Abstract
Acute RSV infection in infancy may produce some asthma-like symptoms and may be followed by a recurrent wheeze later in childhood. It has been proposed that RSV infection stimulates type-2 cytokine responses, resembling those found in atopy and asthma. Peripheral blood cells were obtained from RSV-infected infants (n = 30) and healthy controls (n = 10). After in vitro restimulation of the cells, intracellular IL-4 and interferon-gamma (IFN-γ) were measured by flow cytometry. The cells from RSV-infected infants produced more IL-4 and less IFN-γ than those from healthy controls. IL-4 production was more frequent in CD8 than in CD4 cells, and the bias toward IL-4 production was greatest in infants with mild infections, whereas IFN-γ production increased with disease severity. Our conclusions are that RSV infection is associated with IL-4 production in peripheral T cells, and that peripheral blood in infants with severe disease may be depleted of cytokine-producing cells.
Keywords: respiratory syncytial virus, intracellular cytokines, flow cytometry, IL-4, interferon-gamma, lymphocytes
Introduction
RSV is the cause of one of the most common diseases in infants, despite high levels of maternally transferred specific serum antibodies [1–3]. Primary infections in young infants are frequently manifested as bronchiolitis or pneumonia. In older children and adults, symptoms are usually restricted to the upper respiratory tract. Immaturity of the immune system in infants has been proposed as one of the reasons for the decreased virus-specific clearance [2].
In some children with a genetic predisposition to atopic diseases, RSV infection has been related to the development of episodes of bronchial obstruction and asthma [4].
One possible mechanism by which RSV can induce allergies and asthma could be through increased IgE and IgG4 production. RSV has been demonstrated to induce synthesis of antiviral-specific IgE antibodies in most of the RSV-infected infants [5,6]. This specific IgE response to RSV in infancy could be associated with later recurrence of wheezing [7]. Our finding of increased expression of CD23 in B cells in RSV-infected patients [6] suggests that CD23, a low-affinity IgE receptor, could play a role in IgE regulation and pathogenesis of this disease.
A growing set of data show that RSV with two distinct viral envelope glycoproteins is capable of inducing type-1 and type-2 immune responses. The cytokine profile of CD4+ cell lines specific for these two RSV proteins in mice is well defined. RSV attachment (G) protein-specific CD4 cells have a type-2 profile, producing IL-4, IL-5 and IL-10 with little or no interferon-gamma (IFN-γ), whereas CD4 cells that recognize fusion (F) protein have a type-1 profile producing IFN-γ but little IL-4 [8–11]. An increased production of IL-4 by G protein-specific CD4 cells could therefore up-regulate CD23 and promote class switching to IgE and IgG4 in B cells [12].
Evaluated at both protein (by ELISA) and mRNA levels, cytokine profile in RSV-infected infants produced conflicting results. The mitogen-stimulated peripheral blood mononuclear cells (PBMC) obtained from RSV-infected infants were found to secrete both IL-4 and IFN-γ, but their IL-4 to IFN-γ ratio was higher than in the age-matched controls [13]. The PBMC obtained from RSV+ healthy infants upon restimulation with whole RSV [14] had an increased mRNA level for type-1-associated cytokines, i.e. IFN-γ, with no detectable type-2 cytokine-specific mRNA, i.e. IL-4.
Many studies provide evidence that beside CD4+ T lymphocytes, differentiation may also occur within the CD8 T lymphocyte population under certain clinical conditions [15–17]. In allergic patients, intracellular staining combined with cell surface marker analysis revealed an increased IL-4 production in CD8 T lymphocytes stimulated with phytohaemagglutinin (PHA) [18]. These cells were capable of promoting IgE production by autologous B cells in a contact-independent fashion. Beside allergens, some viruses, e.g. HIV, could also induce a skewing toward type-2 cytokine production in CD8 T lymphocytes and down-regulate their antiviral cytotoxic activity [19,20].
The aim of this study was to determine the cytokine profiles of CD4 and CD8 lymphocytes obtained from infants during an episode of acute respiratory RSV-caused infection. Three-colour immunofluorescence analysis using a flow cytometer allowed us to determine simultaneously the phenotype and cytokine profiles at a single-cell level on short-term polyclonal activation. The possible correlation between the severity of disease and lymphocyte cytokine profiles of lymphocytes in RSV-infected infants was analysed.
SUBJECTS and METHODS
Patients and controls were included in the study upon having obtained written informed consent from their parents and guardians. The study was approved by the Medical Ethics Committees of both Hospitals.
Patients
We studied 30 children (18 boys and 12 girls), mostly infants admitted to the University Hospital for Infectious Diseases and Children's Hospital, Zagreb, Croatia (Table 1). The patients aged 3 weeks to 24 months (mean 5 months) suffered from upper respiratory tract infection (URTI; defined as absence of either wheezing or crackles, O2 saturation > 95%, and normal radiographs), bronchiolitis (defined as wheezing, hypoxia with O2 saturation < 95%, and hyperinflation but infiltrate-free chest radiograph) or pneumonia (defined as crackles on auscultation with or without wheezing, and chest radiograph showing infiltrates) [21]. The patients with URTI were examined in an out-patient Department adjacent to the Hospitals, while others were hospitalized. Most subjects were infants < 6 months old (n = 23). None of the study patients was treated with glucocorticoid drugs. Samples of nasopharyngeal secretions, sera, and heparinized blood were simultaneously obtained at the onset of acute illness and within 8 days thereafter. Bacteriological and other laboratory analyses which included quantification of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and leucocyte count were performed for each infant to exclude possible non-viral infections [22].
Table 1.
Patients and clinical findings
| Clinical syndromes | ||||
|---|---|---|---|---|
| Symptoms and findings | URTI n = 7 | Bronchiolitis n = 17 | Pneumonia n = 6 | Total n = 30 |
| Wheezing (no. of cases) | 0 | 17 | 6 | 21 |
| MOS (%) | 95·1 | 88·6 | 88·7 | 90·5 |
| (95–98) | (82–93) | (86–93) | (82–98) | |
| MRR (/min) | 30 | 40 | 56 | 40 |
| (25–45) | (30–60) | (35–64) | (25–64) | |
| X-ray (no. of cases) | ||||
| Not done | 4 | 2 | 0 | 6 |
| Negative | 3 | 11 | 0 | 14 |
| Hyperinflation | 0 | 4 | 0 | 4 |
| Infiltrate | 0 | 0 | 6 | 6 |
Medians, range (in parentheses) and number of patients (n) were determined. Minimal oxygen saturation (MOS) was measured by percutaneous oxymetry.
URTI, Upper respiratory tract infection; MRR, maximal respiratory rate.
A control group consisted of 10 infants (seven boys and three girls) aged between 1 and 24 months (mean 6 months), who were free from manifest allergic, immune and haematologic disorders, and hospitalized for minor surgery. Only controls and patients with RSV infection and a negative family history of atopy were included in the study.
Virus identification
The presence of RSV infection was determined by detecting the virus in nasopharyngeal secretions by the immunofluorescence test (RSV-direct IF identification; BioMerieux, L'Etoile, France) with antivirus MoAbs. RSV was confirmed by isolation in cell culture using a standard technique [23].
Whole blood culture and cytokine induction
Fresh samples of heparinized whole blood were diluted with equal volumes of RPMI 1640 culture medium (Institute of Immunology, Zagreb, Croatia) prior to in vitro stimulation. Diluted blood (200 μl) was incubated in sterile 12 × 75-mm polystyrene round-bottomed tubes (Falcon, Becton Dickinson, Lincoln Park, NJ). For stimulation 50 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma, St Louis, MO) were used and 0·75 μg/ml ionomycin (Sigma) in the presence of 10 μg/ml brefeldin A (BFA; Sigma) for 6 h at 37°C and 5% CO2. After PMA and ionomycin stimulation, the samples were labelled with peridinin chlorophyll protein (PerCP)-conjugated anti-CD4, anti-CD8, and FITC-conjugated anti-IFN-γ (Becton Dickinson, Heidelberg, Germany) and PE-conjugated anti-IL-4 (PharMingen, San Diego, CA) MoAbs in the following combinations: anti-IFN-γ/anti-IL-4/anti-CD4 and anti-IFN-γ/anti-IL-4/anti-CD8. Isotype-matched controls (Becton Dickinson) were included. Cytokine production in the samples obtained from healthy adults (n = 3) was used to assess whether the stimulation used throughout the study was optimal.
A slightly modified intracellular staining protocol described by Jason et al. [24] was used. In short, after stimulation, diluted whole blood was aliquoted in volumes of 50 μl, washed, and incubated in the dark for 15 min at room temperature with 5 μl of MoAbs for surface markers. After washing in staining buffer (containing 1% fetal calf serum (FCS), 0·1% NaN3 and Dulbecco's PBS), the samples were fixed with a fixation buffer (containing 4% formaldehyde in Dulbecco's PBS) for 30 min at 4°C, washed and permeabilized with a permeabilization buffer (containing 1% FCS, 0·1% NaN3, 0·1% saponin in Dulbecco's PBS) for 15 min at 4°C. Washing in the permeabilization buffer sufficed for erythrocytolysis. Then, 50 μl of the permeabilization buffer were added to the cell pellet and incubated with fluorochrome-conjugated anti-cytokine MoAbs for 30 min at 4°C. The anti-cytokine MoAbs were added in the following concentrations: 0·4 μg/ml anti-IL-4–PE and 1·25 μg/ml anti-IFN-γ–FITC. Finally, the stained cells were washed with permeabilization buffer and resuspended in 0·5 ml of the staining buffer and immediately analysed on a flow cytometer.
To ascertain the specificity of quantified proteins and before adding the samples, the anti-IL-4 antibody was preincubated in separate experiments with 0·2 μg recombinant human IL-4 (R&D Systems, Abingdon, UK) for 30 min. This totally blocked the binding of anti-IL-4 antibody with intracellular IL-4.
Flow cytometry
Cell samples were analysed using the CellQuest Software on a flow cytometer (FACSCalibur; Becton Dickinson, Mountain Valley, CA). A correlated analysis of the forward and right-angle scatters was used to establish the lymphocyte gate. The three-colour analysis followed on obtaining 10 000 cells in lymphocyte gate per sample.
Statistical analysis
The non-parametric Mann–Whitney U-test was used for the study and control group comparisons. The relationship between variables was assessed by means of the Spearman rank order coefficient. We used the StatisticaTM v5.0 (Statsoft Inc., Tulsa, OK) statistical package and considered the probability significant at P < 0·05.
Results
T lymphocyte subsets from RSV+ infants predominantly express type-2 cytokine
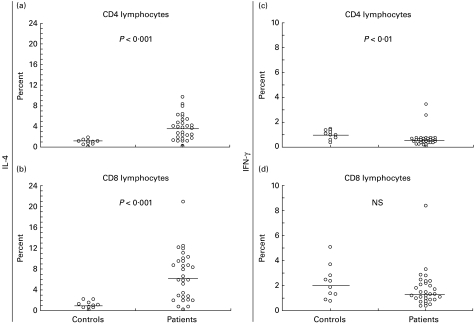
The study involved intracellular cytokine staining of IL-4 and IFN-γ coupled with surface marker analysis for CD4 and CD8 T lymphocytes in RSV-infected infants (n = 30) and age-matched controls (n = 10) (Fig. 1). RSV-infected infants had a higher percentage of IL-4-producing CD4 and CD8 lymphocytes (Fig. 1a,b) upon PMA and ionomycin stimulation, and a lower percentage of IFN-γ-producing CD4 T lymphocytes (Fig. 1c) than healthy donors. Similar results were obtained in infants aged < 6 months (n = 23) presumably suffering from primary RSV infection (data not shown).
Fig. 1.
The percentage of CD4 lymphocytes producing IL-4 (a) and IFN-γ (c), and percentage of CD8 lymphocytes producing IL-4 (b) and IFN-γ (d) upon polyclonal stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin after a 6-h incubation in controls (n = 10) and RSV-infected infants (n = 30). Each symbol characterizes one individual tested. The lines represent medians. The non-parametric Mann–Whitney U-test was used for between-group comparison.
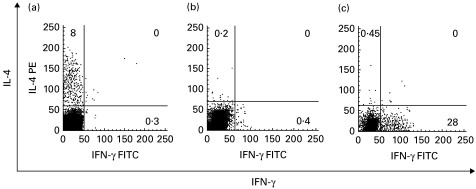
Analysis of cytokine production in the samples obtained from healthy adults (n = 3) revealed that identical stimulation resulted in marked IFN-γ and scarce IL-4 production (Fig. 2c). This showed that the lack of IFN-γ in T cells upon PMA and ionomycin stimulation was not due to low sensitivity of our IFN-γ assay.
Fig. 2.
Representative plots of IL-4 and IFN-γ production within the lymphocyte population in an RSV-infected infant (a), control infant (b) and healthy adult (c) upon 6-h whole-blood activation with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of brefeldin A. Numbers indicate the percentage of single or double-stained lymphocytes.
IFN-γ showed a positive correlation with age in healthy controls and a negative correlation in sick infants (data not shown). No correlation for IL-4 was observed in either sick or healthy infants.
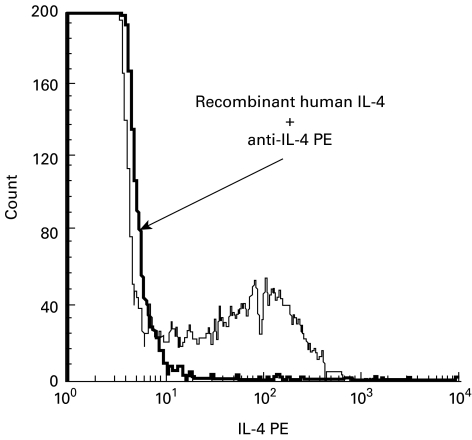
Blocking of intracellular IL-4 staining
To confirm the specificity of IL-4 staining, the anti-IL-4 antibody was first preincubated with recombinant human IL-4 prior to its addition to the cell sample obtained from RSV-infected infants. The binding of recombinant cytokine with anti-IL-4 MoAbs completely eliminated the intracellular IL-4-specific fluorescent staining in all lymphocyte subsets (Fig. 3).
Fig. 3.
Recombinant IL-4 blocks the binding of anti-IL-4 antibody with intracellular IL-4. Intracellular IL-4 staining following polyclonal stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin (thick line), and after the blocking of anti-IL-4 antibody by recombinant human IL-4 (thin line).
The bias toward IL-4 production was highest in children with mild infections
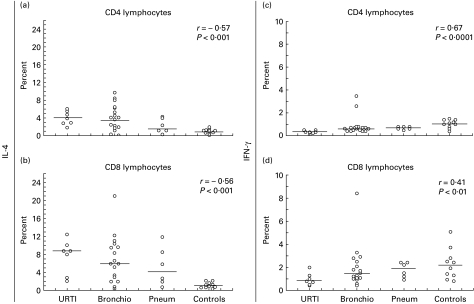
Our patients were a heterogeneous group of RSV-infected infants presenting different clinical pictures of RSV infection. Therefore, RSV-infected infants were divided according to the WHO criteria into groups with URTI (n = 7), bronchiolitis (n = 17), and pneumonia (n = 6). Than we tried to determine whether the observed variations in the percentage of cytokine-producing cells among infants included in the study were due to the severity of illness.
Concerning different RSV-caused clinical syndromes, IL-4 production within CD4 and CD8 subsets appeared to correlate negatively with the severity of disease (Fig. 4a,b). Conversely, IFN-γ production showed a positive correlation with the severity of disease (Fig. 4c,d).
Fig. 4.
Correlation between IL-4 production within CD4 (a) and CD8 (b) and IFN-γ within CD4 (c) and CD8 (d) among the groups of RSV-infected infants divided according to clinical syndromes: upper respiratory tract infection alone (URTI; n = 7), bronchiolitis (Bronchio; n = 17) and pneumonia (Pneum; n = 6), and controls (n = 10). Each symbol represents a tested individual. The relationship between variables was assessed by the Spearman rank order coefficient (r) and P level value. The lines represent median values.
Discussion
It is generally believed that type-1 or type-2 cytokine production by a particular lymphocyte subset determines the clinical outcome of different diseases [16]. Unlike the type-1 response (characterized by the production of IL-2 and IFN-γ), which has been associated with viral disease control and pathogen clearance, the type-2 response (which has been associated with the production of IL-4 and IL-5) has been shown to be incapable of controlling viral disease. In the present study we used intracellular cytokine staining to identify the phenotype of IL-4 and IFN-γ-producing cells. To minimize the amount of blood withdrawn from study children and to avoid the potential influence of gradient separation on isolated cells, we used the modified whole blood activation method first described by Jason et al. [24].
Our results showed the RSV-infected infants to exhibit an increased IL-4 and decreased IFN-γ production on short-term activation with PMA and ionomycin. These results are in agreement with the previous reports of increased IL-4 and decreased IFN-γ secretion in RSV-infected infants [10,13]. However, a major disadvantage of the ELISA method used in these studies is the unknown phenotype of the cells which produced the cytokines that were determined in supernatants within the bulk cell culture. In our tests, PMA and ionomycin stimulation polyclonally activated most of the cells, as confirmed by the expression of CD69, an early activation antigen. However, only a small percentage of cells did produce cytokines. The PMA and ionomycin stimulation appears to be consistent with the physiologically regulated cytokine expression and, when used for short periods of time, it only up-regulates the secretion of cytokines from cells with a defined cytokine pattern of expression [25]. We showed that CD4+ and CD8+ T lymphocyte subsets produced IL-4, a type-2 cytokine, during an RSV infection in infants. Both T cell subsets in age- and gender-matched controls produced IL-4, but in significantly lower amounts than in RSV-infected infants. The importance of skewing the immune response in RSV infection toward type-2 has been demonstrated in IL-4 depletion experiments, resulting in abrogated lung histopathology in mice [26,27]. Our data showed the CD4 lymphocyte skewing into type 2 to be accompanied by decreased IFN-γ production by these cells after short-term polyclonal stimulation in vitro. The ability of CD4+ T lymphocytes to promote an appropriate type-1 immune response was obviously altered.
Generally, in contrast to CD4, the CD8 T lymphocytes produce IFN-γ and exhibit a predominantly cytolytic activity for the elimination of viral and other intracellular pathogens [28]. Recent data show the CD8 T cells to produce type-1 and type-2 cytokines [15–18] and to modulate the humoral immune response [19,20,29]. We showed that CD8 T cells contributed to IL-4 production during RSV infection. A selective IL-4 production by the CD8 T lymphocyte subpopulation might be in correlation with the loss of cytotoxic activity, a phenomenon observed in infants with RSV infection [30]. In an RSV mouse experimental model, the administration of neutralizing anti-IL-4 MoAbs induced a shift from a type-2 to a type-1-like immune response, resulting in an improved clinical outcome and increased CD8 CTL activity due to an enhanced IFN-γ production [31].
Anderson et al. measured the specific memory response to RSV in healthy RSV+ adult donors and infants, showing no IL-2 and IFN-γ secretion, but there was an RSV-specific increase in IL-2 and IFN-γ mRNA. Some patients responded with increased IL-5 mRNA, but none with IL-4 mRNA. They concluded that a natural infection with RSV induced a predominantly type-1 immune response. Unlike our model, Anderson et al. used specific stimulation with purified viral proteins, without infection at the time of experiment [14].
Our data have surprisingly suggested that children develop a mild form of RSV infection if the antiviral response is characterized by predominant IL-4 production in peripheral blood T lymphocytes following polyclonal activation. The percentage of IL-4-producing cells in sick children was significantly higher than in healthy children and adults, and was comparable to the levels observed in adult patients suffering from allergic asthma [32]. Indeed, IFN-γ secretion is suggested to be critical for the development of RSV-induced wheezing [33]. We observed IFN-γ to be higher in children with severe disease. The reason why some previously healthy infants develop bronchiolitis, whereas others have only mild symptoms when exposed to RSV, is not known. We speculate that the immune cell's tendency to produce more IFN-γ in infants with bronchiolitis and pneumonia contributes to the patterns of illness during the antiviral immune response through an as yet unknown mechanism.
A recent finding [34] challenges the hypothesis that immaturity of the immune system in infants is linked with a predominantly type-2 immune response. We have also confirmed the previously published data that IFN-γ production positively correlates with age in healthy subjects; there was a minimal number of IL-4-producing cells across all tested age groups [35].
It remains to be determined in longitudinal studies whether the effect of RSV infection on cytokine profile is a transient phenomenon. We have previously reported [6] that only RSV-infected infants had a transiently increased percentage of CD23+ B cells during an acute illness, which was within the range of control values 4–5 weeks after RSV infection. Sheeran et al. found higher cytokine and chemokine concentrations in nasopharyngeal and tracheal aspirates obtained from RSV-infected infants, with a declining trend during the first 5 days of hospitalization [36]. It should be established whether the production of IL-4 and IFN-γ is affected by the timing of sample collection as well. Further, it may prove useful to investigate whether other viruses, that are not considered as risk factors for asthma development, lack the RSV-associated property to skew the initiated immune response toward type 2.
Acknowledgments
We would like to thank Professor D. Dekaris and Dr A. Sabioncello (Institute of Immunology, Zagreb) for constructive criticism, Professor S. Batinica for providing healthy control blood samples (Zagreb University Hospital Centre, Zagreb), J. Božikov BMath, DSc (School of Medicine, University of Zagreb) for statistical advice, and A. Bezić-Redovniković BA, for language editing. Grant support was form the Croatian Ministry of Science (021-001 and 108-158-GMG).
References
- 1.Martinez FD. Role of respiratory infection in onset of asthma and chronic obstructive pulmonary disease. Clin Exp Allergy. 1999;29:53–58. doi: 10.1046/j.1365-2222.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 2.Everard ML, Milner AD. The respiratory syncytial virus and its role in acute bronchiolitis. Eur J Pediatr. 1992;151:638–51. doi: 10.1007/BF01957564. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki H, Tsutsmi H, Matsuda K, Naga K, Ogra PL, Chiba S. Effect of maternal antibody response in nasopharyngeal secretion in infants and children during primary respiratory syncytial virus infection. J Gen Virol. 1994;75:2115–9. doi: 10.1099/0022-1317-75-8-2115. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–5. [PubMed] [Google Scholar]
- 5.Welliver RC, Sun M, Hildreth SW, Arumugham R, Ogra PL. Respiratory syncytial virus-specific antibody responses in immunoglobulin A and E isotypes to the F and G proteins and to intact virus after natural infection. J Clin Microbiol. 1989;27:295–9. doi: 10.1128/jcm.27.2.295-299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabatić S, Gagro A, Lokar-Kolbas R, et al. Increase in CD23+ B cells in infants with bronchiolitis is accompanied by appearance of IgE and IgG4 antibodies specific for respiratory syncytial virus. J Infect Dis. 1997;175:32–37. doi: 10.1093/infdis/175.1.32. [DOI] [PubMed] [Google Scholar]
- 7.Johnston SL. Viruses and asthma. Allergy. 1998;53:922–32. doi: 10.1111/j.1398-9995.1998.tb03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srikiatkhachorn A, Braciale T. Virus specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–85. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alwan W, Kozlowska W, Openshaw PJM. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson M, Scott R. Different patterns of cytokine induction in cultures of respiratory syncytial (RS) virus-specific human Th cell lines following stimulation with RS virus and RS virus proteins. J Med Virol. 1996;49:161–9. doi: 10.1002/(SICI)1096-9071(199607)49:3<161::AID-JMV2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BA. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–80. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defrance T, Aubry JP, Rousset F, et al. Human recombinant interleukin-4 induces Fcε receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987;165:1459–67. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Román M, Calhoun WJ, Hinton KL, Avendano LF, Simon V, Escobar AM, Gaggero A, Diaz PV. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med. 1997;156:190–5. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 14.Anderson LJ, Tsou C, Potter C, Keyserling HL, Smith TF, Ananaba G, Bangham CR. Cytokine response to respiratory syncytial virus stimulation of human peripheral blood mononuclear cells. J Infect Dis. 1994;170:1201–8. doi: 10.1093/infdis/170.5.1201. [DOI] [PubMed] [Google Scholar]
- 15.Seder RA, Le Gros G. The functional role of CD8+ T helper type 2 cells. J Exp Med. 1995;81:5–7. doi: 10.1084/jem.181.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossman TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 17.Byun DG, Demeure CE, Yang LP, et al. In vitro maturation of neonatal human CD8 T lymphocytes into IL-4 and IL-5 producing cells. J Immunol. 1994;153:4862–71. [PubMed] [Google Scholar]
- 18.Meissner N, Kussebi F, Jung T, et al. A subset of CD8+ T cells from allergic patients produces IL-4 and stimulates IgE production in vitro. Clin Exp Allergy. 1997;27:1402–11. doi: 10.1046/j.1365-2222.1997.1180931.x. [DOI] [PubMed] [Google Scholar]
- 19.Paganelli R, Scala E, Ansotegui IJ, et al. CD8+ lymphocytes provide helper activity for virus-infected patients with hyper-IgE. J Exp Med. 1995;181:423–8. doi: 10.1084/jem.181.1.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maggi E, Giudizi MG, Biagiotti R, et al. Th2 like CD8+ cells showing B cell helper function and reduced cytolytic activity in human immuno-deficiency virus type 1 infection. J Exp Med. 1994;180:489–95. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suppl. 1. National Health, Lung and Blood Inst.; 1995. Pocket guide for asthma management and prevention. [Google Scholar]
- 22.Lukić-Grlić A, Baće A, Lokar-Kolbas R, et al. Clinical and epidemiological aspects of respiratory syncytial virus lower respiratory tract infections. Eur J Epidemiol. 1999;15:361–5. doi: 10.1023/a:1007503302742. [DOI] [PubMed] [Google Scholar]
- 23.Mlinarić-Galinović G, Ugrčić J, Božikov J. Respiratory syncytial virus infections in SR Croatia, Yugoslavia. H Pediatr Pulmonol. 1987;3:304–8. doi: 10.1002/ppul.1950030505. [DOI] [PubMed] [Google Scholar]
- 24.Jason J, Larned J. Single-cell cytokine profiles in normal humans: comparison of flow cytometric reagents and stimulation protocols. J Immunol Methods. 1997;207:13–22. doi: 10.1016/s0022-1759(97)00079-3. [DOI] [PubMed] [Google Scholar]
- 25.Picker LJ, Singh MK, Zdraveski Z, Treer JR, Waldrop SL, Bergstresser PR, Maino VC. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 26.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connors M, Kulkarni AB, Firestone C, Holmes KL, Sotnikov AV, Murphy BR. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunised BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66:7444–51. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufmann SE. Immunity to intracellular microbial pathogens. Immunol Today. 1995;16:338–42. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 29.Renz H, Lack G, Saloga J, et al. Inhibition of IgE production and normalization of airway responsiveness by sensitized CD8 T cells in a mouse model of allergen induced sensitization. Immunology. 1994;152:351–60. [PubMed] [Google Scholar]
- 30.Isaacs D, Bangham CR, McMichael AJ. Cell-mediated cytotoxic response to RSV in infants with bronchiolitis. Lancet. 1987;2:769–71. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- 31.Tang YW, Graham BS. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest. 1994;94:1953–8. doi: 10.1172/JCI117546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagro A, Rabatić S, Ivančić I, et al. Detection of intracellular cytokines in human lymphocytes and monocytes at the single cell level by flow cytometry. Period Biol. 1999;101:17–26. [Google Scholar]
- 33.Van Schaik SM, Tristram DA, Nagpal IS, Hintz KM, Welliver RCII, Welliver RC. Increased production of interferon gamma and cysteinyl leukotrienes in virus-induced wheezing. J Allergy Clin Immunol. 1999;103:630–6. doi: 10.1016/s0091-6749(99)70235-6. [DOI] [PubMed] [Google Scholar]
- 34.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;17:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 35.Chipeta J, Komada Y, Zhang XL, Deguchi T, Sugiyama K, Azuma E, Sakurai M. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and cytotoxic type 1 cell populations with age. Cell Immunol. 1998;183:149–56. doi: 10.1006/cimm.1998.1244. [DOI] [PubMed] [Google Scholar]
- 36.Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sánchez PJ, Ramilo O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18:115–22. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]