have an impaired ability to release TNF-α, upon LPS stimulation.
The corticosteroid treatment had no effect on the large increase in cell and cytokine content in BALF induced by dust exposure. Furthermore, cultured alveolar macrophages were not affected with regard to basal cytokine production, although fluticasone slightly suppressed IL-6 and IL-8, but not TNF-α, release in response to LPS. These results are somewhat surprising, since steroids are known to influence these types of inflammatory responses in other contexts.
The cytokine content found in BALF may originate from many different cell types, but epithelial cells and alveolar macrophages are probably the major sources. We have previously shown that fluticasone propionate, at a concentration of 10−8 m, is a potent inhibitor of LPS-induced IL-6, IL-8 and TNF-α release from human alveolar macrophages and swine dust-induced IL-6 and IL-8 release from epithelial cells in vitro [6]. This concentration of the drug is very likely to be found in the lining fluid of the airway lumen during regular treatment with inhaled fluticasone. In the present study we could not find a similar potent inhibition of dust-induced cytokine release into the lower airways following in vivo administration of fluticasone, nor was there any effect on cytokine release from the cultured alveolar macrophages. A probable reason for this discrepancy between the in vivo and in vitro results is that fluticasone may not reach the alveolar space, and thereby the alveolar macrophages, in concentrations high enough to elicit the same effects as have been shown in vitro [16]. There is also the possibility that cells other than alveolar macrophages may have major importance in producing cytokines in the lower airways and that these cells may not be as steroid-sensitive as epithelial cells and alveolar macrophages have shown to be in vitro. It is known that patients with airway inflammation dominated by neutrophils do not respond as, for example, patients with inflammatory reactions dominated by eosinophils or lymphocytes to treatment with corticosteroids [17]. Steroid treatment stimulates neutrophil survival and has little effect in preventing neutrophil influx in various animal models [17]. An in vivo study by Trapp et al. [18] demonstrated only a mild protective effect of glucocorticoids on grain dust-induced airway inflammation.
There was no effect of salmeterol in influencing the massive cell increase or cytokine release observed after dust exposure, and there was also no salmeterol effect on cultured alveolar macrophages. The ex vivo results however showed a tendency towards enhanced IL-8 secretion upon LPS stimulation following salmeterol treatment. In agreement with this finding, other groups have shown that β-adrenoceptor stimulation in vitro increases the release of IL-8 in human transformed bronchial epithelial cells and in human monocytes [19,20]. This finding however was not confirmed by the in vivo results in the present study, where there was no tendency towards increased IL-8 levels in BALF in the salmeterol group. Hence our study shows that if salmeterol has an effect on IL-8 release, it is not of major importance in healthy individuals following organic dust exposure. The clinical relevance in the treatment of asthma needs to be further evaluated, however.
In conclusion, this study has shown that pretreatment with neither glucocorticoids nor ß2-agonists has any major effect on organic dust-induced lower airway inflammation. The increased cellular and cytokine content in BALF following dust exposure and alveolar macrophages cultured from premedicated and exposed individuals were almost unaffected by medication. A weak inhibition of LPS-induced IL-6 and IL-8, but not TNF-α, release from alveolar macrophages in culture was found, however. Regardless of medication, this study also shows that cultured alveolar macrophages have a reduced capability of releasing cytokines in the basal state after dust-induced airway inflammation.
Acknowledgments
This study was supported by Glaxo Wellcome, Sweden, the Swedish Council for Work Life Research (94-1383), and the Swedish Heart Lung Foundation. The authors would like to thank S. Siljerud and C. Müller-Suur for their skilful technical assistance.
REFERENCES
- 1.Larsson K, Eklund AG, Hansson L-O, Isaksson B-M, Malmberg PO. Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med. 1994;150:973–7. doi: 10.1164/ajrccm.150.4.7921472. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J. 1997;10:381–7. doi: 10.1183/09031936.97.10020381. [DOI] [PubMed] [Google Scholar]
- 3.Larsson B-M, Palmberg L, Malmberg PO, Larsson K. Effect of exposure to swine dust on levels of IL-8 in airway lavage fluid. Thorax. 1997;52:638–42. doi: 10.1136/thx.52.7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Malmberg P, Ek A, Larsson K, Palmberg L. Swine dust induces cytokine secretion from human epithelial cells and alveolar macrophages. Clin Exp Immunol. 1999;115:6–12. doi: 10.1046/j.1365-2249.1999.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmberg L, Larsson B-M, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax. 1998;53:260–4. doi: 10.1136/thx.53.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ek A, Larsson K, Siljerud S, Palmberg L. Fluticasone and budesonide inhibit cytokine release in human lung epithelial cells and alveolar macrophages. Allergy. 1999;54:691–9. doi: 10.1034/j.1398-9995.1999.00087.x. [DOI] [PubMed] [Google Scholar]
- 7.Larsson K, Tornling G, Gavhed D, Müller-Suur C, Palmberg L. Inhalation of cold air increases the number of inflammatory cells in the lungs in healthy subjects. Eur Respir J. 1998;12:825–30. doi: 10.1183/09031936.98.12040825. [DOI] [PubMed] [Google Scholar]
- 8.Helleday R, Sandström T, Stjernberg N. Differences in bronchoalveolar cell response to nitrogen dioxide exposure between smokers and nonsmokers. Eur Respir J. 1994;7:1213–20. doi: 10.1183/09031936.94.07071213. [DOI] [PubMed] [Google Scholar]
- 9.Hallsworth MP, Soh CPC, Lane SJ, Arm JP, Lee TH. Selective enhancement of GM-CSF, TNF-alpha, IL-1ß and IL-8 production by monocytes and macrophages of asthmatic subjects. Eur Respir J. 1994;7:1096–102. [PubMed] [Google Scholar]
- 10.Lohmann-Matthes M-L, Steinmüller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J. 1994;7:1678–89. [PubMed] [Google Scholar]
- 11.Iversen M, Dahl R, Korsgaard J, Hallas T, Jensen EJ. Respiratory symptoms in Danish farmers: an epidemiological study of risk factors. Thorax. 1988;43:872–7. doi: 10.1136/thx.43.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maus U, Rosseau S, Knies U, Seeger W, Lohmeyer J. Expression of pro-inflammatory cytokines by flow-sorted alveolar macrophages in severe pneumonia. Eur Respir J. 1998;11:534–41. [PubMed] [Google Scholar]
- 13.Cavaillon JM. The nonspecific nature of endotoxin tolerance. Trends Microbiol. 1995;3:320–4. doi: 10.1016/s0966-842x(00)88963-5. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler-Heitbrock HWL. Molecular mechanism in tolerance to lipopolysaccharide. J Inflamm. 1995;45:13–26. [PubMed] [Google Scholar]
- 15.Mengozzi M, Fantuzzi G, Sironi M, Bianchi M, Fratelli M, Peri G, Bernasconi S, Ghezzi P. Early down-regulation of TNF production by LPS tolerance in human monocytes: comparison with IL-1ß, IL-6, and IL-8. Lymphokine Cytokine Res. 1993;12:231–6. [PubMed] [Google Scholar]
- 16.Reynolds HY. Bronchoalveolar lavage. Am Rev Respir Dis. 1987;135:250–63. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- 17.Cox G. The role of neutrophils in inflammation. Can Respir J. 1998;5:37A–40A. [PubMed] [Google Scholar]
- 18.Trapp JF, Watt JL, Frees KL, Quinn TJ, Nonnenmann MW, Schwartz DA. The effect of glucocorticoids on grain dust-induced airway disease. Chest. 1998;113:505–13. doi: 10.1378/chest.113.2.505. [DOI] [PubMed] [Google Scholar]
- 19.Lindén A. Increased interleukin-8 release by β-adrenoceptor activation in human transformed bronchial epithelial cells. Br J Pharmacol. 1996;119:402–6. doi: 10.1111/j.1476-5381.1996.tb16000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavelaars A, Pol Mvd Zijlstra J, Heijnen CJ. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J Neuroimmunol. 1997;77:211–6. doi: 10.1016/s0165-5728(97)00076-3. [DOI] [PubMed] [Google Scholar]