Abstract
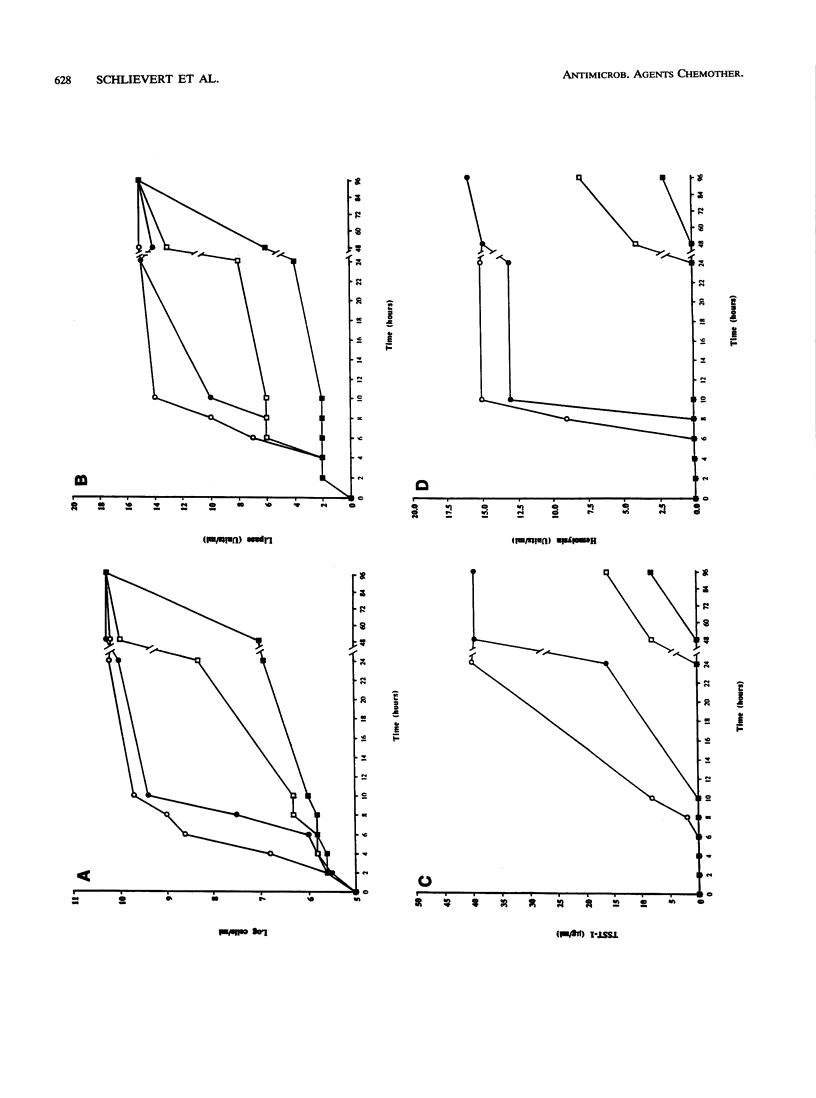
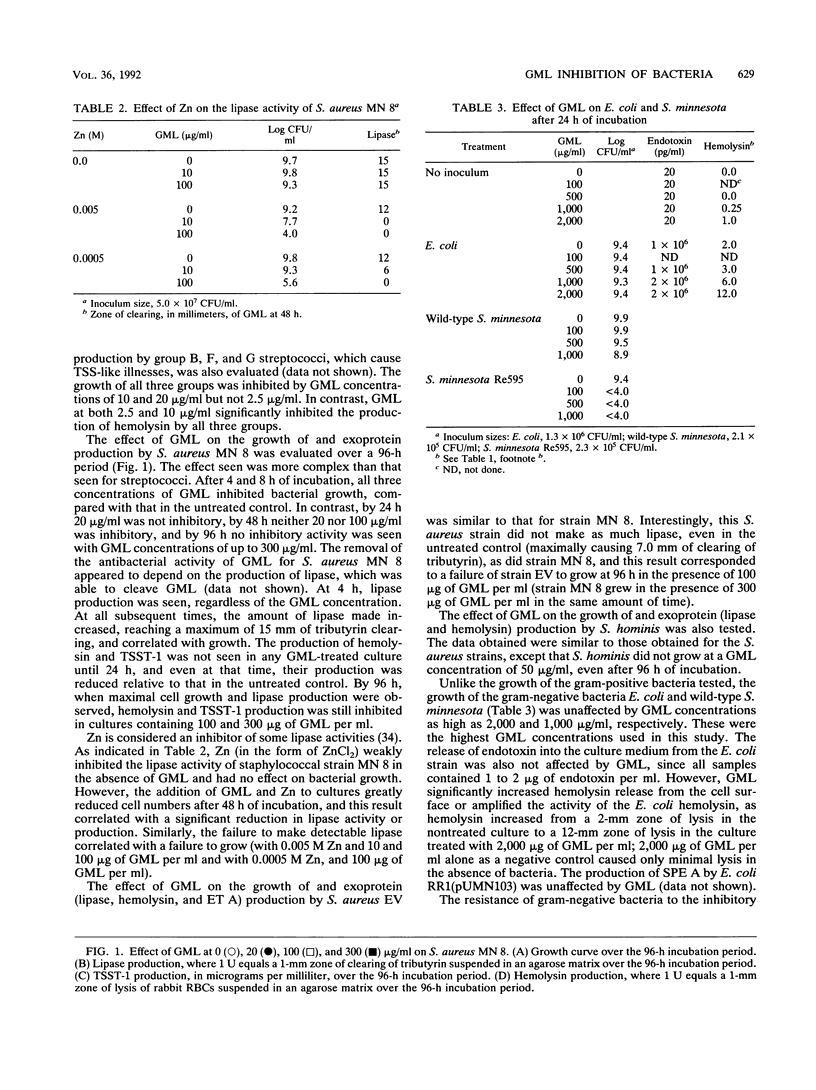
Glycerol monolaurate (GML) is a naturally occurring surfactant that has potential use as an additive to tampons and wound dressings to reduce the incidence of certain bacterial toxin-mediated illnesses. In vitro studies were undertaken to evaluate the effect of GML on the growth of and toxin production by potentially pathogenic bacteria. GML inhibited the growth of clinical isolates of group A, B, F, and G streptococci at concentrations of 10 to 20 micrograms/ml. Exotoxin production, including that of pyrogenic exotoxins and hemolysins, was reduced by concentrations of GML that were below those inhibitory for growth as well as growth inhibitory. The growth of Staphylococcus aureus strains from patients with toxic shock syndrome and scalded skin syndrome was inhibited or delayed in the presence of 100 to 300 micrograms of GML per ml. Growth inhibition by GML could be overcome by the production of lipase. S. aureus elaboration of hemolysin, toxic shock syndrome toxin 1, and exfoliative toxin A was inhibited at GML concentrations below those necessary to inhibit growth. Results similar to those for S. aureus were obtained in tests of S. hominis. Escherichia coli growth and Salmonella minnesota growth were unaffected by GML, but an S. minnesota Re mutant was susceptible to growth-inhibitory activity. Endotoxin release into the medium from E. coli cells was also unaffected by GML, but the release or activity of E. coli hemolysin was increased by GML. Streptococcal pyrogenic endotoxin A production by an E. coli clone was not affectd by GML. These studies indicate that GML is effective in blocking or delaying the production of exotoxins by pathogenic gram-positive bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDER V. G., GILLESPIE W. A., HERDAN G. Production of opacity in egg-yolk broth by Staphylococci from various sources. J Pathol Bacteriol. 1953 Jul;66(1):205–210. doi: 10.1002/path.1700660123. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Drude E., Goldstein C., Anderka M., Alpert S., McCormack W. M. Quantitative bacteriology of the vaginal flora. J Infect Dis. 1977 Aug;136(2):271–277. doi: 10.1093/infdis/136.2.271. [DOI] [PubMed] [Google Scholar]
- Bergdoll M. S., Crass B. A., Reiser R. F., Robbins R. N., Davis J. P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981 May 9;1(8228):1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Kreiswirth B. N., Kornblum J. S., Novick R. P., Schlievert P. M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986 Nov 25;261(33):15783–15786. [PubMed] [Google Scholar]
- Bohach G. A., Fast D. J., Nelson R. D., Schlievert P. M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17(4):251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- Chesney P. J. Clinical aspects and spectrum of illness of toxic shock syndrome: overview. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S1–S7. [PubMed] [Google Scholar]
- Chow A. W., Wittmann B. K., Bartlett K. H., Scheifele D. W. Variant postpartum toxic shock syndrome with probable intrapartum transmission to the neonate. Am J Obstet Gynecol. 1984 Apr 15;148(8):1074–1079. doi: 10.1016/s0002-9378(84)90448-4. [DOI] [PubMed] [Google Scholar]
- Davis J. P., Chesney P. J., Wand P. J., LaVenture M. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980 Dec 18;303(25):1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- Hauser A. R., Schlievert P. M. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol. 1990 Aug;172(8):4536–4542. doi: 10.1128/jb.172.8.4536-4542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. P., Schlievert P. M. Group A streptococcal phage T12 carries the structural gene for pyrogenic exotoxin type A. Mol Gen Genet. 1984;194(1-2):52–56. doi: 10.1007/BF00383496. [DOI] [PubMed] [Google Scholar]
- Kaplan M. H., Chmel H., Hsieh H. C., Stephens A., Brinsko V. Importance of exfoliatin toxin A production by Staphylococcus aureus strains isolated from clustered epidemics of neonatal pustulosis. J Clin Microbiol. 1986 Jan;23(1):83–91. doi: 10.1128/jcm.23.1.83-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCollister B. D., Kreiswirth B. N., Novick R. P., Schlievert P. M. Production of toxic shock syndrome-like illness in rabbits by Staphylococcus aureus D4508: association with enterotoxin A. Infect Immun. 1990 Jul;58(7):2067–2070. doi: 10.1128/iai.58.7.2067-2070.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm M. T., Davis J. P., Gibson R. W., Mandel J. S., Wintermeyer L. A., Helms C. M., Forfang J. C., Rondeau J., Vergeront J. M. Tri-state toxic-state syndrome study. I. Epidemiologic findings. J Infect Dis. 1982 Apr;145(4):431–440. doi: 10.1093/infdis/145.4.431. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Richter A. G., Pier G. B. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect Immun. 1987 May;55(5):1070–1076. doi: 10.1128/iai.55.5.1070-1076.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Regassa L. B., Couch J. L., Betley M. J. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect Immun. 1991 Mar;59(3):955–962. doi: 10.1128/iai.59.3.955-962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkallah M. F., Tolaymat A., Martinez J. S., Schlievert P. M., Ayoub E. M. Toxic shock syndrome caused by a strain of Staphylococcus aureus that produces enterotoxin C but not toxic shock syndrome toxin-1. Am J Dis Child. 1989 Jul;143(7):848–849. doi: 10.1001/archpedi.1989.02150190098031. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Bettin K. M., Watson D. W. Purification and characterization of group A streptococcal pyrogenic exotoxin type C. Infect Immun. 1977 May;16(2):673–679. doi: 10.1128/iai.16.2.673-679.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M., Blomster D. A., Kelly J. A. Toxic shock syndrome Staphylococcus aureus: effect of tampons on toxic shock syndrome toxin 1 production. Obstet Gynecol. 1984 Nov;64(5):666–671. [PubMed] [Google Scholar]
- Schlievert P. M., Blomster D. A. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983 Feb;147(2):236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Kelly J. A. Clindamycin-induced suppression of toxic-shock syndrome--associated exotoxin production. J Infect Dis. 1984 Mar;149(3):471–471. doi: 10.1093/infdis/149.3.471. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986 May 17;1(8490):1149–1150. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- Shands K. N., Schmid G. P., Dan B. B., Blum D., Guidotti R. J., Hargrett N. T., Anderson R. L., Hill D. L., Broome C. V., Band J. D. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980 Dec 18;303(25):1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- Todd J., Fishaut M., Kapral F., Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978 Nov 25;2(8100):1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- Woodburn M., Morita T. N., Venn S. Z. Production of staphylococcal enterotoxins A, B, and C in colloidal dispersions. Appl Microbiol. 1973 May;25(5):825–833. doi: 10.1128/am.25.5.825-833.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


