Introduction
Bare lymphocyte syndrome (BLS) is characterized by a severe down-regulation of HLA class I and/or class II molecules. In type 1 BLS the defect is confined to HLA class I molecules, while in type 2 BLS HLA class II molecules are down-regulated [1]. Characterization of 22 patients with type 1 BLS over the last 22 years has revealed the existence of several clinically and immunologically distinct disease subsets [1–20]. In this review we will focus on a recently characterized group of patients with a distinct disease phenotype due to a defective TAP complex, the peptide transporter complex associated with antigen presentation [2–15]. We will describe clinical manifestations and immunological findings of patients suffering from TAP deficiency syndrome, and discuss the differential diagnosis and therapeutic options.
HLA CLASS I ASSEMBLY and THE ROLE OF THE TAP COMPLEX
HLA class I molecules are highly polymorphic transmembrane glycoproteins expressed to variable levels on the surface of all nucleated cells in the body. HLA class I molecules have the dual role of presenting intracellular antigenic peptides to cytotoxic T lymphocytes (CTL), and modulating the activity of cells bearing HLA class I binding receptors, such as natural killer (NK) cells and γδ T cells [21–24] (Fig. 1). Failure to express HLA class I molecules on the surface of malignant or virus-infected cells sensitizes them for lysis by NK and γδ T cells [25]. Furthermore, presentation of self-peptides via HLA class I molecules is critical for the selection of cytotoxic T cells in the thymus [26].
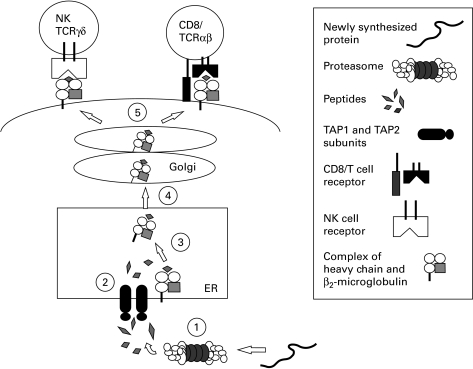
Fig. 1.
1, Proteasome-dependent generation of antigenic peptides from newly synthesized proteins; 2, translocation of peptides from the cytosol into the endoplasmic reticulum (ER) by the transporter associated with antigen presentation (TAP); 3, loading of peptides onto MHC class I/β2-microglobulin (β2-M) complexes; 4, peptide/MHC class I/β2-M complexes leave the ER and enter the Golgi apparatus, where they undergo maturation; 5, expression of peptide/MHC class I/β2-M complexes on the cell surface. Interaction of class I molecules with either NK cells, γδ T cells (TCRγδ) or CD8+ CTL (CD8/TCRS) via NK receptors or the T cell receptor.
During biosynthesis, HLA class I molecules associate with 8–11 amino acid long peptides and β2-microglobulin (β2-M) within the lumen of the endoplasmic reticulum (ER) (as reviewed in [−2627]). Different HLA class I alleles bind to different sets of peptides, most of which are derived from the degradation of cytosolic proteins by a large proteolytic complex called the proteasome. Translocation of peptides derived from degradation of cytosolic proteins into the ER is mediated by the transporter associated with antigen presentation (TAP), a member of the ATP-binding cassette (ABC) superfamily of transporters. TAP is a heterodimer and consists of two subunits, TAP1 and TAP2, which are encoded in the class II region of the HLA locus on chromosome 6. Deletion or mutation of either or both TAP1 and TAP2 proteins severely impairs the translocation of peptides into the ER. The role of TAP1 and TAP2 in peptide presentation is shown in Fig. 1.
Before binding peptides, newly synthesized HLA class I molecules are retained in the ER through interactions with the molecular chaperones calnexin, calreticulin, and ERp57, as well as with the glycoprotein tapasin [28–31]. In the absence of a peptide ligand, the HLA class I/β2-M complex is unstable, resulting in dissociation of β2-M and unfolding of HLA class I molecules [32,33]. Hence, any defect which impairs the translocation of peptides into the ER results in reduced surface expression of HLA class I molecules. Patients with a defective HLA class I presentation pathway can be identified by measuring HLA class I surface expression on peripheral blood mononuclear cells (PBMC) by fluorescence-activated cell sorter analysis (FACS) [2].
Clinically distinct groups of type 1 bls
Since the first report by Touraine in 1978, 22 patients with type 1 BLS have been identified worldwide [1–20]. Three groups of patients can be differentiated on clinical and immunological grounds:
• Group 1. This group includes patients with the most severe disease phenotype with recurrent severe bacterial, fungal and parasitic infections from the age of 4–5 months of life. Only four patients have been described so far, all of whom died within the first 3 years of life from infectious complications [1,17,18]. Besides a severe down-regulation of HLA class I and β2-M on the cell surface, complete lack of antibody production was demonstrated in these patients. Defective transcription of β2-M and HLA class I was ruled out, as both proteins were detectable in the patients' serum. Since patients were heterozygous for the HLA haplotype, it is unlikely that the gene(s) responsible for the HLA class I down-regulation was encoded within the HLA locus.
• Group 2. Patients in this group of type 1 BLS are completely asymptomatic. One family with two affected children has been described so far [19]. Down-regulation of HLA class I and β2-M surface expression was the only observable immunological finding. The two siblings had different heterozygous HLA haplotypes. Northern blot analysis demonstrated a decrease in the mRNA of HLA class I and β2-M, consistent with a transcriptional defect [20].
• Group 3. This is the best characterized subgroup of type 1 BLS, comprising so far 15 patients, in which reduced HLA class I surface expression is associated with recurrent bacterial infections and necrotizing granulomatous skin lesions [2–15]. In contrast to Group 1, all these patients survived into adulthood. Tissue typing of 10 patients demonstrated that they were homozygous for the HLA locus. Further analysis of these 10 patients revealed that a defective TAP complex was responsible for the down-regulation of HLA class I surface expression [2–15]. Five additional adult patients with recurrent infections, but without noticeable skin lesions, were described in 1980 [16,17]. However, the underlying defect in these patients could not be identified at that time.
In the discussion to follow, we refer to the condition of BLS patients in which defects in TAP have been identified as TAP deficiency syndrome.
Clinical presentation of tap deficiency syndrome
To date TAP deficiency syndrome has been diagnosed in 10 patients from seven different families world wide. In six patients a defective TAP1 expression was demonstrated [2,15], while in four patients TAP2 was defective [2–4]. Patients with either TAP1 or TAP2 deficiency had identical clinical manifestations [2,5,9,14]. Clinical symptoms are summarized in Table 1.
Table 1.
Clinical manifestations in 10 patients with TAP deficiency syndrome
| Ear/nose/throat (10/10): |
| Chronic sinusitis |
| Nasal disease (discharge, polyps, septum ulcers) |
| Postnasal drip syndrome |
| Otitis media |
| Mastoiditis |
| Erosion/destruction of facial tissues around the nose |
| Lungs (8/10): |
| Chronic spastic bronchitis |
| Recurrent bacterial pneumonia |
| Bronchiectasis |
| Skin (7/10): |
| Necrotizing granulomatous skin lesions (7/7): |
| Brownish nodular or plaque-like dermal infiltration, on extremities and midface, often ulceration and scar formation; often asymmetrical |
| Leucocytoclastic vasculitisLeucocytoclastic vasculitis (2/7)*: |
| Symmetrical purpura on arms and legs |
| Nervous system (2/10): |
| Cerebral abscess |
| Encephalomyelitis |
| Gastrointestinal tract (2/10): |
| Chronic gastritis |
| Pseudomembranous colitis |
| Other organs (2/10): |
| Non-erosive symmetrical polyarthritis (2/10)* |
| Retinal vasculitis (1/10) |
Simultaneous appearance of polyarthritis and purpura in two patients was clinically interpreted as hypersensitivity vasculitis due to either infection or drugs.
The disease usually manifests within the first 6 years of life with recurrent bacterial infections of the upper respiratory tract, i.e. chronic purulent rhinitis often complicated by nasal septum perforation and nasal polyps, sinusitis and otitis media (unpublished observations and [3,4]). Involvement of the lower respiratory tract typically manifests in the second decade of life with recurrent spastic bronchitis, bacterial pneumonia and eventually bronchiectasis. Isolated pathogens from the respiratory tract commonly include Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella species, Escherichia coli and Pseudomonas aeruginosa, the latter two associated with chronic stages of the disease. Severe viral infections are noticeably absent and normal antibody titres against several viruses could be demonstrated in most patients. Postnasal drip syndrome, which can usually be found in patients with TAP deficiency syndrome, probably promotes bacterial lung infections, especially in those patients who have undergone surgery of the paranasal sinus. Interestingly, histology of the paranasal sinus and nasal polyps may reveal a necrotizing granulomatous inflammation with a close resemblance to Wegener's granulomatosis (WG) ([2], see also [33,34]), while granulomata could not been demonstrated in biopsies from lung and bronchi (unpublished observations).
Before the appearance of bronchiectasis, the erythrocyte sedimentation rate (ESR) and C-reactive protein level, which rise to high levels during acute infections, normalize after the infection has been cleared. Once bronchiectasis is established, ESR levels remain elevated. Similarly, polyclonal hypergammaglobulinaemia develops in most patients in the later stages of the disease (unpublished observations). Progressive lung damage in TAP deficiency syndrome, due to recurrent bacterial infections, may ultimately lead to respiratory failure and death. However, one patient did not suffer from recurrent infections [2].
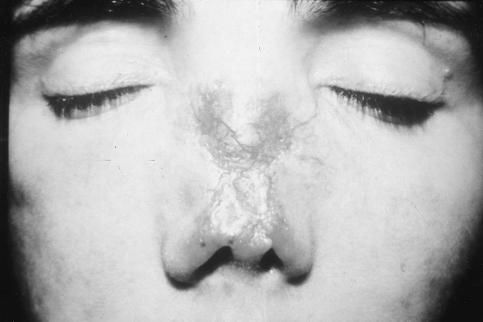
The most striking clinical manifestation in seven out of 10 TAP-deficient patients is necrotizing granulomatous skin lesions, typically located on the extremities and in the midface (Fig. 2). In six out of seven patients these skin lesions appeared only after the age of 15 years. The only patient who developed skin lesions in early childhood did not suffer from recurrent respiratory infections [2]. Typically, a small pustule or a subcutaneous nodule on the lower extremity or around the nostrils slowly expands and finally ulcerates. The lesions heal very slowly, often with the formation of hyperpigmented scars, and they may lead to severe mutilation, especially in the midface, resembling lethal midline granuloma [35]. Three patients without skin lesions, including the sister of a patient with multiple skin ulcers on both legs, all suffered from severe respiratory symptoms.
Fig. 2.
Necrotizing granulomatous skin lesions.
Infection of the nervous system occurred in two patients under treatment with immunosuppressive drugs for suspected WG (unpublished observations). Recurrent leucocytoclastic skin vasculitis and polyarthritis in two patients with prominent bronchiectasis and enhanced circulating immune complexes were interpreted to reflect secondary hypersensitivity vasculitis [36] associated with chronic infection (unpublished observations).
Differential diagnosis
Patients with TAP deficiency syndrome are typically admitted to hospital either because of their respiratory disease, or their skin lesions, or a combination of both symptoms. Table 2 shows the differential diagnosis that may be considered in the course of the disease. Few other systemic diseases present with a combination of granulomatous skin lesions and chronic bacterial infection of the respiratory tract. Some of the primary immunodeficiency syndromes, e.g. chronic granulomatous disease (CGD) and common variable immunodeficiency (CVID) may present with bronchiectasis and granulomatous disease, but they can be easily differentiated from TAP deficiency syndrome by phagocyte function tests, serum electrophoresis and FACS analysis for HLA class I [37–40].
Table 2.
Differential diagnosis
| Systemic diseases: |
| Wegener's granulomatosis |
| Sarcoidosis |
| Midline granuloma |
| Chronic granulomatous disease |
| Common variable immunodeficiency |
| X-linked infantile hypogammaglobulinaemia [47] |
| Ataxia telangiectasia [48] |
| Cystic fibrosis |
| Primary ciliary disorders |
| Prolidase deficiency |
| Mycobacteriosis |
| Leishmaniasis |
| Nocardiosis |
| α1-antitrypsin deficiency |
| Aspergillosis |
| Dermatologic conditions: |
| Necrobiosis lipoidica |
| Granuloma anulare perforans |
| Pyoderma gangrenosum |
Systemic diseases other than immunodeficiency that may present with necrotizing granulomatous skin lesions and chronic inflammation of the upper respiratory tract include lethal midline granuloma (LMG) [35] and WG [34,41,42], the latter also typically involving the lungs and kidneys. However, bronchiectasis is a feature neither of LMG nor of WG. Moreover, the combination of young age of onset, positive family history, lack of glomerulonephritis, absent PR3–c-ANCA and low HLA class I expression helps to exclude these potentially rapidly fatal diseases and to avoid harmful immunosuppressive therapy.
Chronic sarcoidosis also may present with granulomatous skin lesions and bronchiectasis, but ulceration and scar formation of the skin lesions, lack of response to glucocorticoid therapy and low HLA class I expression differentiate TAP deficiency syndrome from sarcoidosis. Moreover, granulomatous inflammation of the lungs has not yet been demonstrated in any of the TAP-deficient patients, but it is found in more than 80% of lung biopsies from patients with chronic sarcoidosis [43].
The differential diagnosis of chronic granulomatosis also includes a long list of pathogens, especially mycobacteria. Although skin tests with purified tuberculin have been positive in some of our patients, the presence of mycobacteria, fungi, leishmania and other pathogens was never demonstrated in the blood or lesional tissue by different techniques including polymerase chain reaction (PCR). Moreover, long-term survival from systemic mycobacteriosis or mycosis on a TAP-deficient background also seems unlikely. Besides, since mycobacterial infection in TAP-deficient mice is rapidly lethal [44].
Finally, the involvement of the respiratory tract in TAP deficiency is indistinguishable from two other autosomal recessive disorders, namely cystic fibrosis and primary ciliary dyskinesia [45,46]. Sweat chloride concentration is normal in TAP deficiency, excluding cystic fibrosis, while electron microscopy of the ciliary apparatus may be needed to exclude primary ciliary dysfunction. TAP deficiency is not associated with infertility.
IMMUNOLOGICAL FINDINGS and IMPLICATIONS ON DISEASE MECHANISMS
Lack of expression of either one of the two TAP1 and TAP2 subunits has been demonstrated to account for the failure of the TAP-heterodimer in all patients so far characterized. In six patients, the underlying genetic defect was shown to involve the generation of a premature STOP codon [2,4,12,15]. Analysis of the patients' lymphocyte repertoire in the peripheral blood revealed an expansion of NK and γδ T cells in most patients [2], while CD8+ αβ T cells were present in low numbers [4]. The activity of NK and γδ T cells was further analysed in several patients, because the cytolytic activity of these cells is negatively modulated by inhibitory HLA class I receptors [22,23]. Autoreactive NK and γδ T cells were demonstrated in four patients [2,6]. In two of these patients, activated NK cells were also found in the skin lesions [2]. The pathogenesis of the granulomatous skin lesions may therefore involve activated NK and γδ T cells, which are both capable of promoting a Th1-type inflammatory response leading to granuloma formation [49]. On the other hand, these cell types may also account to some extent for the lack of severe viral infections, as both NK and γδ T cells can recognize virally infected cells without need for TAP-dependent antigen processing [50,51]. The physiologic importance of the residual population of CD8+ αβ T cells in these TAP-deficient patients is currently under investigation. Evidence for TAP-independent selection of CD8+ αβ T cells comes from the isolation of a cytotoxic CD8+ αβ T cell clone recognizing the Epstein–Barr virus (EBV) protein LMP2 presented by HLA-B molecules in one TAP-deficient patient [52].
Therapeutic approach to tap-deficient patients
The major objectives of therapy for patients with TAP deficiency syndrome are early recognition, aggressive treatment of respiratory infections and prevention of bronchiectasis. In this respect, treatment guidelines are analogous to those in cystic fibrosis [45], with prudent use of antibiotics and chest physiotherapy. For treatment of severe bouts of pneumonia addition of intravenous immunoglobulins to the antibiotic regimen seemed to be useful in some patients. On the other hand, surgical intervention for chronic sinusitis should be avoided in these patients, as this seems to promote progression of nasal disease and postnasal drip syndrome leading to bronchial infection (unpublished observations)
The treatment of skin ulcers in TAP-deficient patients is based on good topical care and involves cleansing, for instance with saline or benzoyl peroxide, to gently debride the ulcers and decrease bacterial colonization. In addition, photochemotherapy with psoralen and UV-A had a positive transient effect in two patients (unpublished observations).
Immunosuppressive therapy in two patients, consisting of steroids in combination with either cyclophosphamide, methotrexate, azathioprine or cyclosporin, was associated with progression of both skin lesions and pulmonary disease and is contraindicated in these patients. Immunomodulatory treatment with interferon-alpha (IFN-α) in two patients and IFN-γin one patient were also associated with progression of the skin lesions and distressing systemic side-effects, e.g. severe fatigue and malaise (unpublished observations).
None of the TAP-deficient patients has yet received an allogeneic organ transplant, e.g. lung or bone marrow. The success of such a therapy cannot be predicted from our current understanding of the disease. Lung transplantation might be beneficial, if lung tissue damage was primarily mediated by activated NK or γδ T cells bearing inhibitory receptors for HLA class I. In contrast, bone marrow transplantation may lead to a severe graft-versus-host disease (GVHD) due to the presence of donor's NK cells. Gene therapy may not be a feasible possibility in TAP deficiency syndrome due to the ubiquitous tissue distribution of HLA class I molecules.
Conclusion
Type 1 BLS comprises a group of aetiologically different and clinically heterogeneous primary immunodeficiencies, of which TAP deficiency syndrome is the best characterized. Typical disease manifestations of TAP deficiency syndrome include recurrent bacterial infections of the respiratory tract and chronic granulomatous skin lesions. Severe complications of the disease are the development of bronchiectasis and respiratory failure, complete destruction of the nose and cerebral abscess. Pathogenesis of the skin lesions has been shown to involve cells bearing inhibitory HLA class I receptors, i.e. NK and γδ T cells, in two patients. Progression of the skin lesions under treatment with either interferons or immunosuppressive drugs is consistent with a disease model in which the skin lesions are mediated by NK and/or γδ T cells after prior activation of these cells via an inflammatory response to pathogen. The primary objective of therapy for patients with TAP deficiency syndrome is tight control of infections in order to prevent the development or progression of bronchiectasis and ultimately respiratory failure.
Acknowledgments
This work was funded by the Medical Research Council of Great Britain. S.D.G. is supported by grants of the Swiss National Science Foundation, Novartis Foundation, and Roche Research Foundation.
References
- 1.Touraine JL, Betuel H, Souillet G, Jeune M. Combined immunodeficiency disease associated with absence of cell surface HLA-A and B antigens. J Pediatr. 1978;93:47–51. doi: 10.1016/s0022-3476(78)80598-8. [DOI] [PubMed] [Google Scholar]
- 2.Moins-Teisserenc HT, Gadola SD, Cella M, et al. Association of a syndrome resembling Wegener' granulomatosis with low surface expression of HLA class I-molecules. Lancet. 1999;354:1598–603. doi: 10.1016/s0140-6736(99)04206-3. [DOI] [PubMed] [Google Scholar]
- 3.Teisserenc H, Schmitt W, Blake N, Dunbar R, Gadola S, Gross WL, Exley A, Cerundolo V. A case of primary immunodeficiency due to a defect of the major histocompatibility gene complex class I processing and presentation pathway. Immunol Letters. 1997;57:183–7. doi: 10.1016/s0165-2478(97)00072-2. [DOI] [PubMed] [Google Scholar]
- 4.De la Salle H, Hanau D, Fricker D, et al. Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science. 1994;265:237–41. doi: 10.1126/science.7517574. [DOI] [PubMed] [Google Scholar]
- 5.Donato L, De la Salle H, Hanau D, Tongio MM, Oswald M, Vandevenne A, Geisert J. Association of HLA class I antigen deficiency related to a TAP2 gene mutation with familial bronchiectasis. J Pediatr. 1995;127:895–900. doi: 10.1016/s0022-3476(95)70024-2. [DOI] [PubMed] [Google Scholar]
- 6.Zimmer J, Donato L, Hanau D, Cazenave JP, Tongio MM, Moretta A, De la Salle H. Activity and phenotype of natural killer cells in peptide transporter (TAP)-deficient patients (type 1 bare lymphocyte syndrome) J Exp Med. 1998;187:117–22. doi: 10.1084/jem.187.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmer J, Donato L, Hanau D, Cazenave JP, Moretta A, Tongio MM, De la Salle H. Inefficient protection of human TAP-deficient fibroblasts from autologous NK cell-mediated lysis by cytokines inducing HLA class I expression. Eur J Immunol. 1999;29:1286–91. doi: 10.1002/(SICI)1521-4141(199904)29:04<1286::AID-IMMU1286>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Maeda H, Hirata R, Chen RF, Suzaki H, Kudoh S, Tohyama H. Defective expression of HLA class I antigens: a case of the bare lymphocyte syndrome without immunodeficiency. Immunogenetics. 1985;21:549–58. doi: 10.1007/BF00395879. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe S, Iwata M, Maeda H, Ishibashi Y. Immunohistochemical studies of major histocompatibility antigens in a case of the bare lymphocyte syndrome without immunodeficiency. J Am Acad Dermatol. 1987;17:895–902. doi: 10.1016/s0190-9622(87)70277-1. [DOI] [PubMed] [Google Scholar]
- 10.Sugiyama Y, Mada H, Okumura K, Takaku F. Progressive sinobronchiectasis associated with the ‘bare lymphocyte syndrome’ in an adult. Chest. 1986;89:398–401. doi: 10.1378/chest.89.3.398. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa H, Yabe T, Watanabe K, et al. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type 1) Human Immunol. 1999;60:32–40. doi: 10.1016/s0198-8859(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa H, Murata S, Yabe T, et al. Splice acceptor site mutation of the transporter associated with antigen processing-1 gene in human bare lymphocyte syndrome. J Clin Invest. 1999;103:649–52. doi: 10.1172/JCI5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa H, Yabe T, Akaza T, et al. Cell surface expression of HLA-E molecules on PBMC from a TAP1-deficient patient. Tissue Antigens. 1999;53:292–5. doi: 10.1034/j.1399-0039.1999.530310.x. [DOI] [PubMed] [Google Scholar]
- 14.Plebani A, Monafo V, Cattaneo R, et al. Defective expression of HLA class I and CD1a molecules in a boy with Marfan-like phenotype and deep skin ulcers. J Am Acad Dermatol. 1996;35:814–28. doi: 10.1016/s0190-9622(96)90091-2. [DOI] [PubMed] [Google Scholar]
- 15.De la Salle H, Zimmer J, Fricker D, et al. HLA class I deficiencies due to mutations in subunit 1 of the peptide transporter TAP1. J Clin Invest. 1999;103:R9–13. doi: 10.1172/JCI5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepage V, Colombani J, Hors J, et al. Decrease of HLA antigen expression in two cases of combined immunodeficiency (CID) Abstract 14.2.15 of the Fourth International Congress of Immunology, Paris. [Google Scholar]
- 17.Touraine JL. The bare lymphocyte syndrome: report on the registry. Lancet. 1981;1:319–21. doi: 10.1016/s0140-6736(81)91922-x. [DOI] [PubMed] [Google Scholar]
- 18.Schuurman RKB, Van Rood JJ, Vossen JM, Schellekens PTA, Feltkamp-Vroom TM, Doyer E, Gmelig-Meyling F, Visser HKA. Failure of lymphocyte-membrane HLA-A and -B expression in two siblings with combined immunodeficiency. Clin Immunol Immunopathol. 1979;14:418–34. doi: 10.1016/0090-1229(79)90094-1. [DOI] [PubMed] [Google Scholar]
- 19.Payne R, Brodsky F, Peterlin BM, Young LM. Bare lymphocytes without immunodeficiency. Hum Immunol. 1983;6:219–27. doi: 10.1016/0198-8859(83)90095-2. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan KE, Stobo JD, Peterlin BM. Molecular analysis of the bare lymphocyte syndrome. J Clin Invest. 1985;76:75–79. doi: 10.1172/JCI111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–58. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 22.Colonna M. Specificity and function of immunoglobulin superfamily NK cell inhibitory and stimulatory receptors. Immunol Rev. 1997;155:127–33. doi: 10.1111/j.1600-065x.1997.tb00945.x. [DOI] [PubMed] [Google Scholar]
- 23.Lanier LL, Phillips JH. Inhibitory MHC class I receptors on NK cells and T cells. Immunol Today. 1996;17:86–91. doi: 10.1016/0167-5699(96)80585-8. [DOI] [PubMed] [Google Scholar]
- 24.Braud VM, Allan DSJ, O'Callaghan CAO, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–9. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 25.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 26.Kisielow P, Teh HS, Blutmann H, von Boehmer H. Positive selection of antigen specific T cells in the thymus by restricting MHC molecules. Nature. 1988;335:730–3. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 27.Cerundolo V, Braud V, Browning M. In: McMichael, editor. Oxford: Bios Scientific Publisher Ltd; 1996. pp. 193–223. HLA and MHC genes, molecules, and function. [Google Scholar]
- 28.Williams DB, Watts TH. Molecular chaperones in antigen presentation. Curr Opin Immunol. 1995;7:77–84. doi: 10.1016/0952-7915(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 29.Hughes EA, Cresswell P. The thiol oxidoreductase ERp57 is a component of the MHC class I peptide-loading complex. Curr Biol. 1998;8:709–12. doi: 10.1016/s0960-9822(98)70278-7. [DOI] [PubMed] [Google Scholar]
- 30.Morrice NA, Powis SJ. A role for the thiol-dependent reductase ERp57 in the assembly of MHC class I molecules. Curr Biol. 1998;8:713–6. doi: 10.1016/s0960-9822(98)70279-9. [DOI] [PubMed] [Google Scholar]
- 31.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class molecules with TAP. Immunity. 1996;5:103–14. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 32.Townsend A, Ohlen C, Bastin J, Ljunggren HG, Foster L, Karre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340:443–8. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 33.Cerundolo V, Alexander J, Anderson K, Lamb C, Cresswell P, McMichael A, Gotch F, Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990;345:449–52. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- 34.Gross WL. Wegener' granulomatosis. In: Madison PJ, Isenberg DA, Woo P, Glass DN, editors. Oxford textbook of rheumatology. Vol. 2. Oxford: Oxford University Press; 1998. pp. 1331–51. [Google Scholar]
- 35.Wolff SM. Midline granuloma. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DL, editors. Harrisons' principles of internal medicine. 13. Vol. 294. New York: McGraw-Hill; 1994. pp. 1686–7. [Google Scholar]
- 36.Martinez-Taboada VM, Blanco R, Garcia-Fuentes M, Rodriguez-Valverde V. Clinical features and outcome of 95 patients with hypersensitivity vasculitis. Am J Med. 1997;102:186–91. doi: 10.1016/s0002-9343(96)00405-6. [DOI] [PubMed] [Google Scholar]
- 37.Curnutte JT, Babior BM. Chronic granulomatous disease, a heterogeneous syndrome. Adv Hum Genet. 1986;19:229–97. doi: 10.1007/978-1-4757-0620-8_4. [DOI] [PubMed] [Google Scholar]
- 38.Liese JG, Jendrossek V, Jansson A, Petropoulou T, Kloos S, Gahr M, Belohradsky H. Chronic granulomatous disease in adults. Lancet. 1995;346:220–3. doi: 10.1016/s0140-6736(96)90403-1. [DOI] [PubMed] [Google Scholar]
- 39.Mechanic LJ, Dikman S, Cunningham-Rundles C. pp. 613–7. Granulomatous disease in common variable immunodeficiency. Ann Intern Med 127: [DOI] [PubMed]
- 40.Cunningham-Rundles C. Clinical and immunologic analyses of 103 patients with common variable immunodeficiency. J Clin Immunol. 1989;9:22–23. doi: 10.1007/BF00917124. [DOI] [PubMed] [Google Scholar]
- 41.Fauci AS, Haynes BF, Kath P, Wolff S. Wegener' granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci A. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 43.DeRemee RA. Sarcoidosis. Mayo Clin Proc. 1995;70:177–81. doi: 10.4065/70.2.177. [DOI] [PubMed] [Google Scholar]
- 44.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–80. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boucher RC. Cystic fibrosis. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DL, editors. Harrisons' principles of internal medicine. 13. Vol. 222. New York: McGraw-Hill; 1994. pp. 1194–7. [Google Scholar]
- 46.Weinberger SE. Bronchiectasis and broncholithiasis. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DL, editors. Harrisons' principles of internal medicine. 13. Vol. 221. New York: McGraw-Hill; 1994. pp. 1191–4. [Google Scholar]
- 47.Fleming MC, Gewurz AT, Pearson RW. Caseating cutaneous granulomas in a patient with X-linked infantile hypogammaglobulinemia. J Am Acad Dermatol. 1991;24:629–33. doi: 10.1016/0190-9622(91)70097-l. [DOI] [PubMed] [Google Scholar]
- 48.Drolet BA, Drolet B, Zvulunov A, Jacobsen R, Troy J, Esterly NB. Cutaneous granulomas as a presenting sign in ataxia-telangiectasia. Dermatology. 1997;194:273–5. doi: 10.1159/000246117. [DOI] [PubMed] [Google Scholar]
- 49.Takashima T, Ihnishi K, Tsuyuguchi I, Kishimoto S. Differential regulation of formation of multinucleated giant cells from concanavalin A-stimulated human blood monocytes by IFN-gamma and IL-4. J Immunol. 1993;150:3002–10. [PubMed] [Google Scholar]
- 50.Schild H, Mavaddat N, Litzenberger C, et al. The nature of major histocompatibility complex recognition by γδ T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 51.Tay CH, Szomolanyi-Tsuda E, Welsh RM. Control of infections by NK cells. Curr Top Microbiol Immunol. 1998;230:193–220. doi: 10.1007/978-3-642-46859-9_12. [DOI] [PubMed] [Google Scholar]
- 52.De la Salle H, Houssaint E, Peyrat MA, et al. Human peptide transporter deficiency: importance of HLA-B in the presentation of TAP-independent EBV antigens. J Immunol. 1997;158:4555–63. [PubMed] [Google Scholar]