Abstract
Fas-mediated apoptosis may be one of the effector pathways leading to the elimination of virus-infected cells. Cytomegalovirus (CMV) infection in two brothers with Fas deficiency associated with autoimmunity and benign lymphoproliferation (ALPS) provided a unique opportunity to study the clinical course of CMV infection in children with defective apoptosis. The clinical courses of two brothers with autosomal dominant ALPS who were infected with CMV in the neonatal period are described. CMV was detected from throat and urine culture from the brothers. ALPS was confirmed by in vitro anti-CD95 MoAb-induced T lymphocyte apoptosis assay and subsequent sequencing and identification of mutations in the Fas gene. A de novo mutation in the Fas gene, leading to a truncated cytoplasmic Fas product, was associated with autosomal dominant ALPS in a mother and her two sons. Both boys had evidence of CMV infection acquired early in infancy which cleared by the age of 2–3 years. There were no neurodevelopmental sequelae. The natural history of CMV infection in two infants with ALPS was similar to that described in normal children.
Keywords: Fas, apoptosis, ALPS, CMV
Introduction
Fas participates in the cellular apoptosis pathway. A defect in the Fas molecule on the surface of activated human T lymphocytes causes ALPS/Canale–Smith syndrome [1–3]. It is characterized by chronic benign lymphadenopathy, splenomegaly and often autoimmune phenomena, especially autoimmune cytopenias [4]. There is expansion of the CD3+TCRαβ+CD4−CD8− lymphocyte population in the lymphoid organs. The syndrome may be confused with T cell lymphoma [5].
Fas-mediated apoptosis is thought to be one of the immune effector pathways leading to the elimination of virus-infected cells, suggesting that lack of Fas or inhibition of Fas pathways by viral products may promote survival of these cells and viral infection [6–8]. Despite in vitro observations, there are no clinical data to suggest that patients with defects in their cellular apoptotic pathways (ALPS) have more severe or prolonged viral infections. Acquisition of cytomegalovirus (CMV) in infancy and early childhood either via breast feeding or at child care facilities is a common occurrence [9,10,11]. The usually benign clinical course of CMV infections in normal infants has been well described; this is the first study describing CMV infections in infants with ALPS.
PATIENTS and METHODS
Patients
All living relatives of the family were assessed for lymphoproliferative syndrome-associated splenomegaly, lymphadenopathy and autoimmunity. Old clinical notes and histology were reviewed where available.
Immunology
Serum immunoglobulin levels, autoantibody screening, peripheral blood lymphocyte subset phenotype determinations (including analyses for CD3+TCRαβ+CD4−CD8− lymphocytes) were determined by standard immune assays. In our laboratory the normal range for CD3+TCRαβ+CD4−CD8− lymphocytes is < 1%. Anti-CD95 monoclonal-induced T lymphocyte apoptosis was measured as previously described. Briefly, isolated peripheral blood mononuclear cells (PBMC) were activated for 6 days with phytohaemagglutinin (PHA) and IL-2 (Genzyme, Cambridge, MA; 20 U/ml), then cultured with Apo-1 MoAb (250 ng/ml) and a rat anti-mouse IgG (Jackson Labs, Westgrove, PA; 10 μg/ml) for 24 h. Apoptotic cells were quantified by resuspending cells in a hypotonic solution containing 0·1% sodium citrate, 0·1% Triton X-100 (Sigma, St Quentin Fallavier, France) and 50 μg/ml propidium iodide (Sigma). Red fluorescence was measured using a FACStar plus flow cytometer (Becton Dickinson, San Diego, CA). Apoptotic cells were counted as hypodiploid. Immunohistochemistry on paraffin-embedded tissue sections was performed using standard techniques with anti-CD3-, CD4- and CD-8 but not TCRαβ-specific MoAbs and an alkaline phosphatase-conjugated second antibody/nitro blue tetrazolium substrate detection system.
Detection of FAS mutation
DNA samples were prepared from activated lymphocytes using standard methods. Single-strand confirmation polymorphisms were assayed as described elsewhere [12]. Genomic DNA segments of 90–350 bp were amplified with primers as previously described. Amplified products were labelled by incorporation of a-dCTP32 (cold dNTPs:a-dCTP32 ratio of 1:10) and boiled single-stranded products were separated on MDE acrylamide gels (AT Biochem, Malvern, PA) and revealed by autoradiography. For cDNA analysis, reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as previously described using Fas-specific primers. Bi-directional sequencing was performed directly on PCR products using the Dye terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Foster City, CA). Evidence that both alleles were examined included the determination of the complete sequence of cDNA as well as the demonstration of previously described polymorphisms. For individuals with homozygous allotypes or for sequences which were unreadable due to insertions or deletions, PCR products were cloned into pGEM T easy vector (Promega, Madison, WI). Subsequently, six to 10 clones were sequenced.
Virology
Throat swabs and urine samples were inoculated to human embryonic fibroblast cells. Standard techniques were used for identification. Antibodies to CMV and Epstein–Barr virus (EBV) were measured using commercially available ELISA. In situ hybridization for detection of EBV and CMV mRNA was carried out using commercially prepared cocktails of FITC-conjugated oligonucleotide probes (Novocastra Laboratories Ltd, Peterborough, UK). Paraffin sections (5 μm) mounted on silane-treated slides were employed. Prior to hybridization, sections were pretreated with Proteinase K. Hybridization was detected using an alkaline phosphatase-conjugated anti-FITC antibody (Dako, Ely, UK) and nitro blue tetrazolium substrate (Sigma). Sections from known positive cases of the specific infections were employed as positive controls.
Results
Proband
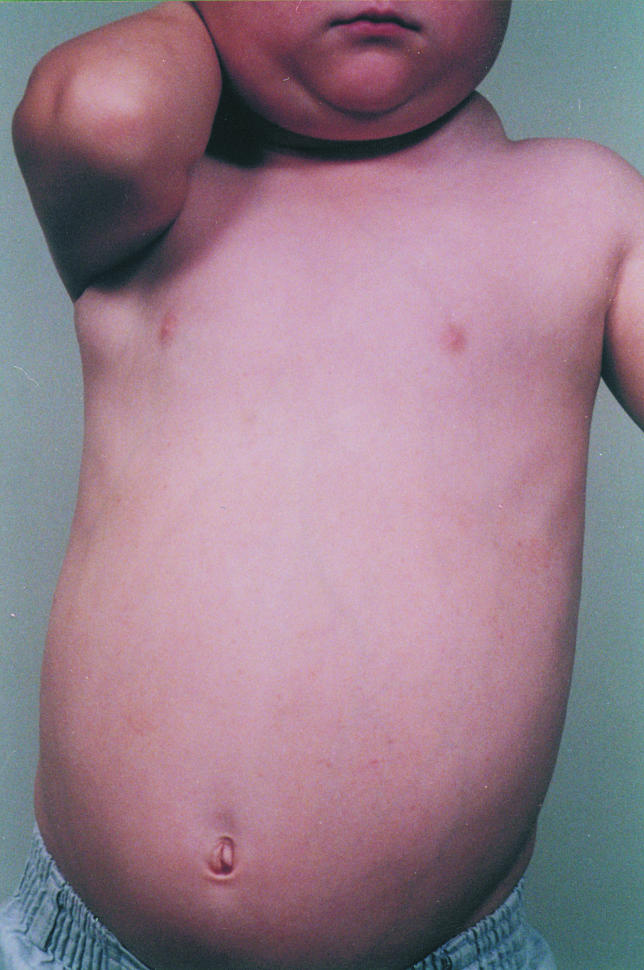
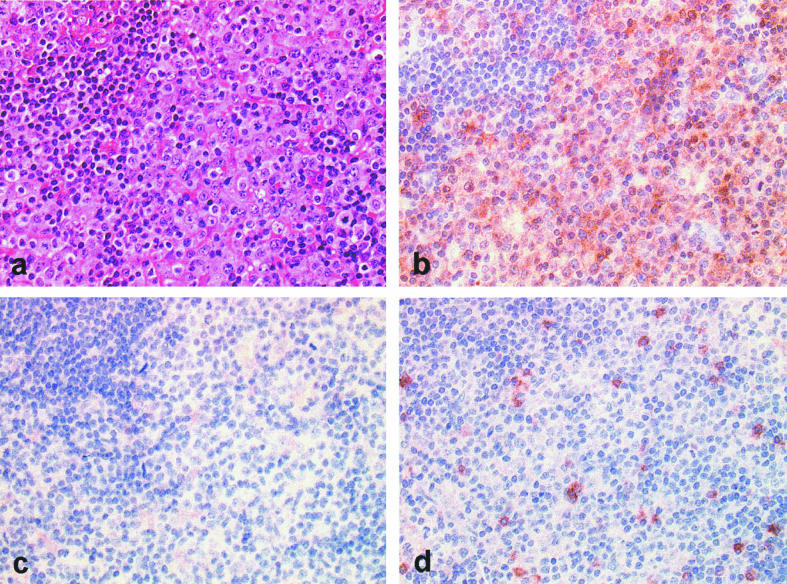
The proband was born to unrelated parents in 1984, after a normal pregnancy with no flu-like illnesses during or just after delivery. He was breast fed for 18 months and fully immunized without adverse effects. Splenomegaly was noted at birth and gross cervical lymphadenopathy from 6 months of age (Fig. 1). Cervical lymph node biopsy at 5 years suggested an indolent T cell lymphoma. Bone marrow aspirate at that time showed an excess of mature lymphocytes but no other abnormalities. Lymph node biopsy repeated at the age of 10 years showed a diffuse parafollicular infiltrate of CD3+CD4−CD8− T lymphocytes with some compression of the node architecture (Fig. 2) and in situ hybridization for EBV mRNA as well as CMV were negative. He is presently 15 years old and his lymphadenopathy has largely resolved, but 12 cm splenomegaly persists. His development has been entirely normal.
Fig. 1.
Proband at the age of 5 years.
Fig. 2.
Immunohistology of proband's lymph node biopsy performed at 10 years old. Stained with haematoxylin and eosin (a) and immunohistochemical labelling for CD3 (b), CD4 (c) and CD8 (d), showing expansion of CD3+CD4−CD8− T lymphocytes in the paracortex.
He chronically excreted CMV in his urine from presentation at 3 months until 3 years of age, when this resolved. At present CMV IgG is positive, but CMV IgM is negative and CMV EA is not detectable in urine or throat swabs and blood PCR is negative. EBV VCA and EBNA IgG have been positive from the age of 3 years, IgM is negative. He has not been troubled by any other infections.
Recent investigations are shown in Table 1. He has a mild lymphopenia and thrombocytopenia, which at the age of 5 years was associated with epistaxis and hypergammaglobulinaemia (first noted at the age of 6 months). No autoantibodies were detected and DCT was negative. Of T lymphocytes, 21% are CD3+TCRαβ+CD4−CD8− (‘double negative’). T cell proliferative responses to PHA, concanavalin A (Con A), phorbol myristate acetate (PMA) and ionophore, anti-CD3, and IL-2 are all normal. PCR analysis for TCR gene rearrangement was consistent with polyclonal lymphocyte expansion in blood (Vβ2 = 6·7%, Vβ5.2/3 = 8·1, Vβ22 = 7·7, Vβ9 = 0·7, Vβ11 = 0·5, Vβ12 = 0·8, other Vβs = 1·0–5·5%).
Table 1.
Laboratory results
| Proband | Brother | Mother | Maternal grandmother | Maternal grandfather | |
|---|---|---|---|---|---|
| Haemoglobin (g/l) | 11·9 | 7·4 | 11·8 | 14·1 | 16·7 |
| Leucocyte count (× 109/l) | 3·0 | 8·0 | 4·8 | 6·6 | 8·2 |
| Platelets (× 109/l) | 90 | 52 | 162 | 225 | 255 |
| IgM (g/l) | 0·26 | 0·08 | 1·10 | 0·45 | 0·64 |
| IgG (g/l) | 22·5 | 6·9 | 12·5 | 9·03 | 9·53 |
| IgA (g/l) | 2·05 | 0·9 | 2·30 | 2·06 | 7·35 (κ paraprotein) |
| Lymphocytes (× 109/l) | 1·3 | 8·6 | 1·2 | 2·7 | 2·9 |
| CD3+ (cells/μl) | 869 | NT | 804 | 2177 | 1759 |
| CD4+ (cells/μl) | 265 | NT | 420 | 1467 | 712 |
| CD8+ (cells/μl) | 428 | NT | 310 | 798 | 1068 |
| CD3+DR+ (% T cells) | 29 | NT | 26 | 18 | 10 |
| CD3+TCRαβ+CD4−CD8− (%) | 21 | NT | 12 | 1 | < 1 |
| CD19 (cells/μl) | 92 | NT | 36 | 154 | 254 |
| CD95 (%) | 100 | NT | 100 | 100 | 100 |
| DCT | –ve | –ve | –ve | –ve | –ve |
| Autoantibodies | –ve | NT | –ve | Thyroid 1/6400 | –ve |
Abnormal results are underlined. NT, Not tested. CD3 = T cells; CD19 = B cells; DR = MHC class II; CD95 = Fas. Brother's immunology is presplenectomy.
Brother
The proband's older brother was born in 1978 after a normal pregnancy and he died of pneumococcal meningitis at the age of 4 years, 2 years post-splenectomy. He was noted to have cervical lymphadenopathy at birth and was breast fed until the age of 2 years. He was fully immunized, including bacille Calmette–Guérin (BCG), without adverse effect. From the age of 2 months, he developed a Coombs negative haemolytic anaemia (Hb 74 g/l, reticulocytes count 15%) associated with mild jaundice, 6 cm splenomegaly, minimal hepatomegaly and generalized lymphadenopathy. He was otherwise well and his general development, vision and hearing were all normal. No rashes were ever present. Investigations showed a normocytic anaemia and thrombocytopenia, but no leucocytosis or hypergammaglobulinaemia (Table 1). ANA was negative. Bone marrow aspirate showed erythroid hyperplasia, no abnormal blasts, atypical lymphocytes or storage cells. Liver function tests were normal except for a bilirubin of 75 μmol/l, and hepatitis B serology was negative. Tests for spherocytosis, erythrocyte enzyme deficiencies and haemoglobinopathies were negative.
From the age of 5 months his haemoglobin decreased to between 50 and 60 g/l and from then on he required monthly blood transfusions. Oral prednisolone was started, but did not reduce the need for transfusions. Repeat bone marrow aspirate and splenic aspirate at the age of 14 months showed a slight excess of mononuclear cells but no other abnormalities. After splenectomy, at the age of 2 years, his haemoglobin returned to normal and he remained transfusion-independent. He did however develop a marked and persistent lymphocytosis (leucocytes 30–40 × 109/l, lymphocytes 20–30 × 109/l) associated with 2% atypical lymphocytes and increased generalized 1–2 cm lymphadenopathy. He remained otherwise well until, he died of pneumococcal meningitis at the age of 4 years, despite penicillin prophylaxis and vaccination with Pneumovax.
CMV was isolated from his urine and throat at his initial presentation at 4 months and then repeatedly for the next 2 years before becoming negative. CMV strain typing was not available to determine whether the brothers acquired the same strain of virus. His CMV IgG titre was 1/40 and his IgM was slightly raised. His mother's CMV IgG titre was 1/40 but IgM was not raised. In situ hybridization for CMV and EBV RNA performed on splenic tissue were negative.
Mother
The boy's mother, born in 1951, was well at birth, but had problems with splenomegaly and lymphadenopathy from early infancy. In infancy and early childhood she suffered from recurrent nose bleeds associated with thrombocytopenia and anaemia requiring transfusions. A lymph node biopsy performed in 1952 at 18 months of age for persisting lymphadenopathy and splenomegaly showed some infiltration by reticulum cells and plasma cells. A possible diagnosis of early Hodgkin's disease was made. A further lymph node biopsy performed in 1958 at the age of 6 years was said to show features more consistent with Hodgkin's disease or Hodgkin paragranuloma. No chemotherapy was offered at this time. At the age of 12 years she was noted to have generalized lymphadenopathy (cervical, axillary and inguinal), splenomegaly extending below the umbilicus but no hepatomegaly. After puberty her signs settled, by 16 years she had only 3 cm of splenomegaly and no lymphadenopathy and by 26 years she had no splenomegaly or lymphadenopathy. She is presently well at the age of 49 years. Results of recent investigations are shown in Table 1.
Other family members
The boys' sister who is now 17 has always been well, as has their father. The boy's maternal grandfather is alive and clinically well at the age of 73 years. Their maternal grandmother has not had problems with lymphadenopathy or splenomegaly, but at the age of 35 years had a subtotal thyroidectomy for Grave's disease and remains well on thyroxine replacement therapy at the age of 72 years. Her recent investigations are also shown in Table 1. One of the boy's three maternal aunts has recently been diagnosed with autoimmune thyroiditis at the age of 47 years, but is otherwise well. All these family members have CD3+TCRαβ+CD4−CD8−T lymphocytes which are ≤ 1% of the total and normal CD95 expression on the lymphocyte surface as measured by flow cytometry.
Investigations leading to the definitive diagnosis
The unusual population of ‘double-negative’ T lymphocytes in the blood of the proband and the mother was in keeping with the diagnosis of ALPS, one possible cause of which is Fas deficiency. All family members had normal expression of Fas (CD95) on the surface of blood lymphocytes as tested by flow cytometry. Using an anti-Fas antibody (Apo-1) it was shown that both the proband and the mother had defective Fas-dependent apoptosis while all other family members tested had normal responses (Fig. 3). A mutation was found in the Fas gene in both the mother and the proband, with G to A change at position +5 in the splice donor site of intron 8, leading to exon-8 skipping and consequently to a premature stop codon at position 205 and thus deletion of the death domain of Fas. Unfortunately, DNA from the brother's splenic tissue could not be amplified. There were no mutations in the Fas genes of the maternal grandparents. Thus in this family, the disease is due to a new mutation in the Fas gene arising in the mother.
Fig. 3.
Anti-CD95 MoAb-induced apoptosis assay. ▪, Control; ▾, proband; ▴, mother; •, grandmother; Δ, grandfather.
Discussion
ALPS is a rare cause of chronic lymphadenopathy and splenomegaly in children and is often confused with chronic viral infection, idiopathic autoimmune disease and lymphoma. In this family, all three latter diagnoses were considered until the definitive diagnosis was finally made, using both a functional in vitro test of blood mononuclear cell apoptosis and gene mutation screening. Mutations in three genes are presently recognized as causing the syndrome: Fas as in this case [13]; Fas ligand [3] and caspase 10 [14]. As in this case, mutations in the Fas gene usually affect the cytoplasmic death domain of the molecule which interacts with intracellular second messengers triggering apoptosis [12].
As far as we are aware, this is the first description of CMV infection in children with ALPS. In both boys, extensive CMV infection was considered as part of the initial differential diagnosis. Infants often acquire CMV infection via breast feeding or from contacts in day care centres. Approximately 40–60% of infants who are breast-fed for over 1 month by seropositive mothers become infected. Infection is usually asymptomatic [15–17]. Virus excretion may continue for > 2 years, with virus usually being cleared from the throat before it is cleared from the urine [18,19]. Clearance depends on the acquisition of specific cell-mediated immunity, rather than the appearance of antibodies against the virus [20]. The occurrence of CMV infection in the two brothers, who were both breast fed for > 1 year by their seropositive mother, and continual excretion of virus for 2–3 years is no different from the natural history of the condition in normal individuals. Neither child attended a day care centre in the first 2 years of their lives. Both boys eventually cleared the virus, as their throat and urine cultures became negative and CMV was not detected in lymph node and splenic tissue, even by in situ hybridization. Thus, it is unlikely that CMV exacerbated their condition or was responsible for their ongoing lymphoproliferation. It is more difficult to know whether CMV infection triggered the onset of the ALPS, but presumably many infective agents can do this. These brothers do however, demonstrate that CMV clearance, even after widespread perinatal infection, is not dependent on Fas-mediated apoptosis of infected cells.
Acknowledgments
This work was supported by grants from Institut National de la Sante et de la Recherche Medicale, Assistance Public-Hopitaux de Paris, Association Française contre les Myopathies, Ligue Nationale contre la Cancer, PHRC European concerted action Biomed-2 no. 963007.
References
- 1.Canale VC, Smith CH. Chronic lymphadenopathy simulating malignant lymphoma. J Pediatr. 1967;70:891–9. doi: 10.1016/s0022-3476(67)80262-2. [DOI] [PubMed] [Google Scholar]
- 2.Rieux-Laucat F, Le Deist F, Hivroz C, et al. Mutation in FAS associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–9. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 3.Jackson CE, Puck JM. Autoimmune lymphoproliferative syndrome, a disorder of apoptosis. Curr Opin Pediatr. 1999;11:521–7. doi: 10.1097/00008480-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Sneller MC, Wang J, Dale JK, et al. Clinical, immunological and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–8. [PubMed] [Google Scholar]
- 5.van der Werff ten Bosch J, Delabie J, Bohler T, Verschuere J, Thielemans K. Revision of the diagnosis of T-zone lymphoma in the father of a patient with autoimmune lymphoproliferative syndrome type II. Br J Haematol. 1999;106:1045–8. doi: 10.1046/j.1365-2141.1999.01643.x. [DOI] [PubMed] [Google Scholar]
- 6.Sieg S, Huang Y, Kaplan D. Viral regulation of CD95 expression and apoptosis in T lymphocytes. J Immunol. 1997;159:1192–9. [PubMed] [Google Scholar]
- 7.Tollefson AE, Hermiston TW, Litchtenstein DL, et al. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature. 1998;392:726–30. doi: 10.1038/33712. [DOI] [PubMed] [Google Scholar]
- 8.Goldmacher VS, Bartle LM, Skaletskaya A, et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci USA. 1999;96:12536–41. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numazaki Y, Yano N, Morizuka T, Takai S, Ishida N. Primary infection with human cytomegalovirus: virus isolation from healthy infants and pregnant women. Am J Epidemiol. 1970;91:410–7. doi: 10.1093/oxfordjournals.aje.a121151. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds DW, Stagno S, Hosty TS, Tiller M, Alford CA., Jr Maternal cytomegalovirus excretion and perinatal infection. New Engl J Med. 1973;289:1–5. doi: 10.1056/NEJM197307052890101. [DOI] [PubMed] [Google Scholar]
- 11.Ahlfors K, Ivarsson SA, Johnsson T, Svensson I. Congenital and acquired cytomegalovirus infections. Virological and clinical studies on a Swedish infant population. Acta Paediatr Scand. 1978;67:321–8. doi: 10.1111/j.1651-2227.1978.tb16328.x. [DOI] [PubMed] [Google Scholar]
- 12.Rieux-Laucat F, Blachere S, Danielan S, et al. Lymphoproliferative syndrome with autoimmunity: a possible genetic basis for dominant expression of the clinical manifestations. Blood. 1999;94:2575–82. [PubMed] [Google Scholar]
- 13.Fisher GH, Rosenberg FJ, Straus SE, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–44. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Zheng L, Lobito A, et al. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 15.Numazaki K, Chiba S. Current aspects of diagnosis and treatment of cytomegalovirus infections in infants. Clin Diagn Virol. 1997;8:169–81. doi: 10.1016/s0928-0197(97)10005-8. [DOI] [PubMed] [Google Scholar]
- 16.Minamishima I, Ueda K, Minematsu T, Umemoto M, Take H, Kuraya K. Role of breast milk in acquisition of cytomegalovirus infection. Microbiol Immunol. 1994;38:549–52. doi: 10.1111/j.1348-0421.1994.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 17.Peckham CS, Johnson C, Ades A, Pearl K, Chin KS. Early acquisition of cytomegalovirus infection. Arch Dis Child. 1987;62:780–5. doi: 10.1136/adc.62.8.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starr JG, Gold E. Prevalence and duration of postnatally acquired human cytomegalovirus infection. J Chron Dis. 1970;22:603–7. doi: 10.1016/0021-9681(70)90036-6. [DOI] [PubMed] [Google Scholar]
- 19.Murph JR, Bale JF. The natural history of acquired cytomegalovirus infection among children in group day care. Am J Dis Child. 1988;142:843–6. doi: 10.1001/archpedi.1988.02150080049020. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka R, Tanaka A, Oizumi Y, Ishida N, Numazaki Y. Virus excretion and cell-mediated immunity during cytomegalovirus infection among healthy infants and children. J Med Virol. 1986;18:21–27. doi: 10.1002/jmv.1890180104. [DOI] [PubMed] [Google Scholar]