Abstract
It is generally believed that neutrophils from HIV-infected patients are functionally competent, but several studies have shown impairment in neutrophil fungal killing and cytokine production. In this study we evaluated the ability of neutrophils from healthy donors and HIV-infected patients to produce IL-12 in response to stimulation with Candida albicans, lipopolysaccharide (LPS) and Cryptococcus neoformans (acapsular and encapsulated), with and without MoAb opsonization. Neutrophils from healthy donors secreted IL-12 in response to LPS or C. albicans but not in response to encapsulated or acapsular C. neoformans, regardless of MoAb opsonization. Surprisingly, neutrophils from HIV-infected patients demonstrated constitutive IL-12 production, although these cells were not responsive to LPS stimulation. The inability of MoAb to C. neoformans capsular polysaccharide to promote IL-12 production by neutrophils excludes phagocytosis and/or CD16 cross-linking in this process, and distinguishes neutrophils from monocytes. Our results provide additional evidence for cytokine dysregulation in neutrophils from HIV-infected patients. Furthermore, the IL-12 response of neutrophils and monocytes to CD16 stimulation appears to be different, suggesting differences in the role of these phagocytic cells during the inflammatory response.
Keywords: IL-12, neutrophils, Cryprococcus neoformans, antibodies, HIV
Introduction
Neutrophils constitute the majority of circulating leucocytes and play a central role in the innate defence against invading microorganisms. In addition to this well-established role for anti-microbial effector cells, recent studies have shown that polymorphonuclear cells (PMN), under certain stimulatory conditions, produce numerous cytokines [1]. These cytokines include tumour necrosis factor-alpha (TNF-α), IL-1α, IL-6 [2], IL-8, interferon-alpha (IFN-α), macrophage inflammatory protein-1α (MIP-1α) and α, macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage CSF (GM-CSF), IL-1 receptor antagonist, and IL-12 [3].
IL-12 is increasingly considered to be a bridge between innate and adaptive immunity and has been shown to be critically important for host defence against many microorganisms [4]. Biologically active IL-12 is a heterodimer of 70 kD (p70) formed by two covalently linked chains of 40 kD (p40) and 35 kD (p35) [4]. IL-12 is produced predominantly by phagocytic cells, as suggested by in vitro and in vivo studies in infectious disease models [1, 5]. Monocytes and PMN produce IL-12 in response to bacterial products such as lipopolysaccharide (LPS) and to live or heat-inactivated bacteria or intracellular pathogens [1,6]. The amount of IL-12 p40 or p70 produced by PMN is lower than that produced by monocytes at the single-cell level. The IL-12 produced by neutrophils is likely to play an important physiological role in the inflammatory response to infection considering that neutrophils comprise the majority of infiltrating cells.
Neutrophils are professional phagocytes that contribute to the initial host inflammatory response in Cryptococcus neoformans infection and may be important cells in resistance to cryptococcal infection in immunocompetent individuals. Their functional status is particularly important in HIV-infected patients because the impaired activity of PMN may facilitate opportunistic infection [7].
Cryptococcosis represents a serious fungal infection in patients with late-stage HIV infection, with an incidence ranging from 5% to 10%. Effective host defence against C. neoformans is ascribed to a combination of multiple mechanisms [8–10], including humoral and cell-mediated [11–14]. Although a number of studies have assessed neutrophil functions in HIV-infected patients' immune responses [15–17], the cytokine response by PMN from HIV-infected patients to the fungal cell has been relatively unexplored. Neutrophils are important for initiation of the inflammatory process and in the protective immune response against C. neoformans, possibly through their ability to produce IL-12. Given that monocytes are poor producers of IL-12 in response to C. neoformans, at least in the early phase of immune response, PMN may be an alternative source of IL-12. To investigate this possibility we evaluated the IL-12 response of PMN from HIV− and HIV+ in the presence and absence of an opsonic MoAb to glucuronoxylomannan (GXM).
SUBJECTS and METHODS
Subjects
Sixteen HIV+ patients, ranging in age from 24 to 40 years, were enrolled in this study. HIV+ donors were recruited from the Clinic of Infectious Disease, University of Perugia, Italy. Sixteen healthy HIV− donors, ranging in age from 20 to 45 years (mean ± s.e.m. CD4 cell count was 1051 ± 75/mm3), were included as controls. At the time of enrolment, all patients were being treated with at least three drugs (didanosine, zidovudine, stavudine, lamivudine) and none of the patients was receiving cytokine therapy. All patients enrolled in this study were eligible for highly active anti-retroviral therapy (HAART).
Reagents and media
RPMI 1640 with glutamine and fetal calf serum (FCS) were obtained from Gibco BRL (Paisley, UK). Human serum (HS) type AB, FITC MoAb sheep anti-mouse IgG (whole molecule) and MoAb mouse isotype control IgG1,k were purchased from Sigma (St Louis, MO). Mouse MoAb to human CD16 (IgG1) was provided by Ancell Corp. (Bayport, ME). Mouse MoAb to human CD32 (IgG1) was purchased from Alexis Corp. (Lëufelfingen, Switzerland). MoAb 18B7 is a murine IgG1 MoAb that binds C. neoformans GXM and the characteristics of this MoAb have been described [18, 19]. MoAb 18B7 was purified by protein G affinity chromatography (Pierce, Rockford, IL). The concentration of MoAb 18B7 was determined by ELISA relative to isotype-matched standards of known concentrations.
All reagents and media used in this study were negative for endotoxin as detected by Limulus amoebocyte lysate assay (Sigma), which had a sensitivity of approximately 0·05–0·1 ng of Escherichia coli LPS per ml.
Microorganisms
Candida albicans (strain PCA-2) was supplied by D. Kerridge (Department of Biochemistry, University of Cambridge, UK). This is an agerminative strain that grows as a pure yeast form in vitro at 28°C or 37°C in conventional media. Two strains of C. neoformans were examined in this study: C. neoformans var. neoformans 6995 (CBS 6995 = NIH 37), a thinly encapsulated isolate of serotype A, and C. neoformans var. neoformans 7698 (CBS 7698 = NIH B-4131), an acapsular mutant. The morphological characteristics and conditions for growth of the two strains of C. neoformans and the C. albicans isolate have been described [13, 14]. Cultures were maintained by serial passage on Sabouraud agar (BioMerieux, Lyon, France) and harvested by suspending a single colony in RPMI 1640. Cells of C. neoformans 6995 and 7698 and C. albicans were killed by autoclaving. In selected experiments, C. neoformans cells were inactivated at 56°C for 30 min before use.
Preparation of PMN
Heparinized venous blood, obtained from healthy donors, was diluted with RPMI 1640, and mononuclear cells were separated by Ficoll–Hypaque density gradient centrifugation [20]. The pellet containing PMN and erythrocytes was treated with hypotonic saline to lyse the erythrocytes. Granulocytes were collected by centrifugation, washed twice in RPMI 1640, counted, and adjusted to the desired concentration. The purity of PMN isolated by this method was always > 98%, as determined by Giemsa staining. PMN preparations contained about 0·5–1% eosinophils. PMN viability evaluated after 18 h of incubation was > 98% in all determinations performed, as assessed by the trypan blue dye exclusion test.
Production of PMN culture supernatants
Isolated PMN were distributed in 0·1-ml volumes into 96-well U-bottomed tissue culture plates (Becton Dickinson, Oxnard, CA) at 4 × 106 cells/ml and incubated for 18 h at 37°C in a 5% CO2 atmosphere with RPMI containing 10% HS with different stimuli as indicated. Supernatant fluids were harvested at the indicated times and stored at −20°C until use.
Cytokine level determination
Cytokine levels in culture supernatant fluids were measured using an ELISA kit for human IL-10 and for human IL-12 (Biosource, Camarillo, CA). The IL-10 and IL-12 kits are a solid-phase ELISA based on the antibody sandwich principle. The assay for human IL-10 recognizes both natural and recombinant human IL-10. The limit of detection of the human IL-10 ELISA was < 5 pg/ml. The assay for human IL-12 recognizes both natural and recombinant human IL-12, as well as the free p40 subunit. The limit of detection of the IL-12 ELISA was < 0·8 pg/ml.
Determination of surface expression of CD16 and CD32 molecules
Freshly isolated human PMN were analysed for CD16 and CD32 surface expression. Briefly, PMN (1 × 106) were fixed with 2% paraformaldehyde in PBS for 10 min at room temperature, washed twice in PBS containing 0·5% bovine serum albumin and 0·1% sodium azide and mixed with isotype-control mouse IgG1 (5 αg/ml) or MoAb to human CD16 (5 αg/ml) or MoAb anti-human CD32 (5 αg/ml). After 30 min of incubation on ice the cells were washed twice and then stained with FITC-conjugated anti-mouse IgG (dilution 1:256) for 30 min. In selected experiments human PMN were preincubated with MoAb 18B7 (10 αg/ml) or with 6995 (E:T = 1:2) preopsonized for 30 min at 37°C with MoAb 18B7 (10 αg/ml), for 10 min or 40 min at 37°C at 5% CO2. CD16 and CD32 surface expression was measured using a FACS (Becton Dickinson, Mountain View, CA). Mean fluorescence of labelled cells was determined using logarithmic-scale histograms. Autofluorescence was assessed using untreated cells.
Preparation of fluorescein-labelled MoAb 18B7
A fluorescein derivative of MoAb 18B7 (18B7 FI) or MoAb isotype control FI was prepared with fluorescein labelling kit (Boehringer Mannheim Biochemica).
Binding of MoAb 18B7 FI to PMN
Freshly isolated human PMN (1 × 106) were incubated for 20 min at 37°C at 5% CO2 with (i) MoAb isotype control FI (50 αg/ml) or MoAb 18B7 FI (50 αg/ml); (ii) 6995 (E:T = 1:2) preopsonized for 30 min at 37°C with MoAb isotype control FI (50 αg/ml) or with MoAb 18B7 FI (50 αg/ml); (iii) 7698 (E:T = 1:2) preopsonized for 30 min at 37°C with MoAb isotype control FI (50 αg/ml) or 18B7 FI (50 αg/ml). Cells were washed twice in PBS containing 0·5% bovine serum albumin (BSA) and 0·1% sodium azide and were then suspended in 0·5 ml of PBS containing 0·5% BSA and 0·1% sodium azide. The binding of 18B7 FI was assessed by flow cytometry (Becton Dickinson).
RNA isolation
Total RNA was isolated by acid guanidine isothiocyanate-phenol-chloroform extraction as described [21]. Briefly, PMN (5 × 106) were lysed with a denaturing solution containing 4 mm guanidine isothiocyanate, 25 mm sodium citrate, 0·5% sarcosyl, 0·1 m 2-mercaptoethanol. Sequentially, 0·1 of 2 m sodium acetate (pH 4), 1 Vol. of water-saturated phenol and 0·2 Vol. of chloroform-isoamylic alcohol mixture (49:1) were added to the lysate and mixed thoroughly after the addition of each reagent. The final mixture was cooled on ice for 15 min and centrifuged at 10 000 g for 20 min at 4°C. The aqueous phase was mixed with an equal volume of isopropanol and precipitated RNA was collected by centrifugation (10 000 g 30 min at 4°C), washed in 75% ethanol, vacuum dried (10 min) and dissolved in DEPC-treated water. The amount of RNA was determined by spectrophotometry.
Reverse transcriptase-polymerase chain reaction analysis of cytokine mRNA and G6PD mRNA
For each sample 1 αg of total RNA was reverse transcribed in a volume of 20 αl (reverse transcriptase (RT)) buffer (Tris–HCl 1 m pH = 8·3, MgCl2 1 m, KCl 1 m, DTT 150 mm, dNTPs 25 mm) containing 20 U RNAsin, 1 αg oligo d(T) primer and 200 U M-MLV reverse transcriptase (Gibco). The RT reaction was performed for 1 h at 37°C using a HYBEID OMN-E Thermocycler and the samples were stored at −20°C until polymerase chain reaction (PCR) analysis was performed. The cDNA was amplified in the presence of 0·05 mm 5″ and 3′ primers (Stratagene), 2 mm deoxynucleotides (Promega, Madison, WI) and 0·5 U of Taq Polymerase (Perkin Elmer) and 10× PCR buffer. Thirty-five PCR cycles were performed as follows: 5 min denaturation at 94°C, 5 min annealing at 60°C, and 1·5 min at 72°C. The primer pair sequences used were: G6PD sense, TTCTTCAACCCCGAGGAGT; G6PD antisense, GGGAAGGAGGGTGGCCGTG; IL-12 sense, GGACCAGAGCAGTGAGGTCTT; IL-12 antisense, CTCCTTGTTGTCCCCTCTGA.
Primary pairs were obtained from Strategene. The sizes of the amplified G6PD and IL-12 DNA were 358 bp and 373 bp, respectively.
PCR products were analysed by electrophoresis on 2% agarose gels stained with ethidium bromide to quantify the size of the banding pattern, and 0·5 αg of 100-bp DNA ladder (M-Medical) was run in parallel. Gels were scanned on a densitometer and analysed using Molecular Analyst.
Quantification of HIV RNA copy numbers in plasma and CD4+ lymphocyte counts
HIV RNA levels (viral load) were measured in EDTA plasma by quantitative reverse PCR (Amplicor Monitor; Roche Diagnostic, Basle, Switzerland). The level of detection was 400 copies/ml. The CD4 lymphocyte count was measured by flow cytometry (FACScan; Becton Dickinson).
Statistical analysis
Differences in IL-12 production between untreated PMN from HIV-infected patients and controls and differences in IL-10 production by PMN between HIV-infected patients and controls were evaluated by the non-parametric Mann–Whitney U-test (two-tailed). P < 0·05 was considered significant.
Differences in IL-12 production between treated versus untreated PMN from HIV-infected patients were evaluated by two-tailed Wilcoxon signed rank test for paired data. For the test with 13 patients P < 0·022 was considered significant, while for the test with eight patients P < 0·024 was considered significant.
Results
The importance of IL-12 in the generation of protective responses to microbial pathogens is widely recognized [22]. PMN secrete IL-12 in response to various stimuli including C. albicans [1,3,23]. Given that C. neoformans stimulates IL-12 secretion by monocytes in the early phase of the immune response [22], one might expect that PMN would also secrete IL-12 in response to this fungus. To test this possibility, PMN from normal individuals and HIV-infected patients were exposed to C. neoformans and the IL-12 concentration in the cell supernatant was determined.
PMN from normal subjects did not produce IL-12 over baseline (5–17 pg/ml) after stimulation with C. neoformans. Addition of the opsonic MoAb 18B7 had no significant effect on IL-12 production by PMN from normal individuals. In contrast, PMN produced IL-12 in response to C. albicans and LPS, as described [3,23]. Surprisingly, the supernatant from PMN of HIV-infected patients had average IL-12 concentrations which were approximately three times that measured for PMN from normal individuals. Furthermore, the IL-12 response of PMN from normal individuals and HIV-infected patients to stimulation with LPS and C. albicans was different. Exposure of PMN from HIV-infected patients to C. albicans or LPS resulted in a significant reduction in IL-12 concentration in the supernatant. The IL-12 production of PMN from HIV-infected patients was also reduced after exposure to C. neoformans, regardless of whether MoAb 18B7 was present. Hence, PMN from normal individuals and HIV-infected patients differed with regard to baseline IL-12 production and in their response to stimulation with LPS and C. albicans (Table 1). To sort out whether there was a block release or an inhibition of IL-12 production, we tested cultured PMN for 18 h in the presence of fungi, then the cells were lysed mechanically and IL-12 was determined in supernatants. The IL-12 levels were slightly higher but the difference was not statistically different from those of intact cells (data not shown). We considered the possibility that killing by autoclaving could conceivably affect the results by altering fungal antigens. To evaluate this possibility we compared IL-12 release from selected samples (four from HIV-infected patients and four from control patients) using C. neoformans cells killed at 56°C for 30 min. The results were similar to those obtained by autoclaving (data not shown). Hence, lack of responsiveness to C. neoformans by PMN was not the result of changes in fungal cells resulting from autoclaving.
Table 1.
IL-12 (pg/ml) production by polymorphonuclear neutrophils (PMN) exposed to acapsular (7698) or encapsulated (6995) Cryptococcus neoformans from normal subjects or HIV-infected patients
| Subjects | CD4/mm3 | RNA copies/ml | None | LPS (10 µg/ml) | MoAb 18B7 | C. albicans | C. albicans + MoAb 18B7 | 7698 | 7698 + MoAb 18B7 | 6995 | 6995 + MoAb 18B7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls‡ | 1051 ± 75 | 0 | 12 ± 1 | 39 ± 4 | 13 ± 2 | 40 ± 5 | 32 ± 3 | 16 ± 2 | 14 ± 2 | 15 ± 2 | 14 ± 1 |
| Pt no. 5 | 819 | 76 980 | 60 | 30 | ND | 21 | 18 | 11 | 10 | 16 | 15 |
| Pt no. 1 | 815 | 89 843 | 21 | 20 | 18 | 7 | 9 | 11 | 9 | 9 | 10 |
| Pt no. 6 | 600 | 8577 | 19 | 35 | ND | ND | ND | 22 | ND | 9 | 9 |
| Pt no. 16 | 445 | 92 633 | 22 | 19 | ND | 17 | ND | 13 | ND | 20 | 11 |
| Pt no. 3 | 384 | 12 764 | 25 | 15 | 18 | 10 | 9 | 12 | 13 | 12 | 14 |
| Pt no. 15 | 384 | 45 678 | 22 | 128 | 20 | 22 | ND | 24 | ND | 14 | 15 |
| Pt no. 8 | 160 | 830 400 | 80 | 15 | ND | ND | ND | 6 | 7 | 12 | 7 |
| Pt no. 9 | 130 | 490 776 | 5 | 5 | 2 | 3 | 1 | 0 | 1 | 2 | 1 |
| Pt no. 7 | 128 | 1401 144 | 65 | 12 | ND | 6 | 7 | 2 | 3 | 10 | 8 |
| Pt no. 12 | 93 | 730 769 | 91 | 129 | 85 | 76 | 78 | 92 | 94 | 85 | 87 |
| Pt no. 13 | 82 | 5388 | 74 | 80 | ND | ND | ND | 30 | 27 | 33 | 35 |
| Pt no. 10 | 80 | 564 932 | 50 | 21 | ND | ND | ND | 12 | 10 | 9 | 12 |
| Pt no. 14 | 26 | 1928 691 | 96 | 85 | 91 | 52 | 48 | 90 | ND | 82 | 84 |
| Mean | 318 ± 78 | 482·96 | 48 ± 9† | 46 ± 12 | 39 ± 16 | 24 ± 8* | 24 ± 11 | 25 ± 8** | 19 ± 10 | 24 ± 7 | 24 ± 8*** |
| ± s.e.m. | ± 169·07 |
PMN (4 × 106) were exposed for 18 h to the indicated fungi at an E:T ratio of 1:2. Supernatants fluids were harvested and cytokine levels were determined by ELISA. ND, Not determined eight patients.
P < 0·024 (HIV-infected treated versus HIV-infected untreated cells), 13 patients
P < 0·022 (HIV-infected 7698-treated versus HIV-infected untreated cells), 13 patients
P < 0·022 (HIV-infected MoAb plus 6995 treated vs. HIV-infected untreated cells)
P < 0·014 (HIV-infected versus control cells).
The results are the mean ± s.e.m. of experiments with cells from 13 different donors.
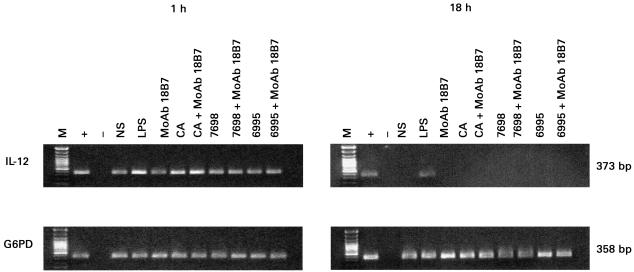
Given that IL-10 has been reported to be a potential inhibitor of IL-12 production, we investigated whether the differences in IL-12 production between PMN from normal individuals and those with HIV infection were the result of differences in IL-10 production. The conditions under which PMN produce IL-10 are uncertain. One study reported that PMN did not secrete IL-10 in response to various stimuli [24], whereas another reported IL-10 gene expression in response to C. albicans stimulation [23]. In our experimental system low concentrations of IL-10 were produced by PMN from normal individuals constitutively or after stimulation with either C. albicans or C. neoformans. However, appreciable levels of IL-10 were measured in PMN supernatants after stimulation with LPS; in contrast, IL-10 production by PMN from HIV-infected patients was low, even after LPS stimulation (Table 2). Analysis of PMN mRNA for IL-12 showed constitutive expression and significant increases only in response to LPS and C. albicans (Fig. 1).
Table 2.
IL-10 (pg/ml) production by polymorphonuclear neutrophils (PMN) exposed to acapsular (7698) or encapsulated (6995) Cryptococcus neoformans from normal subjects or HIV-infected patients
| Subjects | CD4/mm3 | RNA copies/ml | None | LPS (10 αg/ml) | MoAb 18B7 | C. albicans | C. albicans + MoAb 18B7 | 7698 | 7698 + MoAb 18B7 | 6995 | 6995 + MoAb 18B7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls† | 1051 ± 75 | 0 | 7 ± 1 | 98 ± 8 | 5 ± 1 | 16 ± 2 | 18 ± 2 | 15 ± 1 | 14 ± 2 | 10 ± 2 | 7 ± 1 |
| Pt no. 5 | 819 | 76 980 | 4 | 20 | 0 | 6 | 8 | 0 | 0 | 7 | 5 |
| Pt no. 1 | 815 | 89 843 | 8 | 60 | 6 | 9 | 10 | 6 | 5 | 11 | 7 |
| Pt no. 6 | 600 | 8577 | 2 | 10 | 3 | ND | ND | 4 | ND | ND | ND |
| Pt no. 2 | 515 | 260 114 | 7 | 26 | 4 | 8 | 7 | ND | ND | ND | ND |
| Pt no. 4 | 445 | 253 200 | 6 | 41 | 4 | 6 | 6 | 6 | ND | 18 | 8 |
| Pt no. 3 | 384 | 12 764 | 2 | 34 | 2 | 4 | 5 | 0 | 0 | 12 | 10 |
| Pt no. 8 | 160 | 830 400 | 15 | 19 | 8 | 12 | ND | 6 | ND | 12 | 7 |
| Pt no. 9 | 130 | 490 776 | 4 | 4 | 2 | 10 | 11 | 4 | 4 | 2 | 2 |
| Pt no. 7 | 128 | 1401 144 | 0 | 3 | 0 | 4 | 3 | ND | ND | 0 | 2 |
| Pt no. 11 | 102 | 910 067 | 3 | 5 | 3 | ND | ND | 2 | 3 | 3 | ND |
| Pt no. 10 | 80 | 564 932 | 5 | 5 | 3 | ND | ND | 2 | 2 | 1 | 2 |
| Mean | 380 ± 85 | 445·34 ± | 5 ± 1 | 20 ± 5* | 3 ± 1 | 7 ± 1* | 7 ± 1* | 3 ± 1* | 2 ± 1* | 7 ± 2 | 5 ± 1 |
| ± s.e.m. | 135·02 |
PMN (4 × 106) were exposed for 18 h to the indicated fungi at an E:T ratio of 1:2. Supernatant fluids were harvested and cytokine levels were determined by ELISA. ND, Not determined.
P < 0·05 (HIV-infected versus control cells).
The results are the mean ± s.e.m. of data from 11 different donors.
Fig. 1.
Analysis of IL-12 gene expression by polymorphonuclear neutrophils (PMN; 5 × 106/ml) unstimulated (NS) or stimulated for 1 h and 18 h with: (i) lipopolysaccharide (LPS; 10 αg/ml); (ii) MoAb 18B7 (10 αg/ml); Candida albicans (CA, E:T = 1:2) or acapsular (7698) or encapsulated (6995) Cryptococcus neoformans (E:T = 1:2) strains in the presence or absence of MoAb 18B7 (10 αg/ml). No DNA (–) was added to amplification mixture during polymerase chain reaction. M, DNA markers. +, positive control. The results reported are from a representative experiment of three performed with similar results.
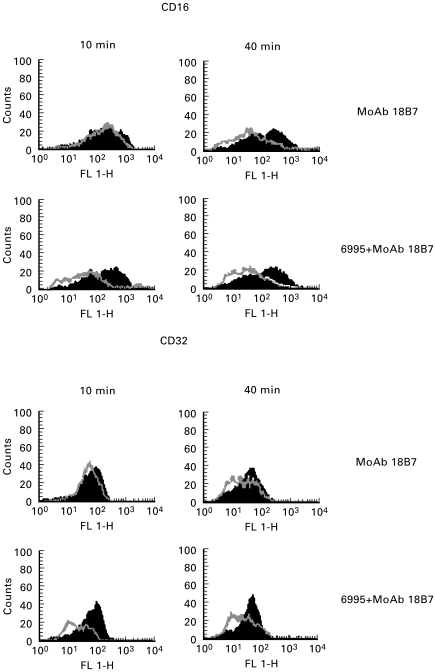
We recently demonstrated that specific MoAb to GXM facilitates IL-8 release and the killing capacity of PMN when combined with the encapsulated strain [25,26]. Consequently, one might expect that the phagocytic process or cross-linking of Fc receptors would selectively affect cytokine production by neutrophils. Given that CD16 ligation has been reported as a potential stimulus for IL-12 induction by monocytes/macrophages [22,27] and that MoAb to GXM enhances IL-12 production by monocytes stimulated with C. neoformans, probably via CD16 ligation [27], we analysed the receptor to neutrophils that preferentially binds MoAb to GXM to exclude the contribution of both phagocytosis and Fc receptor cross-linking for IL-12 induction by neutrophils. In the first series of experiments, we considered the possibility that MoAb to GXM (IgG1) binds to neutrophils via CD16. To this end, C. neoformans strains (encapsulated or acapsular) were mixed with MoAb (10 αg/ml) for 30 min at 37°C, washed and then mixed with neutrophils. In addition, neutrophils were treated with specific MoAb to GXM (5 αg/ml). At various times CD16 expression was analysed by cytofluorometric analysis. The results showed that CD16 expression on neutrophil surface was significantly inhibited when MoAb to GXM was used in combination with the encapsulated strain. In contrast, neutrophils treated with the acapsular strain, previously mixed with MoAb to GXM and then washed, did not manifest a change in CD16 expression (data not shown). Similar results were observed for CD32 expression (Fig. 2).
Fig. 2.
CD16 and CD32 surface expression by polymorphonuclear neutrophils (PMN) from normal subjects untreated (black lines) or treated (grey lines) with MoAb 18B7 (10 αg/ml) or with 6995 (E:T = 1:2) plus MoAb 18B7 (10 αg/ml) for 10 min or 40 min. 6995 alone did not regulate CD16 or CD32 expression at the indicated time. The results reported are from a representative experiment of four performed with similar results.
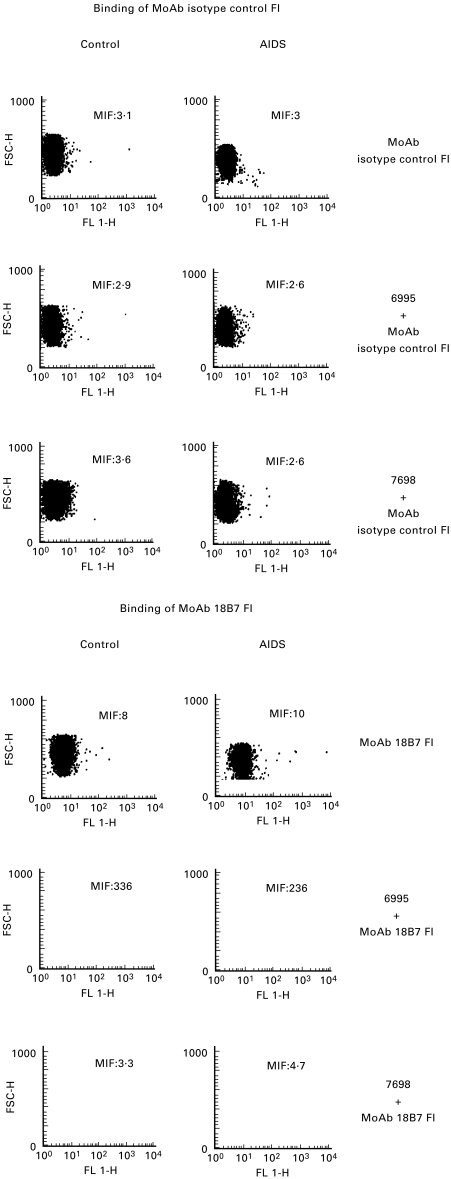
To analyse the amount of immunocomplexes binding to neutrophils, MoAb to GXM was labelled with FITC (50 αg/ml) and mixed with the encapsulated or acapsular strain. After washing, opsonized cryptococcal cells were added to neutrophils. Cytofluorometric analysis showed an extensive ligation of immunocomplexes on the neutrophil surface (Fig. 3).
Fig. 3.
Cytofluorometric analysis of polymorphonuclear neutrophils from HIV-infected patients and normal subjects (control) treated with: (i) MoAb isotype control FI (50 αg/ml) or MoAb 18B7 FI (50 αg/ml); (ii) 6995 (E:T = 1:2) preopsonized for 30 min at 37°C with MoAb isotype control FI (50 αg/ml) or MoAb 18B7 FI (50 αg/ml); (iii) 7698 (E:T = 1:2) preopsonized for 30 min at 37°C with MoAb isotype control FI (50 αg/ml) or with MoAb 18B7 FI (50 αg/ml). The results reported are from a representative experiment of three performed with similar results.
Discussion
We previously demonstrated that neutrophils from healthy donors are able to secrete IL-8 in response to C. neoformans [28]. The encapsulated strain elicited more IL-8 production than the acapsular strain. Although the major mechanism for IL-8 release is C3a and C5a generation, that in turn stimulates neutrophils [28], phagocytosis appears to be an adjunctive or alternative pathway facilitating IL-8 release. This is consistent with the capability of MoAb to GXM to up-regulate IL-8 secretion by neutrophils [25,26].
IL-12 has been established as a key cytokine in the generation of Th1 responses in many fungal, bacterial, and parasitic infections [29–33]. Monocytes and macrophages are believed to be the major physiological source of IL-12 for the immune response. However, we recently demonstrated that these cell types are not efficient producers of IL-12 after stimulation with C. neoformans, at least in the early phase of the immune response. Since PMN constitute an important source of IL-8, we hypothesized that PMN could compensate for the low levels of IL-12 produced in response to C. neoformans by monocytes and macrophages. Here we demonstrate that PMN from healthy donors secrete IL-12 when stimulated by LPS and C. albicans. These results are consistent with the findings of Romani et al. that murine PMN produce significant amounts of IL-12 in response to C. albicans [23]. However, in sharp contrast to the effect of C. albicans, incubation of PMN with either encapsulated or acapsular C. neoformans cells had no significant effect on IL-12 production.
The phagocytic process appears to influence many effector and secretory functions of neutrophils. Enhanced killing activity and up-regulation of IL-8 secretion was observed in neutrophils exposed to C. neoformans yeast cells [25,26] in the presence of MoAb to GXM. The inability of MoAb to GXM to influence IL-12 in response to encapsulated C. neoformans argues against a role for internalization in the modulation of IL-12, and strongly suggests that IL-12 signal transduction in neutrophils is independent of receptors involved in the antibody-mediated phagocytic process. This is consistent with evidence that encapsulated C. neoformans treated with MoAb to GXM binds to human neutrophils via CD16.
Here we demonstrate that, in contrast to monocytes which are producers of IL-12 also via CD16 perturbation [22], human neutrophils are unable to secrete IL-12 following CD16 ligation. We provide evidence that the phagocytic process and FcRγ receptor perturbation do not trigger IL-12 release by neutrophils. In contrast, internalization of particulate antigens or microorganisms has been described as a main event in inducing IL-12 release from monocytes/macrophages [6]. However, because the phagocytic process is probably involved in IL-8 up-regulation by neutrophils, the internalization process selectively influences cytokine release.
LPS and C. albicans stimulation resulted in a lower production of IL-10 and IL-12 from PMN of HIV-infected patients relative to PMN from healthy controls. This may be due to an intrinsic HIV-related PMN hyporesponsiveness. It should be noted that LPS appeared to be the only stimulus able to moderately induce IL-10 production, suggesting potential IL-10 secretion by PMN from HIV-infected patients in response to Gram-negative bacteria.
PMN from HIV-infected patients produced significantly more IL-12 at baseline than PMN from normal individuals. This may reflect a dysregulation of cytokine production observed in HIV-infected patients and, in particular, could mirror a state of chronic activation previously observed with proinflammatory cytokines, including IL-12. In particular, a decreased secretion of IL-12 was observed in mononuclear cells from HIV-infected patients in response to Staphylococcus aureus [34]. The failure of neutrophils from HIV-infected patients to produce IL-12 in response to LPS or C. albicans extends to neutrophils, the unresponsiveness of IL-12 to pathogens, or to T cell-dependent stimuli previously observed in mononuclear cells [35,36].
Taken together, our results indicate that (i) C. neoformans does not stimulate IL-12 production from PMN; (ii) PMN from HIV-infected patients constitutively produce significantly higher levels of IL-12 than those from normal individuals; (iii) PMN from HIV-infected patients respond to LPS and C. albicans with reduced IL-12 production; and (iv) neither phagocytosis nor CD16 ligation trigger IL-12 synthesis in PMN. Our results provide additional evidence for functional differences between PMN from healthy individuals and those with HIV infection, and show that the interactions of PMN with C. albicans and C. neoformans are quite different with regard to IL-12 production. Furthermore, our results highlight fundamental differences in the response of monocytes/macrophages and PMN with regard to IL-12 production in response to phagocytosis and Fc receptor cross-linking.
Acknowledgments
The authors thank Eileen Mahoney Zannetti for dedicated secretarial and editorial support. This study was supported by the National Research Project on AIDS ‘Opportunistic Infections and Tuberculosis’, contract 50B.39, Italy.
References
- 1.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 2.Retini C, Vecchiarelli A, Monari C, Tascini C, Bistoni F, Kozel TR. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect Immun. 1996;64:2897–903. doi: 10.1128/iai.64.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romani L, Mencacci A, Cenci E, et al. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–56. [PubMed] [Google Scholar]
- 4.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 6.D'Andrea A, Rengaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (NKSF/IL-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy PM, Lane HC, Fanci AS, Galli JL. Impairment of neutrophil bactericidal capacity in patients with AIDS. J Infect Dis. 1988;158:627–30. doi: 10.1093/infdis/158.3.627. [DOI] [PubMed] [Google Scholar]
- 8.Hidore MR, Nabavi N, Sonleitner F, Murphy JW. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991;59:1747–54. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MF, Mitchell TG. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991;59:24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozel TR, McGaw TG. Opsonization of Cryptococcus neoformans by human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect Immun. 1979;25:255–8. doi: 10.1128/iai.25.1.255-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall A, Sharff MD. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med. 1991;174:151–60. doi: 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–8. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vecchiarelli A, Dottorini M, Beccari T, Cociani C, Todisco T, Bistoni F. Inhibition of candidacidal activity of polymorphonuclear cells by alveolar macrophage-derived factor from lung cancer patients. Am Rev Respir Dis. 1993;147:414–9. doi: 10.1164/ajrccm/147.2.414. [DOI] [PubMed] [Google Scholar]
- 14.Vecchiarelli A, Dottorini M, Pietrella D, Monari C, Retini C, Todisco T, Bistoni F. Role of human alveolar macrophages as antigen-presenting cells in Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 1994;11:130–7. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- 15.Pitrak DL, Bak PM, De Marais P, Novak RM, Andersen BR. Depressed neutrophil superoxide production in human immunodeficiency virus infection. J Infect Dis. 1993;167:1406–11. doi: 10.1093/infdis/167.6.1406. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Ivanova A, Serebrovskaya L, Shepeleva GK, Pokrovsky V. Clinical significance of neutrophil functional activity in HIV infection. Scand J Infect Dis. 1994;26:41–47. doi: 10.3109/00365549409008589. [DOI] [PubMed] [Google Scholar]
- 17.Roilides E, Holmes A, Blake C, Pizzo PA, Walsh TJ. Impairment of neutrophil antifungal activity against hyphae of Aspergillus fumigatus in children infected with human immunodeficiency virus. J Infect Dis. 1993;167:905–11. doi: 10.1093/infdis/167.4.905. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee J, Feldmesser M, Scharff MD, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan enhance fluconazole activity. Antimicrob Agents Chemother. 1995;39:1398–405. doi: 10.1128/aac.39.7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee J, Zukier LS, Scharff MD, Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994;38:580–7. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vecchiarelli A, Monari C, Baldelli F, Pietrella D, Retini C, Tascini C, Francisci D, Bistoni F. Beneficial effect of recombinant human granulocyte colony-stimulating factor on fungicidal activity of polymorphonuclear leukocytes from patients with AIDS. J Infect Dis. 1995;171:1448–54. doi: 10.1093/infdis/171.6.1448. [DOI] [PubMed] [Google Scholar]
- 21.Netea MG, Drenth JPH, De Bont N, Hijmans A, Keuter M, Dharmana E, Demacker PNM, van der Meer JWM. A semi-quantitative reverse transcriptase polymerase chain reaction method for measurement of mRNA for TNF-α and IL-1β in whole blood cultures: its application in typhoid fever and exentric exercise. Cytokine. 1996;8:739–44. doi: 10.1006/cyto.1996.0098. [DOI] [PubMed] [Google Scholar]
- 22.Retini C, Casadevall A, Pietrella D, Monari C, Palazzetti B, Vecchiarelli A. Specific activated T cells regulate IL-12 production by human monocytes stimulated with Cryptococcus neoformans. J Immunol. 1999;162:1618–23. [PubMed] [Google Scholar]
- 23.Romani L, Bistoni F, Puccetti P. Initiation of T-helper cell immunity to Candida albicans by IL-12: the role of neutrophils. Chem Immunol. 1997;68:110–35. doi: 10.1159/000058688. [DOI] [PubMed] [Google Scholar]
- 24.Reglier H, Arce-Vicioso M, Fay M, Gourgerot-Porcidalo MA, Chollet-Martin S. Lack of IL-10 and IL-13 production by human polymorphonuclear neutrophils. Cytokine. 1998;10:192–8. doi: 10.1006/cyto.1997.0272. [DOI] [PubMed] [Google Scholar]
- 25.Monari C, Casadevall A, Retini C, Baldelli F, Bistoni F, Vecchiarelli A. Antibody to capsular polysaccharide enhances the function of neutrophils from patients with AIDS against Cryptococcus neoformans. AIDS. 1999;13:653–60. doi: 10.1097/00002030-199904160-00005. [DOI] [PubMed] [Google Scholar]
- 26.Monari C, Casadevall A, Pietrella D, Bistoni F, Vecchiarelli A. Neutrophils from patients with advanced HIV infection have impaired complement receptor function and preserved Fcγ receptor function. J Infect Dis. 1999;180:1542–9. doi: 10.1086/315099. [DOI] [PubMed] [Google Scholar]
- 27.De la Salle H, Galon J, Bausinger H, et al. Soluble CD16/FcγRIII induces maturation of dendritic cells and production of several cytokines including IL-12. Adv Exp Med Biol. 1997;417:345–52. doi: 10.1007/978-1-4757-9966-8_56. [DOI] [PubMed] [Google Scholar]
- 28.Vecchiarelli A, Retini C, Casadevall A, Monari C, Pietrella D, Kozel TR. Involvement of C3a and C5a in interleukin-8 secretion by human polymorphonuclear cells in response to capsular material of Cryptococcus neoformans. Infect Immun. 1998;66:4324–30. doi: 10.1128/iai.66.9.4324-4330.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romani L, Puccetti P, Bistoni F. Interleukin 12 in infectious diseases. Clin Microbiol Rev. 1997;10:611–36. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–9. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 31.Coutelier JP, Van Broeck J, Wolf SF. Interleukin-12 gene expression after viral infection in the mouse. J Virol. 1995;69:1955–8. doi: 10.1128/jvi.69.3.1955-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sartori A, Ma X, Gri GM, Showe L, Benjamin D, Trinchieri G. Interleukin-12: an immunoregulatory cytokine produced by B cells and antigen-presenting cells. Methods. 1997;11:116–27. doi: 10.1006/meth.1996.0395. [DOI] [PubMed] [Google Scholar]
- 33.Okamura H, Tsutsi H, Komatsu M, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 34.Chougnet C, Wynn TA, Clerici M, et al. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 35.Chehimi J, Starr SE, Frank I, D'Andrea A, Ma X, MacGregor RR, Sennelier J, Trinchieri G. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–6. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanham G, Penne L, Devalck J, Kestens L, Colebunders R, Bosmans E, Thielemans K, Ceuppens JL. Decreased CD40 ligand induction in CD4 T cells and dysregulated IL-12 production during HIV infection. Clin Exp Immunol. 1999;117:335–42. doi: 10.1046/j.1365-2249.1999.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]