Abstract
C57Bl/10 mice have a superior ability to control chronic infections with virulent strains of the intracellular bacteria Brucella abortus compared with BALB/c mice. While a number of differences in the cytokines produced by lymphocytes following infection of these two strains of mice have been shown, macrophages have not been evaluated for their role in conveying relative resistance. The importance of macrophages in control of brucella infections is demonstrated by the observations that intracellular survival of various strains of B. abortus directly correlates with their virulence in vivo, and the ability of macrophages to control brucellae in vitro has been shown to correlate with a brucella-resistant phenotype in ruminants. While both BALB/c and C57Bl are Nramp-susceptible mouse strains, additional differences in macrophage function outside of the Nramp1 gene effects could influence susceptibility to brucellosis. The studies conducted here comparing the ability of macrophages from C57Bl/10 and BALB/c mice indicate that the macrophages from resistant mice did not control intracellular growth of B. abortus strain 2308 more efficiently than those from the susceptible mice, either in the absence of, or following, interferon-gamma activation or iron supplementation. A number of different conditions for culturing macrophages were evaluated to rule out the influence of antibiotics on the conclusions drawn from the results.
Keywords: Brucella, macrophages, intracellular bacteria, interferon-gamma
Introduction
Brucella abortus is a Gram-negative facultative intracellular bacterium that survives and replicates in host macrophages. Inbred mouse strains which differ in their ability to control infection with B. abortus have been identified [1,2]. C57Bl/10 mice more effectively control the initial replication of the virulent B. abortus strain 2308 than do BALB/c mice and clear the infection by 16 weeks post-infection, whereas BALB/c mice remain infected [2]. There are no published reports on the contribution of macrophages in conveying relative resistance to B. abortus among mouse strains, although control by BALB/c macrophages of attenuated (B. abortus 19) and virulent (B. abortus 2308) strains of B. abortus correlates with resistance and susceptibility, respectively, to infection with these bacterial strains [3].
The role of murine macrophages in mediating resistance or susceptibility among mouse strains to some intracellular pathogens has been demonstrated in studies of the Ity/Lsh/Bcg resistance model. These studies have shown that resistance to Salmonella typhimurium, Leishmania donovani and mycobacterial species is regulated by the polymorphism of the Nramp1 gene which controls macrophage function [4–6]. Moreover, in cattle monocytes from naturally resistant animals have been shown to have an enhanced ability to control intracellular B. abortus in vitro [7,8]. Cattle have a polymorphism of the Nramp1 gene which may be responsible for this [9]. However, control of B. abortus infections is known to be a multigenic trait [1] and both brucella-resistant and brucella-susceptible phenotypes [2] are Nramp-susceptible (Ity/Lsh/Bcgs) mouse strains [10]. Here we compared the nature of anti-brucella activities of macrophages from the resistant C57Bl/10 and the susceptible BALB/c mouse strains to determine whether a difference in macrophage control of intracellular brucellae contributed to the difference in resistance.
Materials and methods
Macrophages
Macrophages were obtained from 8–12-week-old virus-free female mice (Harlan Sprague Dawley, Indianapolis, IN) maintained in barrier housing with filtered inflow air in a restricted-access room. Unless indicated otherwise, peritoneal macrophages were elicited with peptone in all studies by injecting 1 ml of 5% proteose peptone (Difco Labs, Detroit, MI) intraperitoneally and harvesting macrophages 5 days later as described [11]. Resident macrophages were obtained by peritoneal wash-out and thioglycollate-elicited macrophages by injecting 3 ml of 4% thioglycollate (BBL Microbiology Systems, Cockeysyville, MD) intraperitoneally and harvesting macrophages 3–5 days later as described above. Macrophages were plated in multiwell plates after suspension in RPMI 1640 with 10% heat-inactivated fetal bovine serum (FBS), 5 × 10−5m 2-mercaptoethanol (2-ME), and 50 μg/ml l-glutamine.
Infection of macrophages and determination of colony-forming units of bacteria
Macrophages were infected with the virulent B. abortus strain 2308 as described by us previously [11,12]. Briefly, 1·67 × 104 or 3 × 104 macrophages/well were cultured in 96-well plates and infected with bacteria at a ratio of 100 bacteria to one macrophage for 2 h by centrifuging the bacteria onto the macrophages at 1800 g for 20 min and then incubating at 37°C for 1 h 40 min. After this the cultures were washed three times by removing the culture medium, adding fresh medium to the wells followed by centrifugation of the plate at 800 g for 1 min to pellet loosened macrophages without pelleting bacteria. Unless indicated otherwise, 100 μg/ml of gentamycin was added to control extracellular bacteria in the cultures similar to that described by us previously [12]. Different concentrations of antibiotics and timing of addition were used as described below and in Results.
Recombinant murine interferon-gamma (IFN-γ) (rMuIFN-γ; Boehringer, Indianapolis, IN) was added at 100 U/ml either 24 h before infection or immediately following the 2-h infection period as indicated for the individual experiment. To supplement with iron either Fe-Tf was added 24 h before infection or iron salt (FeNTA) was added after infection as described previously [11]. Colony-forming units (CFU) per macrophage culture were determined as described elsewhere [12] by detergent (0·2% deoxycholic acid) lysis of the macrophages, centrifugation of the lysate to pellet bacteria, resuspension in PBS, serial dilution and plating on brucella agar plates.
Experimental design to evaluate effect of antibiotics on recovery of intracellular bacteria
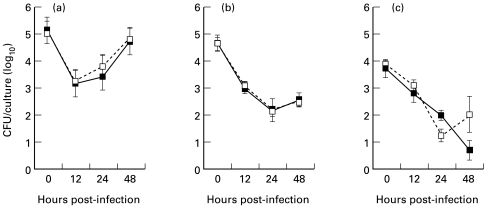
This is the design for results presented in Fig. 3. Several protocols were compared to assess the influence of antibiotics on intracellular bacterial replication using peptone-induced macrophages. They are as follows: (i) 100 μg/ml of gentamycin was added after infection of the macrophages and remained in the cultures until macrophages were to be lysed for assessing intracellular CFU; (ii) same as protocol (i) except that 5 μg/ml of gentamycin was used; (iii) 100 μg/ml of gentamycin was added following infection for 2 h only, and subsequently the cells were washed three times by centrifuging the microtitre plates, removing the culture medium and replenishing with antibiotic-free medium, following which the cultures were allowed to proceed in antibiotic-free medium until 2 h prior to macrophage lysis, at which time 100 μg/ml of gentamycin were again added for 2 h before removing it and lysing the macrophages as described in (i); (iv) antibiotics were never added to the cultures, but extracellular bacteria were minimized by washing the macrophage cultures both immediately after infection and just prior to lysing macrophages for assessing intracellular bacteria.
Fig. 3.
Peptone-elicited peritoneal macrophages from BALB/c or C57Bl/10 mice were infected with Brucella abortus strain 2308. Results represent mean and s.e.m. of colony-forming units (CFU) from replicate cultures for one of two experiments performed for each mouse strain and treatment. Macrophages were infected with brucellae and cultured with (a) 100 μg/ml gentamycin throughout, (b) 5 μg/ml gentamycin throughout, or (c) 100 μg/ml gentamycin for only 2 h immediately after infection and for 2 h prior to lysing. Results from macrophages cultured in the presence of 100 U/ml rIFN-γ following infection (- - ○ - -) or in the absence of rIFN-γ (––•––) are shown. The CFU recovered from C57Bl/10 cultures was never significantly less than that recovered from equivalent BALB/c cultures.
Experimental design to evaluate accumulation of antibiotics in macrophages with and without IFN-γ activation
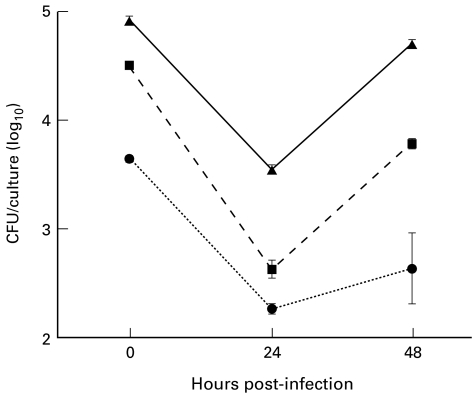
This is the design for the results presented in Fig. 2. BALB/c macrophages were cultured in Teflon tubes in medium either without antibiotics or with 100 μg/ml of gentamycin for 1 h or 12 h prior to infection. Medium containing antibiotics was then removed and the macrophages washed and resuspended in antibiotic-free medium. Macrophages were counted and distributed into wells of a multiwell plate and infected for 2 h as described above. They were either lysed immediately following infection or cultured for an additional 24 h or 48 h to determine whether the preincubation with antibiotics influenced recovery of brucellae. During the subsequent incubation, antibiotics were not present until 2 h prior to lysis, when it was necessary to add 100 μg/ml of gentamycin for 2 h to kill extracellular bacteria. Macrophages were washed before lysing to remove antibiotics as described above.
Fig. 2.
Peptone-elicited peritoneal macrophages from BALB/c mice were precultured in medium either with 100 μg/ml gentamycin for 12 h (•) or 100 μg/ml gentamycin for the final 1 h (▪), or without gentamycin (▴) and then washed and infected with Brucella abortus 2308. Results represent mean and s.e.m. of colony-forming units (CFU) obtained from replicate cultures for each treatment. These results are representative of one of two experiments performed. At all time points the cultures were significantly different from one another (P < 0·05).
A second similar type of experiment (for results presented in Table 2) employed macrophages from both BALB/c and C57Bl/10 mice cultured in the absence or presence of rMuIFN-γ (100 U/ml) and with or without 100 μg/ml of gentamycin for 24 h prior to infection. Following this, macrophages were washed to remove antibiotics and IFN-γ and then infected with brucellae in antibiotic-free medium. After the 2-h infection period, 100 μg/ml of gentamycin were added to the cultures for 1 h to kill extracellular brucellae, then removed and the macrophages lysed and bacteria plated to determine CFU. The number of CFU in macrophages preincubated with antibiotics was subtracted from that in macrophages that were preincubated in medium without antibiotics to determine whether antibiotics accumulated intracellularly and caused a decrease in the number of intracellular bacteria that survived following phagocytosis, and more specifically, whether IFN-γ activation increased the antibiotic-induced intracellular anti-brucella effect.
Table 2.
Effect of antibiotic treatment on recovery of intracellular brucellae immediatley following infection of macrophages
| BALB/c | C57Bl/10 | |||
|---|---|---|---|---|
| Pre-infection treatment of macrophages* | Mean CFU ± s.e.m. (log10)† | Change in CFU due to antibiotics in culture ‡ (log10) | Mean CFU ± s.e.m. (log10)† | Change in CFU due to antibiotics in culture ‡ (log10) |
| Medium | 4·74 ± 0·07 | 4·62 ± 008 | ||
| Medium + gentamycin | 2·49 ± 0·09 | −2·25 | 2·66 ± 0·05 | −1·96 |
| IFN-γ | 4·04 ± 0·11 | 3·52 ± 0·02 | ||
| IFN-γ + gentamycin | 2·68 ± 0·07 | −1·36 | 2·44 ± 0·13 | −1·08 |
Macrophages were cultured for 12 h in complete culture medium alone or in the presence of gentamycin (100 μg/ml) and/or recombinant murine interferon-gamma (rMuIFN-γ; 100 U/ml) in Teflon tubes. Antibiotics and cytokines were removed by washing before macrophages were distributed into multiwell plates and infected with brucellae.
There was no significant difference between BALB/c and C57Bl/10 macrophages with regard to the colony-forming units (CFU) recovered in equivalent cultures, except for the IFN-γ-treated culture where it was significantly less for the C57Bl/10 mice (P < 0·05). This difference was not consistently observed, as noted in Fig. 3.
A decrease in CFU is indicated as a negative value of the net decrease in log10 units.
Statistical analysis
Groups were compared by two-tailed Student's t-test and P < 0·05 was considered to be significant.
Results
Effects of modes of induction of macrophage populations on control of brucella survival and growth
Initial experiments compared various BALB/c macrophage populations (resident, thioglycollate-elicited and proteose peptone-elicited) for their ability to control growth of intracellular B. abortus including following activation with IFN-γ. An increase in CFU of brucellae occurred between 24 h and 48 h after infection in all three types of non-IFN-γ-activated macrophage populations ranging from log10 0·5 to 1·5. Addition of IFN-γ to macrophage cultures 24 h prior to infection resulted in a similar decrease in recovery of CFU (compared with cultures that did not receive IFN-γ) among the macrophage populations ranging from log10 1·9 to 2·3 at 48 h. Since no striking difference in the pattern of control of intracellular brucellae was observed among the macrophage populations, all further experiments were conducted using peptone-elicited peritoneal exudates which increased the macrophage yield per mouse and thus reduced the use of animals.
Comparison of BALB/c and C57Bl/10 macrophages for control of brucellae following IFN-γ activation and iron supplementation
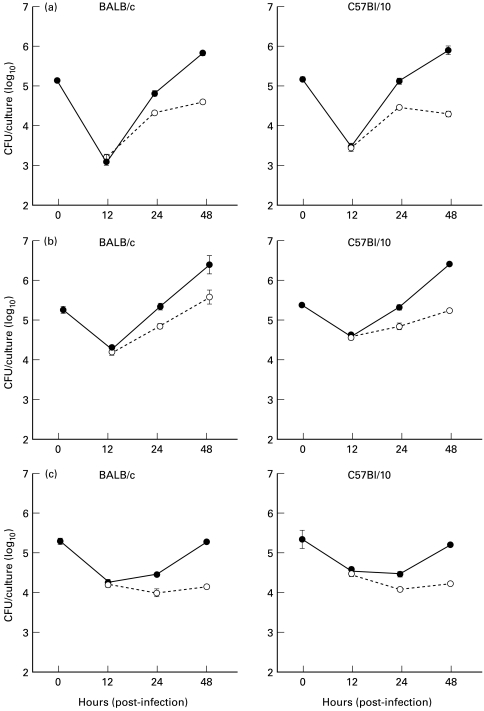
When BALB/c and C57Bl/10 macrophages were compared for control of B. abortus, we found the results to be virtually identical between mouse strains. In medium cultures (Fig. 1a) the intracellular brucellae increased between 12 h and 48 h. Since macrophages activated with IFN-γ prior to phagocytosing brucellae are likely to be most effective at controlling the bacteria [12], we evaluated macrophage cultures treated in this way. Also because preactivation with IFN-γ decreased bacteria recovered at 0 h we compared the results between mouse strains by treatment. While there was no difference between strains, unlike the medium cultures the number of CFU did not increase in the IFN-γ cultures between 12 h and 48 h (Fig. 1b). We have also previously shown that iron in the form of iron-bound transferrin (FeTf) or as a soluble salt (FeNTA) increases the brucellacidal activity of BALB/c macrophages and that the effect when combined with IFN-γ activation is especially profound [11]. In preliminary experiments we found that iron also increased anti-brucella activities of C57Bl/10 macrophages (Table 1). Comparisons made for BALB/c and C57Bl/10 macrophages precultured with IFN-γ and FeTf indicated that while the iron/IFN-γ-supplemented cultures (Fig. 1c) had more variation between the two mouse strains than the medium or IFN-γ-supplemented cultures, the CFU recovered were not significantly different between the two mouse strains.
Fig. 1.
Comparison of colony-forming units (CFU) recovered from peptone-elicited peritoneal macrophages from BALB/c (––––) and C57Bl/10 (- - - -) mice infected with Brucells abortus strain 2308. The mean and s.e.m. of CFU indicated are from eight experiments for BALB/c mice and three for C57Bl/10 mice. Two of the experiments evaluated the BALB/c and C57Bl/10 macrophages together and found they mirrored the values represented by the means presented here. Macrophages were cultured in complete tissue culture medium with (a) no additives, (b) medium with IFN-γ, or (c) medium with IFN-γ and iron-bound transferrin (FeTf). Additives were added for 24 h before infection and remained throughout the culture period. The numbers of CFU recovered from the macrophage cultures treated with IFN-γ alone or IFN-γ/FeTf were significantly lower than the numbers in the medium controls at 48 h after infection for all experiments (P < 0·01), but there was no significant difference between macrophages from the two mouse strains.
Table 1.
Effect of iron on Brucella abortus survival and growth in C57Bl/10 macrophages
| Exp. No. | Treatment | CFU (log10)* | CFU recovered relative to medium (log10)† |
|---|---|---|---|
| 1 | Medium | 5·37 | |
| FeTf | 4·76 | −0·61 | |
| FeNTA | 3·86 | −1·51 | |
| 2 | Medium | 4·74 | |
| FeTf | 3·88 | −0·86 | |
| FeNTA | 3·26 | −1·48 |
Mean colony-forming units (CFU) 48 h after infection. The variation (s.e.m.) incurred from determining CFU from replicate plates and multiple sample dilutions was < 5% in all experiments (not shown). Peptone-elicited peritoneal macrophages were cultured in medium alone or with either iron-bound transferrin (FeTf) for 24 h before infection or with iron salt (FeNTA) after infection only.
Relative log10 CFU represents the change in CFU in experimental groups with iron relative to the medium controls. Iron significantly decreased the number of intracellular brucellae recovered compared with the medium controls in both experiments (P < 0·05).
Evaluation of the contribution of intracellular antibiotics to recovery of brucellae
The above experiments were done in the presence of antibiotics, since attempts to perform the macrophage infection experiments in the absence of antibiotics were unsuccessful. Despite rigorous washing steps, extracellular bacteria overgrew the cultures to the extent that addition of gentamycin (100 μg/ml) 2 h prior to harvesting the macrophages for plating bacterial CFU was ineffective in killing the extracellular organisms (data not shown). However, antibiotics can also accumulate in macrophages and potentially influence the recovery of CFU. To quantify the influence of antibiotics on our results, BALB/c macrophages were preincubated with 100 μg/ml of gentamycin before infection with B. abortus. Incubation for even 1 h reduced the number of bacteria recovered at 0, 24 and 48 h and the overall reduction increased with preincubation time (Fig. 2). Further experiments showed the effect of antibiotics was comparable for macrophages from BALB/c and C57Bl/10 mice (Table 2).
Since it is possible that the effect of IFN-γ shown above was due to increased antibiotic uptake, thus lowering the recovery of CFU, macrophages were also precultured in the presence of antibiotics with IFN-γ present. The decrease due to preculture with antibiotic was less when the macrophages were simultaneously activated with IFN-γ (Table 2).
Control of intracellular brucellae by C57Bl/10 and BALB/c macrophages using various antibiotic protocols
More extensive comparisons were made between the mouse strains using three different antibiotic protocols to minimize the impact of antibiotics on results. In these experiments IFN-γ was added after infection of macrophages. The minimal concentration of antibiotics necessary to control extracellular brucellae was either 5 μg/ml if present throughout the culture or 100 μg/ml when the presence of antibiotic was restricted to a 2-h pulse immediately after the infection period and again for 2 h immediately prior to harvesting the macrophages (data not shown). Both of these protocols resulted in a greater recovery of bacteria at 12 h post-infection than when 100 μg/ml of gentamycin were present throughout the culture period (Fig. 3), suggesting that the decrease in CFU at this time may have been enhanced by antibiotic uptake. This was consistently observed. Other differences in bacterial recovery or growth pattern among protocols, such as the greater rate of growth in macrophages when 100 μg/ml of gentamycin were present as shown in Fig. 3, were not consistently observed. Significant differences in recovery of CFU between macrophages from the two strains of mice never occurred with any of the three protocols with either macrophages activated or not activated with IFN-γ.
Discussion
We conclude that superior control of infections with the virulent strain B. abortus 2308 by C57Bl/10 mice relative to that by BALB/c mice is unlikely to be due to a superior ability of macrophages to control replication of intracellular brucellae in vivo. We have evidence however, that resistance and susceptibility by C57Bl/10 and BALB/c mice is at least partially a consequence of differences in the T cell cytokines produced or their effectiveness during a brucella infection [13,14]. The two most important cytokines are IFN-γ, without which the mice die (Murphy et al., submitted for publication), and IL-10, which decreases IFN-γ production [13,14]. Thus it was particularly important to be sure that the IFN-γ effect was not magnified by differences in the ability of macrophages from the two strains of mice to be activated by it. Our results indicate there was no strain-associated difference following IFN-γ activation when macrophages were pretreated with IFN-γ either before infection (see Fig. 1) or after infection (see Fig. 3).
We were also able to conclude that the decreased recovery of CFU from IFN-γ-activated macrophages is not due to increased accumulation of antibiotics and thus truly reflects enhanced killing by the macrophages. Previously we have shown that the enhanced control following IFN-γ activation can be overcome by addition of inhibitors of reactive oxygen intermediates [15]. These observations support the contention that the IFN-γ effect is a result of cellular products and not antibiotic accumulation. We attempted to use green fluorescent protein-transfected brucellae to establish an antibiotic-free system to assess infection rates in cells. Because the flow cytometer can be manipulated to gate out free bacteria, large numbers of extracellular bacteria would not affect the results. However, killing of the brucellae by reactive oxygen intermediates was not found to diminish fluorescence, so this method could not distinguish between viable and dead intracellular brucellae (E. L. Murphy and C. L. Baldwin, unpublished observations).
Because it was impossible to establish an antibiotic-free system to conduct these assays, we evaluated a number of protocols for comparing C57Bl/10 and BALB/c macrophages and established two which used the minimal concentration of gentamycin that could be employed to kill extracellular brucellae effectively (5 μg/ml, which had to be present throughout the culture period) as well as the minimal time that gentamycin could be applied and still be effective (2 h, but required 100 μg/ml of antibiotics). We chose gentamycin for our studies because it is reported to have a moderate to low accumulation rate in cells compared with other antibiotics such as lipid soluble antibiotics and those that are actively transported [16]. It has been shown by Drazek et al. [17] that in the cultures of human monocyte-derived macrophages infected with B. melitensis 1 or 50 μg/ml of gentamycin results in a slightly lower recovery of brucellae at 24 h and 48 h than that from cultures with no antibiotic present, although the general pattern of recovery is the same. They attributed this to killing of extracellular bacteria. Our results indicate that gentamycin actually accumulated in murine macrophages and affected the number of intracellular B. abortus organisms. Nevertheless, none of the experimental results presented here indicates a superior ability of C57Bl/10 macrophages to control brucellae. Also, the observation that the bacteria began to grow in the murine macrophages after 12 h suggests that they transit to a compartment inside the macrophage that is less accessible to antibiotics. Detilleux and colleagues have shown that B. abortus is found in the lumen of the rough endoplasmic reticulum of HeLa cells [18]. Recent studies suggest that this occurs through a process of autophagy and a retrograde transport system that allows brucellae access to the endoplasmic reticulum [19,20]. The endoplasmic reticulum may be inaccessible to gentamycin. It has also been shown that Mycobacterium tuberculosis, which resides in phagosomes as does B. abortus, can remodel the intracellular compartment to exclude molecules [21]. Perhaps brucellae have a similar capacity which results in exclusion of antibiotics. In our system, we showed that once the brucellae began replicating the rate of increase in CFU was virtually identical between the two mouse strains.
In conclusion, there is no evidence from multiple systems for analysis and the multiple analyses made to indicate that the difference in resistance to brucellosis between C57Bl/10 and BALB/c is related to differences in the ability of their macrophages to control intracellular B. abortus strain 2308. Therefore, our studies are continuing to focus on differences in the expression of cytokine genes and their receptors, particularly those involved in activation of macrophages.
Acknowledgments
This work was supported by a grant from USDA/NRI Competitive Grants Program Award no. 9303615 to C.L.B. We wish to thank Rita Benson, Dancella Fernandes, Bryan Leonard and Erin Murphy for contributions to the development of the study, and Alex Winter for extensive discussion of these experiments and critical review of the manuscript.
References
- 1.Ho M, Cheers C. Resistance and susceptibility of mice to bacterial infection. IV. Genetic and cellular basis of resistance to chronic infection with Brucella abortus. J Infect Dis. 1982;146:381–7. doi: 10.1093/infdis/146.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Montaraz JA, Winter AJ. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986;53:245–51. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones SM, Winter AJ. Survival of virulent and attenuated strains of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect Immun. 1992;60:3011–4. doi: 10.1128/iai.60.7.3011-3014.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crocker PR, Blackwell JM, Bradley DJ. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984;43:1033–40. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lissner CR, Swanson RN, O'Brien AD. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–13. [PubMed] [Google Scholar]
- 6.Stokes RW, Orme IM, Collins FM. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacteria avium infections in mice. Infect Immun. 1986;54:811–9. doi: 10.1128/iai.54.3.811-819.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price RE, Templeton JW, Smith R, III, Adams LG. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect Immun. 1990;58:879–86. doi: 10.1128/iai.58.4.879-886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qreshi T, Templeton JW, Adams LG. Intracellular survival of Brucella abortus, Mycobacterium bovis BCG, Salmonella dublin, and Salmonella typhimurium in macrophages from cattle genetically resistant to Brucella abortus. Vet Immunol Immunopathol. 1996;50:55–56. doi: 10.1016/0165-2427(95)05492-8. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Li Y, Hashad M, Schurr E, Gros P, Adams LG, Templeton JW. Bovine natural resistance associated macrophage protein 1 (Nramp1) gene. Genome Res. 1996;6:956–64. doi: 10.1101/gr.6.10.956. [DOI] [PubMed] [Google Scholar]
- 10.Skamene E, Gros P, Forget A, Kongshavn PAL, St Charles C. Genetic regulation of resistance to intracellular pathogens. Nature. 1982;297:506–9. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Baldwin CL. Iron augments macrophage-mediated killing of Brucella abortus alone and in conjunction with interferon-γ. Cell Immunol. 1993;148:397–407. doi: 10.1006/cimm.1993.1121. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Baldwin CL. Effect of cytokines on the ability of macrophages to control intracellular Brucella abortus. Infect Immun. 1993;61:124–34. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes DM, Jiang X, Jung JH, Baldwin CL. Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus. FEMS Micro Immunol. 1996;16:193–203. doi: 10.1111/j.1574-695X.1996.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes DM, Baldwin CL. IL-10 downregulates protective immunity to Brucella abortus. Infect Immun. 1995;63:1130–3. doi: 10.1128/iai.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Leonard B, Benson R, Baldwin CL. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 1993;151:309–19. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JD, Hand WL, Francis JB, King-Thompson N, Corwin RW. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980;95:429–39. [PubMed] [Google Scholar]
- 17.Drazek ES, Houng HS, Crawford RM, Hadfield TL, Hoover DL, Warren RL. Deletion of purE attenuates Brucella melitensis 16M for growth in human monocyte-derived macrophages. Infect Immun. 1995;63:3297–301. doi: 10.1128/iai.63.9.3297-3301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detilleux PG, Deyoe BL, Cheville NF. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect Immun. 1990;58:2320–8. doi: 10.1128/iai.58.7.2320-2328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel JP. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–24. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pizarro-Cerda J, Moreno E, Sanguedolce V, Mege JL, Gorvel JP. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–92. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturgill-Koszycki P, Schlesinger H, Chakrobborty P, et al. Lack of acidification in Mycobacterium phagosomes produce by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–81. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]