Abstract
The level of the terminal complement components secreted by human umbilical vein endothelial cells (HUVEC) was measured by a sensitive ELISA which allows the detection of 30–50 pg/ml of these components. C7 was the only terminal component detected in measurable amounts in the cell supernatant. The mean value was 11 ng/106 cells at 96 h and was slightly higher than that of C3 (9 ng/106 cells). HUVEC and serum C7 analysed by SDS–PAGE and immunoblot exhibited the same electrophoretic mobility. A proportion of C7 secreted by HUVEC was incorporated into the terminal complement complex (TCC) assembled spontaneously in the supernatant of cells cultured in C7-deficient human serum, and was not detected by the standard ELISA for C7 measurement. By adding the amount of C7 present in the TCC to that of free C7, the total amount of the component released by HUVEC was calculated to be approximately 35 ng/106 cells. Further TCC was produced following complement activation of the cell supernatant through the alternative pathway. Synthesis of C7 by HUVEC was confirmed by inhibition experiments in the presence of cycloheximide and by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of C7 mRNA expression. Addition of IL-1α and tumour necrosis factor-alpha to the cell culture stimulated the secretion of C3, but had no effect on the synthesis of C7. By contrast, interferon-gamma had only a marginal effect on the production of C3, but markedly down-regulated the synthesis of C7 as assessed both by ELISA and RT-PCR.
Keywords: C7, endothelial cells, cytokines
INTRODUCTION
The complement (C) system is an important humoral constituent of innate immunity and includes more than 25 glycoproteins between C components and regulators. The majority of the C proteins circulating in blood are produced by the liver, which should therefore be regarded as the primary site of their biosynthesis [1]. In addition, many other tissues and cell types have been shown to synthesize and secrete C components and regulators at extravascular sites where they contribute to induce local inflammatory reaction [2,3].
Endothelial cells represent one of the extrahepatic sources of C components and regulators, and, because of their strategic position along the surface of the vessel wall, they may supply both the circulating blood and the extravascular fluids with these proteins. In vitro studies have provided evidence indicating that endothelial cells in culture synthesize C1s and C1-inhibitor [4], C3 and factor B as well as factors H and I [5–9]. Synthesis of C components and regulators by endothelial cells can be modulated by various cytokines, including interferon-gamma (IFN-γ), which up-regulates the production of factors H and I [9], and IL-1, which stimulates that of C3 and factor B [10].
We have previously shown that endothelial cells can also secrete the terminal components using a model system of cell co-culture with agarose beads to bind the secreted components, which were then revealed by radiolabelled polyclonal antibodies [11]. C7 was the only late component that was not detected in this study, though the assembly of the terminal complement complex (TCC) on the beads was proven by its reaction with a MoAb, which recognizes the neoantigen of C9 exposed in the complex but not in the native molecule [12]. In subsequent studies we found that IFN-γ strongly reduces the formation of TCC when added to endothelial cells co-cultured with agarose beads [13].
The intriguing observation that TCC could be detected on agarose beads despite our inability to measure C7 led us to re-evaluate the production of the terminal C components by endothelial cells under basal conditions and after stimulation with cytokines. We focused our attention particularly on the production of C7 by endothelial cells, since analysis of C7 allotype conversion in liver transplant recipients clearly indicate that a substantial amount of plasma C7 is synthesized at extrahepatic sites [14]. Similar studies performed in bone marrow transplant recipients suggest that the bone marrow is one of these sites and contributes approximately 20% to the total circulating C7 level [15]. Circulating leucocytes may be an additional source of C7 present in plasma. Monocytes are known to synthesize C7, though they are unlikely to contribute significantly to the systemic level of C7 due to their limited numbers in blood [16]. Polymorphonuclear leucocytes may be a better source of C7 since they store large amounts of this component, which is released following cell degranulation [17]. However, this process is more likely to occur at extravascular sites, where the local level of C7 may increase during an inflammatory process, rather than in the circulation. Endothelial cells may be a better candidate as a cellular source of circulating C7 since they cover a large surface area.
In this study we have measured the amount of the late C components secreted by endothelial cells by ELISA and analysed their mRNA expression by reverse transcriptase-polymerase chain reaction (RT-PCR). Data will be presented indicating that these cells actively synthesize C7 and, moreover, that IFN-γ markedly down-regulates the secretion of C7.
MATERIALS AND METHODS
Reagents and C components
Human recombinant IL-1α (Boehringer Mannheim GmbH, Mannheim, Germany), tumour necrosis factor-alpha (TNF-α; Saxon Biochemicals GmbH, Hannover, Germany) and IFN-γ (Genentech, San Francisco, CA) were used at concentrations of 10 U/ml, 100 ng/ml and 100 U/ml, respectively. Cycloheximide (CHX) was purchased from Sigma-Aldrich S.r.l. (Milan, Italy) and used at a concentration of 1 μ g/ml to inhibit protein synthesis. Human purified C3 and all the terminal C components from C5 to C9 were from Quidel (San Diego, CA). Complement C7-deficient human serum was obtained from a patient with a history of meningococcal disease and was identified in the laboratory of one of the authors (F.T.) because of an undetectable haemolytic activity of the serum, which was selectively reconstituted by the addition of purified C7, and a markedly low immunochemical level of C7 (40 ng/ml) as opposed to 50 μ g/ml of C7 in pooled human sera. This patient was also found to have half-normal levels of C6. Endotoxin-free gelatin and medium 199, heparin, penicillin, streptomycin and fungizone were all purchased from Sigma-Aldrich.
Antisera
Goat antisera to human C3 and each of the terminal components were obtained from Quidel, except for goat antiserum to C8 which was purchased from Atlantic Antibodies (Windham, ME). The IgG were purified by affinity chromatography on protein G-Sepharose column (HiTrap™ Protein G column; Pharmacia Biotech, Milan, Italy) according to the instructions of the manufacturer. The purified IgG were labelled with biotin using biotinamidocaproate n-hydroxysuccinimide ester (Sigma-Aldrich) as the labelling reagent according to a previously described procedure [18]. This includes overnight dialysis of the IgG fraction against 0·1 m sodium bicarbonate buffer pH 8·8 and incubation with biotin at a protein–biotin ratio of 10:1 (w/w) for 15 min at room temperature, addition of 10 μ l of 1 m ammonium chloride (pH 6·5) to block the biotin–protein reaction and final dialysis against PBS to remove free biotin. The murine MoAb aE11 recognizing a neoantigen expressed in poly(C9) was a kind gift of Professor T. Lea (Oslo, Norway) [12].
Endothelial cell cultures
Endothelial cells were isolated from umbilical cord veins (HUVEC) by collagenase treatment (Sigma-Aldrich) according to the method of Jaffe et al. [19] and cultured in tissue culture flasks (Nunc, Mascia Brunelli, Milan, Italy) coated with 2% endotoxin-free gelatin (Sigma-Aldrich). The cells were maintained in endotoxin-free medium 199 supplemented with endothelial cell growth factor isolated from bovine hypothalamus (50 μ g/ml), heparin (50 μ g/ml), penicillin (50 U/ml), streptomycin (50 μ g/ml), fungizone (2·5 μ g/ml) and 20% newborn bovine serum (NBS; Hyclone Labs Inc., Logan, UT). This was used as a control medium throughout the study. For some experiments NBS was replaced by 15% C7-deficient human serum. After reaching confluence, the cells were subcultured in 25-cm2 flasks using trypsin-EDTA (Sigma-Aldrich) and used at their first passage and occasionally at the second passage. The experiments were performed on a monolayer of cells kept in 5 ml of medium containing serum and one-fifth of the supernatant was collected daily and replaced by fresh medium. The number of endothelial cells was counted using a Coulter counter (Coulter Electronic Ltd, Luton, UK) after detaching the adherent cells with trypsin-EDTA.
Quantification of secreted C components by ELISA
The amount of C components released in the supernatant of the endothelial cell culture was tested by a previously reported immunoenzymatic assay [20]. Briefly, the wells of 96-well ELISA plates (Corning Costar, Acton, MA) were coated with an optimal concentration of the IgG fraction of the various antibodies by overnight incubation in 0·1 m sodium bicarbonate buffer pH 9·6 at 4°C. After washing the wells with PBS containing 0·1% Tween 20, the residual free sites were blocked with PBS containing 1% bovine serum albumin (BSA) for 1 h at 37°C. The antibodies were then allowed to react with the cell supernatant overnight at 4°C. Binding of the C components was revealed by their reaction with optimal dilutions of biotin-labelled specific antibodies for 1 h at 37°C followed by 30 min incubation at 37°C with 1/4000 streptavidin-alkaline phosphatase (Sigma-Aldrich). The enzymatic reaction was developed using p-nitrophenyl phosphate (Sigma-Aldrich; 1 mg/ml) as a substrate in 0·1 m glycine buffer pH 10·4 containing 1 mm MgCl2 and 1 mm ZnCl2 and read kinetically at 405 nm using a Titertek Multiskan ELISA reader (Flow Labs, Milan, Italy). Calibration curves were set up with increasing concentrations of the purified C components obtained from Quidel.
Immunoaffinity chromatography
Anti-C7 immunoaffinity column was prepared by coupling goat IgG anti-C7 with cyanogen bromide (CNBr)-Sepharose 4B (Sigma-Aldrich), which had previously been washed three times with 1 mm HCl, by overnight incubation at 4°C on a rotator. After blocking the residual active site with 0·1 m Tris–HCl pH 8·3, the Sepharose beads were washed and finally equilibrated in 10 mm Tris–HCl buffer pH 7·4 containing 0·14 m NaCl. Approximately 120 ml of HUVEC supernatant were passed through a 1-ml column at a flow rate of 10 ml/h and the bound C7 was eluted with 4 m guanidine–HCl (Sigma-Aldrich) in 0·1 m sodium phosphate pH 7·4 containing 2 m NaCl.
SDS–PAGE and immunoblotting
The HUVEC supernatant was subjected to SDS–PAGE on a 10% gel under non-reducing conditions according to Laemmli [21] followed by electrophoretic transfer onto nitrocellulose membrane (Hybond ECL; Amersham Italia, Srl, Milan, Italy) using the semidry Semiphor transfer unit (Heifer Scientific Instruments, San Francisco, CA). After soaking the nitrocellulose sheet in 50 mm Tris–HCl pH 7·6 containing 0·5 m NaCl and 4% skimmed milk for 1 h at 37°C to block the free binding sites, the C7 band was revealed by incubation with 1/1000 biotin-labelled goat IgG anti-C7 for 1 h at 37°C followed by 1/4000 alkaline phosphatase conjugated to streptavidin (Sigma-Aldrich) for 30 min at 37°C. The enzymatic reaction was developed using nitroblue tetrazolium (0·60 mg/ml) and 5-bromo-4-chloro-3-indolyl phosphate (0·30 mg/ml), both purchased from Sigma-Aldrich and diluted in 0·1 m Tris–HCl pH 9·5 containing 0·1 m NaCl and 5 mm MgCl2. Rainbow RPN 756 (Amersham Italia) was used as a mixture of defined molecular markers.
Functional activity of C7
The activity of C7 was evaluated by the incorporation of C7 into TCC, which was measured by ELISA following a previously described procedure [22] with slight modifications. Briefly, solid-phase bound MoAb aE11 was used at a dilution of 1/5000 to bind the complex for 1 h at 37°C and, after washing, the bound TCC was evaluated by its reaction with 1/1000 biotin-labelled goat IgG anti-C5 for 1 h at 37°C followed by 30 min incubation at 37°C with 1/4000 alkaline phosphatase conjugated to streptavidin (Sigma-Aldrich). After addition of p-nitrophenyl phosphate (1 mg/ml) in 0·1 m glycin buffer pH 10·4 containing 0·1 mm MgCl2 and 0·1 mm ZnCl2, the enzymatic reaction was developed at 37°C and read kinetically at 405 nm. Assembly of TCC was also induced by incubating for 60 min at 37°C a mixture of HUVEC supernatant (200 μ l) and C7-deficient serum (50 μ l) with a suspension of yeast cell wall (12 μ l) prepared and titrated as described by Harrison & Lachmann [23]. Similar experimental conditions were followed to set up a standard curve with increasing concentrations of purified C7 instead of HUVEC supernatant.
Reverse transcription and PCR
Total RNA was extracted from resting and activated HUVEC with a commercially available solution (RNAzol B, Tel-Test Inc. Friendswood, TX). Reverse transcription was performed with M-MLV Reverse Transcriptase (Gibco-BRL, Milan, Italy) according to the instructions using 1 μ g of RNA and random hexanucleotide primers (Boehringer Mannheim). PCR was carried out with 2 μ l of cDNA, 0·2 mm dNTP, 1·4 U Taq DNA polymerase (Finnzymes OY, Espoo, Finland) and 0·5 μm specific 20mer oligonucleotide primers (Pharmacia-Biotech) in a MJ Research PTC-100 thermal cycler (Watertown, MA) (30 cycles). The amplified products were run through a 1·5% agarose gel containing ethidium bromide for UV detection. Controls for specificity included treatment of the samples with Taq DNA polymerase to check for the presence of DNA and, in the case of positive results, treatment with DNase and also sequencing of the amplified product. The EMBL accession numbers, the amplified sequences and the expected size of the amplified products were as follows: C3: K02765, 4216–4769, 554 bp [24]; C7: J03507, 2761–3330, 570 bp; β-actin: M10278, 311–838, 528 bp [25].
Statistical analysis
Data are reported as mean ± s.d. Student's t-test was used to compare two groups of data.
RESULTS
Immunochemical level of the terminal C components secreted by HUVEC
The amount of the late C components released by HUVEC into the culture supernatant was evaluated using cells grown at near confluence in medium supplemented with 20% NBS. Under these conditions the cells survive reasonably well in culture for several days provided that the medium is partly replaced every day by fresh medium. The presence of NBS in the cell supernatant did not interfere with the assay of the C components since the serum neither increased the absorption value obtained with serum-free medium, nor changed the standard curves established with the purified C components (data not shown).
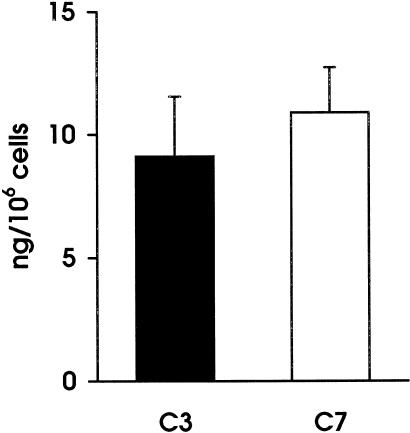
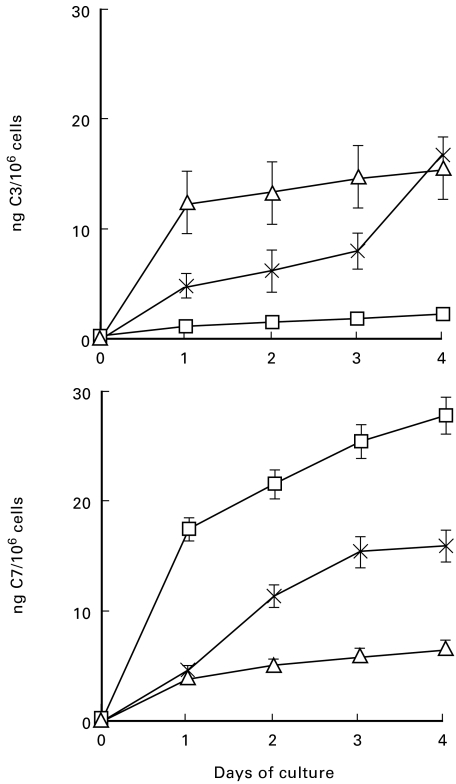
Immunochemical analysis of the late C components in the culture supernatant revealed a preferential secretion of C7 by unstimulated HUVEC. The level of this component in the spent medium reached an average value of 11 ng/106 at 96 h and was slightly higher than that of C3, which was 9 ng/106 cells (Fig. 1) All the other late components were undetectable even when tested in the supernatant of 4 day cultures, except for a marginal increase of C9 just above background level. The secretion of C3 and C7 was followed kinetically on samples of supernatants harvested daily and partly replaced by fresh medium. As shown in Fig. 2, both these components were already detected at 24 h in measurable amounts, which varied in different cell cultures, but progressively increased with time up to 4 days. It is interesting to note that, despite the fact that the secretion of C3 and C7 by each batch of cell culture exhibited a similar kinetic pattern, the absolute amount of the two components produced by the same cells was sometimes different. Both C3 and C7 were undetectable in samples of supernatant collected 60 min after the start of the cell culture, thus excluding that these components may have been passively acquired from plasma and subsequently released from the cell surface. Moreover, addition of CHX to the cell culture markedly inhibited the secretion of C3 and C7, indicating that they were both synthetic products of endothelial cells (Fig. 3).
Fig. 1.
Secretion of C3 and C7 by unstimulated human umbilical vein endothelial cells (HUVEC) at 96 h. The cells were used at their first passage and grown in medium containing 20% newborn bovine serum (NBS). The experiments were performed on nearly confluent cell culture and the amount of secreted C3 and C7 was evaluated by ELISA. Values are the mean ± s.d. for duplicate determinations of six different cultures.
Fig. 2.
Kinetic of secretion of C3 and C7 by human umbilical vein endothelial cells (HUVEC). The cells obtained from three individuals indicated with different symbols were cultured in medium containing newborn bovine serum (NBS) for 4 days. Samples of supernatants were collected every day and replaced by fresh medium. The level of secreted C3 and C7 was measured by ELISA. The data represent the mean values ± s.d. for triplicate determinations of each cell culture.
Fig. 3.
Effect of cycloheximide (CHX) on the secretion of C3 and C7 by human umbilical vein endothelial cells (HUVEC). The cells were cultured in medium supplemented with 20% newborn bovine serum (NBS) with (· · ·· ·) or without (———) CHX (1 mg/ml) and the level of the two C components was evaluated on samples of supernatant harvested every day. Values are the mean ± s.d. for triplicate determinations of two experiments.
Molecular analysis and functional activity of secreted C7
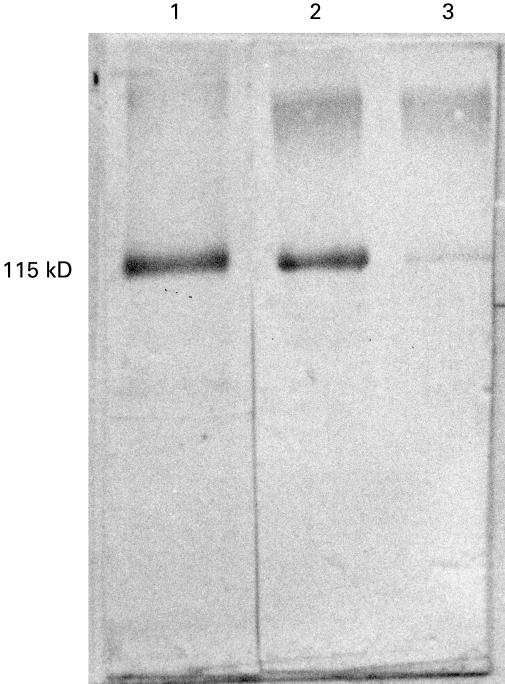
To characterize further the C7 produced by HUVEC, the cells were grown for 4 days in medium supplemented with NBS and the supernatant was purified through an anti-C7 immunoaffinity column. The bound proteins were eluted with guanidinium chloride, concentrated by lyophilization after extensive dialysis and analysed by SDS–PAGE and Western blot (Fig. 4). A distinct band of approximately 115 kD with an electrophoretic mobility corresponding to that of serum C7 was readily identified in the lane loaded with the HUVEC eluate. A very faint band was observed in the lane containing C7-deficient human serum due to the presence of a residual amount of C7 in this serum.
Fig. 4.
Immunoblot analysis of C7 secreted by human umbilical vein endothelial cells (HUVEC). C7 (20 ng) purified from cell supernatant by affinity chromatography (lane 1), 0·2 μ l of normal human serum (lane 2) and 0·2 μ l of C7-deficient human serum (lane 3) were subjected to SDS–PAGE on a 10% gel and blotted to nitrocellulose membrane. The immunoblot was then developed with biotin-labelled anti-C7 antibodies followed by streptavidin-alkaline phosphatase. The mol. wt of the C7 band is indicated with reference to the relative mobility of known molecular weight markers.
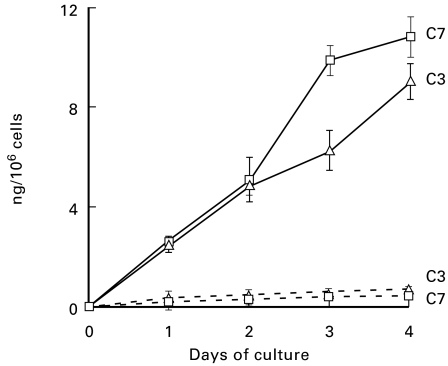
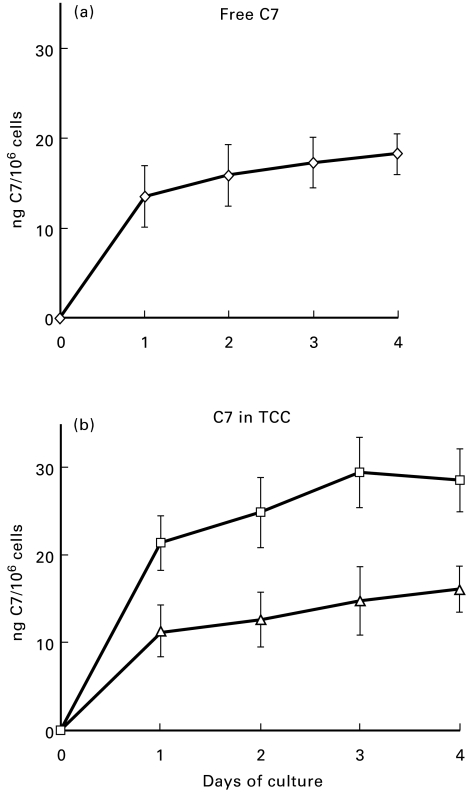
We next evaluated the incorporation of HUVEC C7 into TCC as a marker of the functional activity of this late component. The assembly of the complex was ascertained by its reaction with MoAb aE11, which recognizes the C9 neoantigen expressed only in TCC. For these experiments, HUVEC were maintained in medium containing 15% C7-deficient human serum since aE11 only reacts with human TCC and does not recognize the complex formed in NBS. Samples of the culture supernatant were collected daily for 4 consecutive days and the level of C7 was measured by ELISA. As shown in Fig. 5, the secretion of C7 was not affected by the addition of C7-deficient human serum to the culture medium and was comparable to that produced by the cells kept in NBS. Analysis of the supernatant for spontaneous formation of TCC revealed that this complex was already present in the 24 h supernatant in detectable amounts that increased in the samples collected in the next days of culture (Fig. 5). Not all the C7 produced by HUVEC was incorporated into the spontaneously assembled TCC, since more complexes were formed upon stimulation of the alternative pathway with yeast cell walls (Fig. 5).
Fig. 5.
Measurement of C7 in the human umbilical vein endothelial cell (HUVEC) supernatant as free molecule or as a component of terminal complement complex (TCC). The cells were cultured in 15% C7-deficient human serum and samples were harvested daily for the assessment of the level of C7 (a) and of TCC (b). The lower curve of (b) (Δ) represents the TCC assembled spontaneously in the medium containing C7-deficient serum. The upper curve of (b) (□) represents the TCC formed following yeast cell wall treatment of medium containing further 20% C7-deficient serum to a final concentration of 35%. The results of TCC levels are expressed as equivalent amount of C7 used to set up a standard curve of TCC formed in the activated mixture of C7-deficient serum and increasing amounts of purified C7. Values are the mean ± s.d. for triplicate determinations of three cell cultures.
Regulation of C7 biosynthesis by cytokines
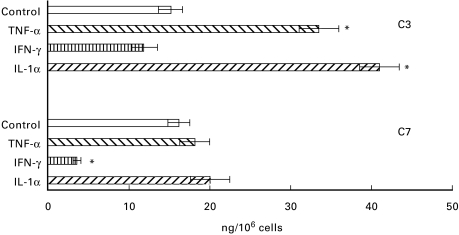
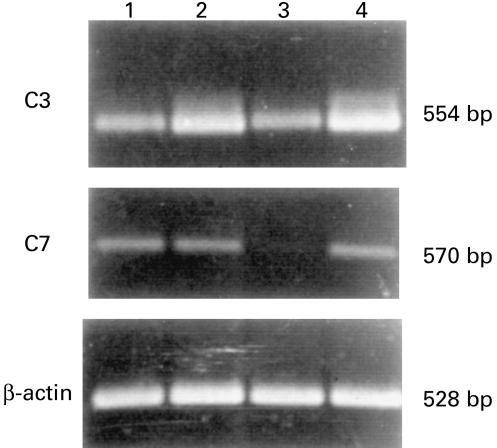
HUVEC were cultured in the presence of IL-1α (10 U/ml), TNF-α (100 ng/ml) and IFN-γ (100 U/ml) or in control medium for 96 h. Samples of supernatant were harvested daily to measure the levels of C3 and C7, whereas the cells were analysed for the presence of C3 and C7 mRNA transcripts by RT-PCR. As shown in Fig. 6, the secretion of C3 and C7 by unstimulated cells was approximately 15 ng/106 cells for both C components. The addition of IL-1α and TNF-α to HUVEC induced a two-fold increase in the production of C3, but had no effect on the secretion of C7. By contrast, IFN-γ had only a negligible negative effect on the secretion of C3 while markedly and significantly reducing that of C7. These results were essentially confirmed by RT-PCR analysis (Fig. 7).
Fig. 6.
Effect of cytokines on the secretion of C3 and C7 by human umbilical vein endothelial cells (HUVEC). The cells at confluence were incubated with IL-1α (10 U/ml), tumour necrosis factor-alpha (TNF-α) (100 ng/ml), IFN-γ (100 U/ml) or control medium for 4 days and the concentration of C3 and C7 in the supernatant was measured by ELISA. Values are mean ± s.d. ofr triplicate determinations of two different experiments. *P < 0·05 versus control.
Fig. 7.
Reverse transcriptase-polymerase chain reaction analysis of mRNA expression of C3 and C7 of human umbilical vein endothelial cells (HUVEC) unstimulated (1) or stimulated by tumour necrosis factor-alpha (2), IFN-γ (3), IL-1α (4) for 48 h.
DISCUSSION
Endothelial cells synthesize several active products involved in important biological processes, such as inflammation, coagulation and regulation of the vascular tone [26]. C components of both classical and alternative pathways as well as C regulatory proteins are among the secretory proteins of endothelial cells [4–11]. The results of the present study indicate that HUVEC actively synthesize C7, which is the terminal C component preferentially secreted by these cells. This conclusion is supported by the ELISA data obtained with a polyclonal anti-C7 antiserum that reacts specifically with purified C7. The finding that the secretion of C7 was completely inhibited by CHX added to the cell culture proves that this C component was de novo synthesized by the endothelial cells and not just absorbed from plasma and released thereafter.
The observation that endothelial cells secrete measurable amounts of C7 was unexpected, since Johnson & Hetland in a previous study [11] were unable to detect this C component in the culture supernatant of endothelial cells. The technical approaches followed in these two studies however are quite different, and this may explain, at least in part, the discordant results obtained. Thus, Johnson & Hetland co-cultured HUVEC with agarose beads and measured the C7 present in the TCC bound to the beads during the culture period. To avoid the risk that the anti-C7 antibodies may not recognize the C7 hidden in the complex, we tested the cell supernatant for the presence of free C7 molecules by ELISA. The only terminal C component that could accurately be measured in the culture medium was C7, despite the high sensitivity of the assay system that allows the detection of as little as 30–50 pg/ml of all these C components. A small amount of C9 was secreted by HUVEC, but the value obtained was just above background level and could not be precisely quantified. It is possible that the assay system employed by Johnson & Hetland allows the detection of a small number of molecules bound to agarose beads and this number is below the limit of sensitivity of our enzymatic assay.
HUVEC C7 examined by SDS–PAGE and Western blot exhibited molecular characteristics similar to those of serum C7, since they both migrated as distinct bands with the same electrophoretic mobility. Moreover, C7 secreted by endothelial cells was functionally active, as indicated by its ability to be incorporated into TCC. This was shown by cultivating HUVEC in C7-deficient human serum and measuring the TCC assembled in the supernatant once C7 became available. The level of the terminal complex formed under these conditions was related to the amount of C7 secreted by the cells and could easily be calculated from a standard curve established by activating mixtures of C7-deficient human serum and increasing concentrations of purified C7 with yeast cell wall. Surprisingly, a substantial amount of TCC was already detected in the supernatant of cells grown in C7-deficient human serum without the need of prior C activation, suggesting that TCC was spontaneously assembled in our culture conditions. One possible explanation for this observation is that the terminal complex was formed as a result of C activation through the alternative pathway by the extracellular matrix, as recently reported by Hindmarsh & Marks [27]. Exposure of matrix in our culture conditions cannot be excluded, since the experiments of C biosynthesis were usually performed using cells that had reached a near confluent state. Alternatively, the assembly of TCC in C7-deficient serum may have been initiated by the low amount of C5b6 detected in this serum. It is well known that the bimolecular complex C5b6 is functionally stable [28] and is capable of reacting with newly synthesized C7 and the remaining terminal components provided by the C7-deficient serum. Although C activation by the extracellular matrix and C5b6 present in the deficient serum may both account for the spontaneous formation of the terminal complex in the cell culture, C5b6 is more probably the main initiating factor since not all the C7 secreted by HUVEC was incorporated into TCC. The finding that C activation of the cell supernatant with yeast cell wall promoted further assembly of TCC containing additional C7 molecules suggests that the low level of C5b6 in C7-deficient serum was the limiting factor involved in the spontaneous formation of TCC. Platonov et al. [29] reported a similar low haemolytic activity of C5b6 in four C7-deficient patients and considered the C5b6 complex responsible for the formation of measurable amounts of TCC in their sera. One of these patients, like our own patient, exhibited low amounts of C7, which in their case was attributed to the combined effect of low levels of C5b6 and the homozygous defect of C6 characterized by mutation at exon 15/intron 15 boundary [30,31].
The amount of C7 measured in the cell supernatant by ELISA was slightly higher than that of C3 and the latter was within the range observed by other groups [10,32]. The levels of both C7 and C3 would probably have been higher if, instead of using endothelial cells obtained from umbilical cord veins, which may not be a good producer of these C components, we had worked with endothelial cells isolated from other sources. Thus, the endothelial cells isolated from human skin seem to secrete a higher amount of C3 than HUVEC [5]. The level of C7 evaluated by ELISA in the culture medium does not reflect the true amount of the component secreted by HUVEC, since the immunoenzymatic assay only measures the concentration of the free molecules and does not detect the C7 incorporated into TCC. The failure of goat IgG anti-C7 to detect C7 in the complex using the standard assay to measure C7 suggests that this C component is not available for reaction with the biotin-labelled IgG anti-C7 when the TCC is trapped by the same polyclonal antibodies. In evaluating the total amount of C7 produced by HUVEC, one should therefore take into account not only the level of the free molecules, but also the amount of C7 present in TCC. Consequently, the true concentration of C7 in the cell supernatant can be calculated to be around 35 ng/106 cells. Assuming that the amount of C3 secreted by HUVEC cultivated in C7-deficient serum does not differ from that produced by the same cells grown in NBS, as is the case for C7, we conclude that the endothelial cells synthesize more C7 than C3. Thus, the low C7/C3 ratio of 1/20 in the circulation, which reflects the higher synthetic rate of C3 by the liver cells, is inverted in the supernatant of endothelial cell culture and becomes 2/1. The amount of 35 ng/106 cells may seem scant at first sight, but we feel that it is biologically relevant in view of the large surface area of several square metres covered by the endothelium in the vascular bed with a total number of approximately 1–6 × 1013 cells [33]. This makes the endothelial cells an additional source of plasma C7, since previous studies have clearly established that a substantial part of this C component is synthesized extrahepatically [14,15].
By contributing to the circulating pool of C7, the endothelial cells participate in the host defence whenever the terminal complex is required to destroy the infectious agents circulating in the blood. The assembly of the membrane attack complex of the C system on the target cells requires binding of C7 to C5b6 to form C5b-7. This stabilized complex establishes firm interaction with the phospholipid bilayer and binds C8 and C9. An additional and probably more important role of C7 produced by the endothelial cells is to control the cytolytic effect of TCC formed in the proximity and capable of damaging these cells. This is made possible by the rapid decay of the binding site for the membrane phospholipids transiently exposed on the C7 of circulating C5b-7, though this complex is still able to interact with C8 and C9. In addition, the C5b-7 complex assembled at some distance from the cell surface has very little chance to reach the endothelial cells while still in an active state because C7 of the complex quickly reacts with the serum inhibitor vitronectin or S protein [34]. Loss of cytolytic activity however does not prevent binding of the inactive complex to endothelial cells, which can be stimulated to express adhesion and procoagulant molecules [22]. It is therefore conceivable that endothelial C7 may protect these cells from the destructive effect of the cytolytic complex and permits binding of the inactive complex that promotes more defensive functions, such as inflammation and coagulation.
The cytokines IL-1α and TNF-α, known to up-regulate the synthesis of C3 by endothelial cells [10,32,35], are unable to modulate the production of C7, supporting the conclusion that C7 is not an acute-phase protein [36]. On the other hand, IFN-γ down-regulates the secretion of C7, leaving unaltered the level of C3. This effect of IFN-γ on C7 contrasts with the stimulating activity of IFN-γ on the production of H and B by endothelial cells [7,9]. The present data however confirm previous observations by Berge et al. [13], who have shown that IFN-γ down-regulates the production of the late C components. These authors suggested that IFN-γ may control the damaging effect deriving from an excessive activation of the terminal pathway.
In conclusion, we have documented a selective synthesis of C7 among the terminal C components by endothelial cells. The secretion of this C component is higher than that of C3 and is not up-regulated by IL-1α and TNF-α, but is markedly reduced by the addition of IFN-γ.
Acknowledgments
The Authors wish to thank Paolo Macor for his excellent technical assistance and Professor T. Lea (Institute of Immunology and Rheumatology, University of Oslo, Norway) for kindly providing the MoAb aE11. This work was supported by grants received from the Italian Ministry of University and Scientific Research and Italian CNR (target project on Biotechnology No. 99.00496.PF49), and by the Eu-Biomed 2 grant (BMH4-CT96-1005).
REFERENCES
- 1.Morris KM, Aden DP, Knowles BB, et al. Complement biosynthesis by the human hepatoma derived cell line HepG2. J Clin Invest. 1982;70:906–13. doi: 10.1172/JCI110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colten HR, Garnier G. Regulation of complement protein gene expression. In: Volanakis JE, Frank MM, editors. The human complement system in health and disease. New York: Marcel Dekker; 1998. pp. 217–40. [Google Scholar]
- 3.Johnson E, Hetland G. Mononuclear phagocytes have the potential to synthesize the complete functional complement system. Scand J Immunol. 1988;27:489–93. doi: 10.1111/j.1365-3083.1988.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 4.Gulati P, Lemercier C, Guc D, et al. Regulation of the C1 subcomponents and C1-inhibitor. Behring Inst Mitt. 1993;93:193–203. [PubMed] [Google Scholar]
- 5.Ueki A, Sai T, Oka H, et al. Biosynthesis and secretion of the third component of complement by human endothelial cells in vitro. Immunology. 1987;61:11–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Warren HB, Pantazis P, Davies PF. The third component of complement is transcribed and secreted by cultured human endothelial cells. Am J Pathol. 1987;129:9–13. [PMC free article] [PubMed] [Google Scholar]
- 7.Brooimans RA, Hiemstra PS, van der Ark AAJ, et al. Biosynthesis of complement factor H by human umbilical endothelial cells. Regulation by T cell growth factor and IFN-γ. J Immunol. 1989;142:2024–30. [PubMed] [Google Scholar]
- 8.Julen N, Dauchel H, Lemercier C, et al. In vitro biosynthesis of complement factor I by human endothelial cells. Eur J Immunol. 1992;22:213–7. doi: 10.1002/eji.1830220131. [DOI] [PubMed] [Google Scholar]
- 9.Ripoche J, Mitchell JA, Erdei A, et al. Interferon γ induces synthesis of complement alternative pathway proteins by human endothelial cells in culture. J Exp Med. 1988;168:1917–22. doi: 10.1084/jem.168.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dauchel H, Julen N, Lemercier C, et al. Expression of complement alternative pathway proteins by endothelial cells. Differential regulation by interleukin 1 and glucocorticoids. Eur J Immunol. 1990;20:1669–75. doi: 10.1002/eji.1830200808. [DOI] [PubMed] [Google Scholar]
- 11.Johnson E, Hetland G. Human umbilical vein endothelial cells synthesize functional C3, C5, C6, C8 and C9 in vitro. Scand J Immunol. 1991;33:667–71. doi: 10.1111/j.1365-3083.1991.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 12.Mollnes TE, Lea T, Harboe M, et al. Monoclonal antibodies recognizing a neoantigen of poly (C9) detect the human terminal complement complex in tissue and plasma. Scand J Immunol. 1985;22:183–95. doi: 10.1111/j.1365-3083.1985.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 13.Berge V, Johnson E, Nazir M. Effect of gamma-interferon on the synthesis of the functional alternative and terminal complement pathways by human umbilical vein endothelial cells in vitro. APMIS. 1994;102:554–8. doi: 10.1111/j.1699-0463.1994.tb05205.x. [DOI] [PubMed] [Google Scholar]
- 14.Wurzner R, Joysey VC, Lachmann PJ. Complement component C7. Assessment of in vivo synthesis after liver transplantation reveals that hepatocytes do not synthesize the majority of human C7. J Immunol. 1994;152:4624–9. [PubMed] [Google Scholar]
- 15.Naughton MA, Walport MJ, Wurzner R, et al. Organ-specific contribution to circulating C7 levels by the bone marrow and liver in humans. Eur J Immunol. 1996;26:2108–12. doi: 10.1002/eji.1830260922. [DOI] [PubMed] [Google Scholar]
- 16.Hetland G, Johnson E, Falk RJ, et al. Synthesis of complement components C5, C6, C7, C8 and C9 in vitro by human monocytes and assembly of the terminal complement complex. Scand J Immunol. 1986;24:421–8. doi: 10.1111/j.1365-3083.1986.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 17.Hogasen AKM, Wurzner R, Abrahamsen TG, et al. Human polymorphonuclear leukocytes store large amounts of terminal complement components C7 and C6, which may be released on stimulation. J Immunol. 1995;154:4734–40. [PubMed] [Google Scholar]
- 18.Tedesco F, Roncelli L, Petersen BH, et al. Two distinct abnormalities in patients with C8α-γ deficiency. Low level of C8β chain and presence of dysfunctional C8α-γ subunit. J Clin Invest. 1990;86:884–8. doi: 10.1172/JCI114789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffe EA, Nachman RL, Becker CG, et al. Culture of human endothelial cells derived from umbilical veins. Identification by morphological and immunological criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perisutti S, Tedesco F. Effect of cytokines on the secretion of the fifth and eighth complement components by HepG2 cells. Int J Clin Lab Res. 1994;24:45–48. doi: 10.1007/BF02592409. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Tedesco F, Pausa M, Nardon E, et al. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–27. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison RA, Lachmann PJ. Complement technology. In: Weir DM, Herzenberg LA, Blackwell C, Herzenberg Leonore A, editors. Handbook of experimental immunology. Vol. 2. London: Blackwell Scientific Publications; 1986. chapter 39. [Google Scholar]
- 24.Montinaro V, Serra L, Perisutti S, et al. Biosynthesis of C3 by human mesangial cells. Modulation by proinflammatory cytokines. Kidney Int. 1995;47:829–36. doi: 10.1038/ki.1995.125. [DOI] [PubMed] [Google Scholar]
- 25.Ponte P, Ng SY, Engel J, et al. Evolutionary conservation in an untranslated region of actin mRNAs: DNA sequence of a human betaactin cDNA. Nucl Acid Res. 1984;12:1687–96. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffe EA. Biochemistry, immunology and cell biology of endothelium. In: Colman RW, Hirsch J, Marder VJ, Saltzman EW, editors. Hemostasis and thrombosis: basic principles and clinical practice. Philadelphia: Lippincott Co; 1994. pp. 718–44. [Google Scholar]
- 27.Hindmarsch EJ, Marks RM. Complement activation occurs on subendothelial extracellular matrix in vitro and is initiated by retraction or removal of overlaying endothelial cells. J Immunol. 1998;160:6128–36. [PubMed] [Google Scholar]
- 28.Lachmann PJ, Thompson RA. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970;131:643–57. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platonov AE, Würzner R, Beloborodov VB, et al. Paradoxical reconstitution of complement activity following plasma transfusion of an individual with deficiency of the seventh component of complement. Immunology. 1994;81:142–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Würzner R, Platonov AE, Beloborodov VB, et al. How partial C7 deficiency with chronic and recurrent bacterial infections can mimic total C7 deficiency: temporary restoration of host C7 levels following plasma transfusion. Immunology. 1996;88:407–11. doi: 10.1046/j.1365-2567.1996.d01-663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernie BA, Würzner R, Orren A, et al. Molecular basis of combined subtotal deficiencies of C6 and C7. Their effects in combination with other C6 and C7 deficiencies. J Immunol. 1996;157:3648–57. [PubMed] [Google Scholar]
- 32.Brooimans RA, van der Ark AAJ, Buurman WA, et al. Differential regulation of complement factor H and C3 production in human umbilical vein endothelial cells by IFN-γ and IL-1. J Immunol. 1990;144:3835–40. [PubMed] [Google Scholar]
- 33.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and pathophysiology of vascular disorders. Blood. 1998;91:3527–61. [PubMed] [Google Scholar]
- 34.Podack ER, Kolb WP, Müller-Eberhard HJ. The C5b-9 complex: formation, isolation, and inhibition of its activity by lipoprotein and S-protein of human serum. J Immunol. 1978;120:1841–8. [PubMed] [Google Scholar]
- 35.Berge V, Johnson E, Berge KE. Interleukin-1α, interleukin 6 and tumor necrosis factor α increase the synthesis and expression of the functional alternative and terminal complement pathways by human umbilical vein endothelial cells in vitro. APMIS. 1996;104:213–9. doi: 10.1111/j.1699-0463.1996.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 36.Pepys MP. Acute phase proteins. In: Roitt IM, Delves PJ, editors. Encyclopedia of immunology. London: Academic Press; 1992. pp. 16–18. [Google Scholar]