Abstract
Pseudomonas aeruginosa-resistant BALB/c and susceptible C57Bl/6 (B6) mice were immunized with heat-killed Pseudomonas either in the foot pad or via the trachea, and panels of Pseudomonas-specific T cell clones were developed from lymph nodes and lungs. All clones from either strain, whether of lymph node or lung origin, were CD3+CD4+CD8−TCRαβ+. The efficacy of cloning from lymph node cells was comparable between BALB/c and B6 mice. All lymph node BALB/c clones proliferated in response to Pseudomonas antigen in a dose-dependent manner, and this response was MHC class II-restricted. Vigorous proliferation by a considerable proportion of B6 T cell clones occurred in the absence of specific antigen. Lymph node clones from either strain could be categorized as either Th1 or Th0 on the basis of interferon-gamma (IFN-γ)/IL-4 production. In either mouse strain the efficacy of cloning from lung tissue was substantially lower than from lymph nodes, but the efficacy of cloning from BALB/c compared with B6 lungs was higher. Four lung T cell clones from BALB/c and two from B6 mice were expanded for further analyses, and an interstrain difference was observed in cytokine production. Both B6 lung T cell clones were Th1-like and produced IFN-γ but not IL-4 and IL-10, whereas four BALB/c lung T cell clones were Th2-like and produced IL-4 and IL-10 but not IFN-γ. These observations suggest that differences in the CD4+ Th response in the lung may contribute to differences among inbred mouse strains in the level of resistance to bronchopulmonary Pseudomonas infection.
Keywords: Pseudomonas aeruginosa, T cell clones, specificity, cytokines, lungs
Introduction
Pseudomonas aeruginosa, a commensal Gram-negative bacillus commonly found in soil and water, is the major cause of chronic and often fatal bronchopulmonary infection in individuals with cystic fibrosis (CF). While it is clear that excessive neutrophilic inflammation contributes to persistent Pseudomonas infection and tissue damage in the CF lung [1], the exact nature of protective immune responses, particularly the role of T cells, remains unclear. Earlier studies showed that transfer of immune T cells protected Pseudomonas-infected intact as well as nude and granulocytopenic mice [2,3]. Studies by Markham & Powderly [4] demonstrated that T cell-mediated immunity to Pseudomonas was protective even in the absence of antibody production. Alternatively, T cells may provide B cell help for the production of protective antibody. In humans, T cells from normal individuals proliferate in vitro in response to Pseudomonas [5], as well as to bacterial components [6–8]. Evaluation of T cell function in CF patients demonstrated decreased T cell proliferation to Pseudomonas [9–11]. More recently, it was observed that there is a decreased percentage of peripheral Th cells as well as diminished function of these cells in terms of proliferation to Pseudomonas and help for antibody production by B cells in CF patients [12,13].
We have established a mouse model of chronic bronchopulmonary Pseudomonas infection and compared the level of resistance and of T cell responses among inbred mouse strains ([14], unpublished observations). Following intratracheal infection with mucoid Pseudomonas enmeshed in agar beads, BALB/c mice were found to clear the infection from their lungs more efficiently than C57Bl/6 (B6) mice through 3 weeks post-infection. Lung T cells from BALB/c mice exhibited significantly higher in vitro proliferative responses to heat-killed Pseudomonas than cells from B6 mice and were less sensitive to immune suppression mediated by adherent lung cells via production of nitric oxide and prostaglandins. In addition, BALB/c mice were found to have high Pseudomonas-specific DTH and low antibody responses in vivo, while B6 mice had low DTH and high antibody levels, in particular IgG2b and IgM. More recently, we have shown that the resistant phenotype of BALB/c mice is expressed as only limited granulomatous inflammation in the lung following challenge, without any major alterations in lung architecture [15–17]. In contrast, an acute, predominately neutrophilic inflammation, evident in the interstitium as well as the bronchoalveolar space, with tissue damage is characteristic of susceptible B6 mice. Moreover, the CD4/CD8 ratio was found to be significantly greater in BALB/c mice by 3 weeks post-infection. These results, which suggest the importance of T cells in acquired immunity to Pseudomonas, prompted us to study Pseudomonas-specific T cell responses at the clonal level. Here, we report on development and partial characterization of T cell clones obtained from lymph nodes and lungs of BALB/c and B6 mice following local immunization with heat-killed Pseudomonas.
Materials and methods
Mice
Age-matched BALB/cJCit (BALB/c) and C57Bl/6JCit (B6) female mice were used for T cell cloning, and mice of either sex of B10.A(4R), B10.MBR, CBA, B10.S and B10.D2 strains were used as the source of allogeneic antigen-presenting cells (APC). Mice were bred under conventional conditions in the animal facility of the Central Institute of Tuberculosis, Moscow, Russia. Water and food were provided ad libitum.
Bacteria
Pseudomonas aeruginosa, strain 508, was kindly provided by Dr J. Lagacé (Université de Montréal, Montréal, Québec, Canada). This strain has a mucoid appearance when grown on blood agar, and was originally isolated from the sputum of a CF patient at St-Justine Hospital, Montreal. Heat-killed (1 h, 60°C) bacteria were used throughout the study. Aliquots containing 2·5 × 1010 colony-forming units (CFU)/ml in sterile saline were stored at −20°C until use.
Immunization and T cell cloning
To develop T cell clones from lymph nodes, two mice of each strain were immunized in the foot pads with 50 µl per mouse containing 2·5 × 107 CFU, in the form of a 1:1 mixture of the heat-killed Pseudomonas suspension and Freund’s incomplete adjuvant (FIA; Sigma, St Louis, MO). Seven days after immunization, popliteal lymph nodes were removed aseptically and homogenized in Hanks’ balanced salt solution (HBSS) containing 2% fetal calf serum (FCS) and antibiotics (HyClone, Cramlington, The Netherlands). Aliquots of 4 × 106 cells/ml were cultured in RPMI 1640 containing 10% FCS, 10 mm HEPES, 4 mm l-glutamine, 5 × 10−5 m 2-mercaptoethanol (2-ME), and antibiotics (all components from HyClone) in 24-well plates (Costar, Cramlington, The Netherlands) for 48 h in the presence of 2·5 × 107 CFU/ml heat-killed bacteria as antigen. Live cells, enriched for blasts, were isolated by centrifugation at 2500 g for 20 min at 23°C on Lympholyte M (Cedarlane Labs, Hornby, Ontario, Canada). Cells were cloned by limiting dilution, starting at 1000 cells with 10-fold dilutions, in 96-well flat-bottomed plates (Costar) in the presence of 1·5 × 106/ml syngeneic APC, γ-irradiated at 12 Gy from a 60Co source, and antigen. Culture medium was supplemented with 5 μg/ml indomethacin (Sigma) and 10% conditioned medium (40 h supernatant from concanavalin A (Con A)-activated murine splenocytes, absorbed with 10 mg/ml α-methylmannoside) as a source of cytokines. To expand growing clones, every 10–14 days positive wells were restimulated, either in situ or split into new wells. This was achieved by replacement culture supernatants with fresh culture medium containing irradiated APC, antigen, conditioned medium and indomethacin. Two independent T cell cloning experiments were performed.
To develop T cell clones from lungs, three mice of each strain were immunized via the trachea with 109 heat-killed bacteria in 40 µl of sterile saline. The surgical procedure was previously described [14]. Briefly, in anaesthetized mouse the trachea was visualized by a transverse cervical incision, intubated with a flexible 22 G cannula attached to a 1-ml syringe and inoculated with 109 heat-killed bacteria in 40 μl of sterile saline. The incision was closed by suture. Seven days later, lung cell suspensions were prepared using a previously described modification [14,18] of the method described by Holt et al. [19]. Briefly, mice were anaesthetized, and blood vessels were washed by perfusion of the vena cava with 0·02% EDTA–HBSS. Repeated bronchoalveolar lavage with EDTA–HBSS via cannulated trachea was performed to remove alveolar cells. Lungs were removed, sliced into pieces, and enzymatically disrupted by incubation with 2 mg/ml collagenase (Sigma), 50 μg/ml DNase (Sigma) and 10 U/ml elastase (Boehringer, Mannheim, Germany). After vigorous pipetting and washing, single-cell suspensions of lung cells in complete RPMI 1640 were enriched for T cells by adherence on plastic for 1 h at 37°C followed by passage through a nylon wool (Polyscience, Warrington, PA) column. After incubation for 1 h at 37°C, cells were eluted with warm culture medium. Two independent T cell cloning experiments were performed as described above for lymph node cells, starting at 5000 cells per well.
Proliferation assay
Aliquots of 4 × 104 cells of each T cell clone plus 3 × 105 irradiated splenic APC from either syngeneic or allogeneic donors were co-cultured in 96-well plates at 37°C, 5% CO2 for 48 h. For each clone, two to three experiments were performed, either in duplicate or in triplicate depending on the cell yield. Antigen-specific stimulation was determined by adding 2·5 × 107 CFU/ml; non-stimulated wells served as controls. For the last 18 h, cultures were pulsed with 0·5 μCi/well 3H-methyl-thymidine. Cells were harvested onto fibreglass filters using a semiautomatic cell harvester (Skatron, Oslo, Norway) and radioactivity was measured in a liquid scintillation counter (Wallac Laboratory, Turku, Finland). Results are expressed as either mean ct/min or Δcpm (ct/minAg – ct/mincontrol) ± s.e.m. To establish whether BALB/c T clones were class II-restricted, a mixture of supernatants of anti-IAd hybridoma MKD6 and anti-IE hybridoma 4.14.4S (a kind gift from Dr V. Yurin, Institute for Genetics of Industrial Microorganisms, Moscow, Russia) was added to cultures at 1:20 final dilution.
Stimulation/rest protocol for generation of long-term T cell lines
Polyclonal T cell lines were generated from immune lymph nodes as previously described [20]. Briefly, 2 × 106 immune cells isolated from popliteal lymph nodes were cultured in complete RPMI 1640 for 10–12 days in the presence of antigen. Live immune cells, which by this point had reached the ‘rest’ phase of the cycle, were isolated on Lympholyte M as described above and restimulated by co-culturing 2 × 105 cells with 1·5 × 106 APC in the presence of antigen for another 10–12 days. This cycle was repeated until T cells were either lost or started to grow as a stable cell line.
Cell phenotyping
Cells of individual clones were harvested 3–5 days following antigen stimulation and live T cells were isolated on Lympholyte M. Cells were resuspended to 2–10 × 105/ml, washed twice in PBS containing NaN3 and 0·5% bovine serum albumin (BSA) and incubated for 30 min at 4°C in the same buffer containing 20% normal mouse serum to block non-specific antibody binding. Cells were single-stained with PE-labelled anti-CD3 or FITC-labelled anti-TCRαβ, or double-stained with PE-labelled anti-CD8 and FITC-labelled anti-CD4 antibodies diluted according to the instructions of the manufacturer (PharMingen, San Diego, CA) for 30 min at 4°C. Isotype-matched control antibodies were included in each staining. Stained cells were washed twice, fixed with 1% paraformaldehyde and analysed by flow cytometry on an EPICS ELITE flow cytometer (Coulter Corp., Hialeah, FL) equipped with a Cyonics argon laser (Uniphase, San Jose, CA). At least 104 cells per sample were analysed and the data processed with Multigraph software (Coulter). Unstained cells were analysed as a control for each T cell clone.
Cytokine assays
Cells of individual clone (2 × 105) and 1·5 × 106 irradiated splenic APC were co-cultured in 1 ml of culture medium in the presence or absence (control culture) of 2·5 × 107 heat-killed bacteria. Levels of interferon-gamma (IFN-γ), IL-4, IL-5, IL-10 and IL-12 were determined in 48-h culture supernatants by ELISA. Capture and detecting (biotinylated) MoAbs specific for mouse cytokines were purchased from PharMingen. ELISAs were performed following the manufacturer’s instructions for each cytokine. A standard curve for each assay was generated with mouse rIL-4 (PharMingen), rIL-5 (PharMingen), rIL-10 (Sigma), rIL-12 (PharMingen), and rIFN-γ (Genzyme, Boston, MA).
Results
Establishment of T cell clones
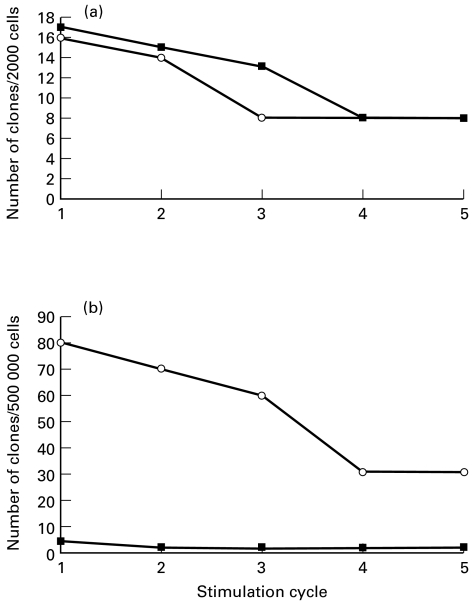
Despite vigorous T cell proliferation in bulk cultures, our initial attempts to develop Pseudomonas-specific T cell clones were unsuccessful. When the culture medium was supplemented with 5 μg/ml indomethacin, conventional growth of T cell clones occurred. As shown in Fig. 1a, T cell clones were cultured with equal ease from BALB/c and B6 lymph nodes, and no apparent defect in T cell responsiveness was evident in B6 lymph nodes. In total, panels of 12 BALB/c and 16 B6 T cell clones from lymph nodes were expanded.
Fig. 1.
Efficacy of T cell cloning from lymph nodes (a) and lungs (b) of BALB/c (○) and B6 mice (▪) immunized in the foot pad or via the trachea, respectively, with heat-killed Pseudomonas. T cell clones were established from lymph node and lung cells of immunized mice by limiting dilution in the presence of 1·5 × 106 syngeneic, γ-irradiated, splenic antigen-presenting cells (APC) and 2·5 × 107 colony-forming units (CFU)/ml heated-killed Pseudomonas. The number of cells indicated on ordinate of each panel corresponds to the total number of immune cells per plate.
The efficacy of T cell cloning from lung tissue of either mouse strain was very low (Fig. 1b). However, about 10-fold more T cell clones showed initial growth and a much slower decline in the numbers of surviving clones with time was observed in cells from BALB/c compared with B6 mice. The T cell response in the lungs of the Pseudomonas-resistant host, thus, is more vigorous and stable than that of the susceptible mouse strain. In total, we established four T cell clones from BALB/c lungs and two from B6 mice. Flow cytometry showed that the phenotype of all BALB/c and B6 clones, whether lymph node- or lung-derived, was CD3+CD4+CD8−TCRαβ+.
Antigen-specificity and genetic restriction
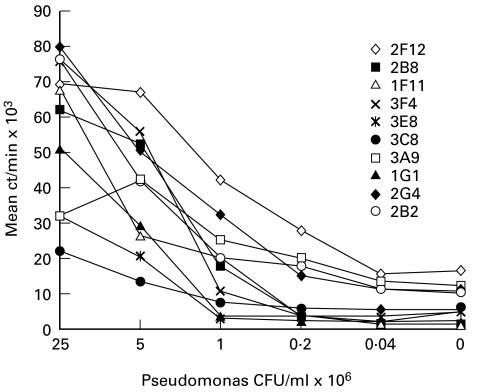
Next, T cell clones from both panels were characterized with respect to their antigen specificity and genetic restriction. As shown in Fig. 2, all BALB/c lymph node T cell clones showed a gradual loss of proliferative activity along the Pseudomonas antigen titration curve, i.e. all clones were antigen-specific. No or marginal responses of these H-2d clones were observed in the presence of allogeneic APC from B6 (H-2b), CBA (H-2k) and B10.S (H-2s) mouse strains, whereas the response in the presence of B10.D2, H-2d-matched, APC did not differ from that in the fully syngeneic system (data not shown). Addition of a mixture of MKD6 (anti-IAd) and 4.14.4S (anti-IE) antibodies to the cultures totally abrogated the response of all 10 BALB/c T clones tested (data not shown). Thus, all BALB/c T clones developed in our system were antigen-specific and class II-restricted, i.e. they were conventional CD4+ T cell clones.
Fig. 2.
Antigen specificity of T cell clones obtained from lymph nodes of BALB/c mice immunized in the foot pad with heat-killed Pseudomonas. Proliferation was determined between 14 and 18 days following the last stimulation of the clones. Clones (see legend for symbols and names ascribed to individual clones) were stimulated in the presence of syngeneic, irradiated, splenic antigen-presenting cells (APC) with indicated doses of heat-killed Pseudomonas (range 4 × 104−2·5 × 107 colony-forming units (CFU)/ml) for 48 h and pulsed for the last 16 h with 3H-thymidine. The results of one of three replicate experiments are shown. Data are presented as mean ct/min of triplicate cultures; the s.d. never exceeded 15%.
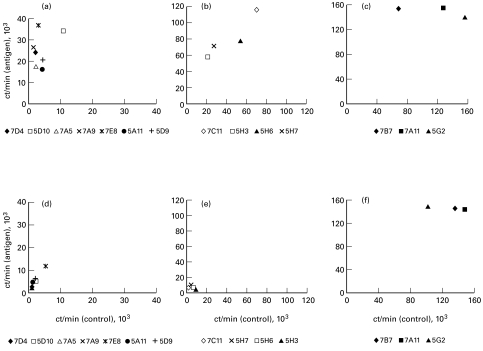
When the antigen specificity of B6 lymph node T cell clones was tested, many clones did not follow a normal antigen titration curve but vigorously proliferated in the absence of antigen. When the values of 3H-thymidine incorporation by individual clones in the presence and absence of antigen were plotted against each other at least two, and possibly three, distinct patterns of response were seen (Fig. 3). Seven of 14 B6 clones, which were low responders, clearly showed antigen specificity (stimulation index (SI) = (ct/minAg)/(ct/mincontrol), between 3 and 12, Fig. 3a). In contrast, much more vigorously proliferating clones showed no or marginal dependence on the presence of specific antigen (SI between 1 and 2·5, Fig. 3b,c).
Fig. 3.
Antigen specificity (a–c) and genetic restriction (d–f) of lymph node T cell clones from B6 mice immunized in the foot pad with heat-killed Pseudomonas. Proliferative responses of individual clones in the presence of syngeneic, irradiated, splenic antigen-presenting cells (APC) (a–c) and allogeneic APC from B10.MBR mice (d–f). Clones were stimulated with heat-killed Pseudomonas (2·5 × 107 colony-forming units (CFU)/ml) and mean ct/min values obtained in triplicate wells of control and antigen-stimulated cultures were plotted against each other. The results of one of three replicate experiments are shown; the s.d. never exceeded 25%.
To study the MHC restriction pattern of the response of B6 T cell clones, we used APC from intra-H-2 recombinant mouse strains sharing alleles of individual H-2 loci with B6 mice. In the presence of APC from the B10.A(4R) mouse strain, which differs by H-2K and IA alleles but shares D-end alleles with B6 mice, neither clone proliferated, irrespective of the presence of Pseudomonas antigen (data not shown). On the other hand, in the presence of APC from B10.MBR mice sharing the H-2Kb allele with B6 and the IAk allele with 4R, the response of all Pseudomonas-specific (Fig. 3d) and of some non-specific (Fig. 3e) T clones was abrogated, whereas the most vigorously responding three non-specific T clones continued to proliferate (Fig. 3f). One possible explanation for this result is that the clones displayed in Fig. 3b,e react against some product which is presented by the self IAb molecule (the latter is absent in B10.A(4R) and B10.MBR mice), whereas the latter three clones with unrestricted recognition pattern react with the product(s) presented by both self IAb and foreign IE molecules (the latter is not expressed by either B6 or B10.A(4R) cells). In both cases the response is not aimed against Pseudomonas antigens, although the initial activation of T cells might be dependent upon the presence of bacteria-derived substances.
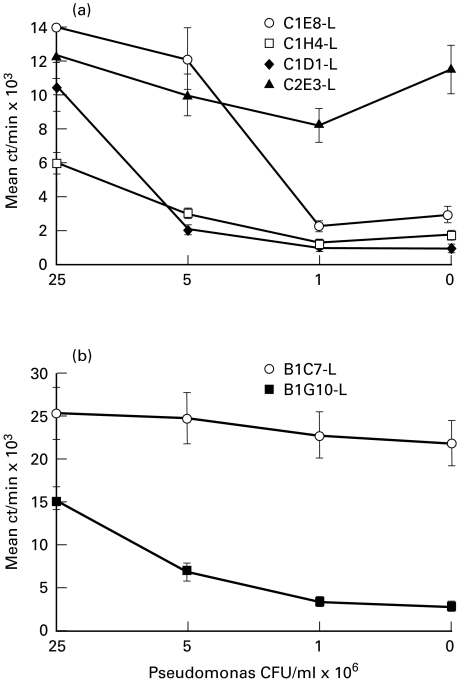
We also examined the antigen specificity of the lung-derived T cell clones. Interestingly, one out of four BALB/c clones, 2F3 (Fig. 4a), and one out of two B6 clones, 1C7 (Fig. 4b), were not antigen-specific.
Fig. 4.
Antigen specificity of T cell clones obtained from lungs of BALB/c (a) and B6 (b) mice immunized via the trachea with heat-killed Pseudomonas. Proliferation was determined between 14 and 18 days following the last stimulation of the clones. Clones (see legend for symbols and names ascribed to individual clones) were stimulated in the presence of syngeneic, irradiated, splenic antigen-presenting cells (APC) with indicated doses of heat-killed Pseudomonas (range 4 × 104−2·5 × 107 colony-forming units (CFU)/ml) for 48 h and pulsed for the last 16 h with 3H-thymidine. The results of one of three replicate experiments are shown. Data are presented as mean ct/min of triplicate cultures; the error bars represent s.e.m.
Polyclonal response of long-term T cell lines to Pseudomonas
B6 mice had defective Pseudomonas-specific T cell responses in vitro and in vivo following infection with live bacteria [14] and appeared to readily develop non-specific T cell response following immunization with heat-killed Pseudomonas. Thus we studied the ability of BALB/c and B6 T cells to retain long-term Pseudomonas-specific polyclonal responses in vitro. To address this question, immune lymph node cells were isolated and subjected to repeated cycles of rest and antigen stimulation.
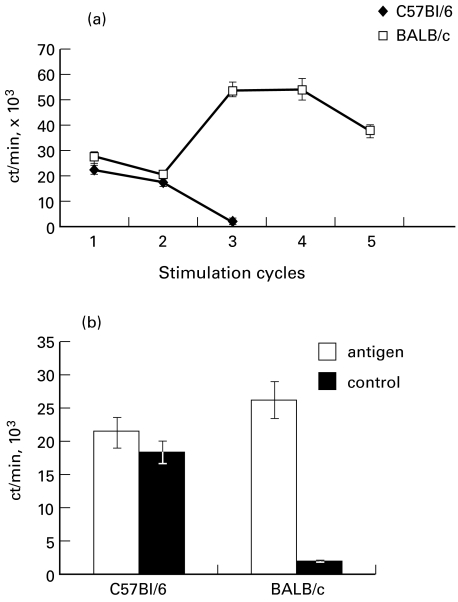
As shown in Fig. 5, repeated stimulation of T cells from BALB/c mice resulted in development of a stable antigen-specific T cell line following the fourth stimulation cycle. In contrast, the T cells from B6 mice were lost after the third in vitro stimulation cycle (Fig. 5a). Thus, the defect in T cell response of susceptible B6 mice was confirmed in this in vitro system. Interestingly, a relatively high level of proliferation in B6 lymph node cells freshly isolated ex vivo was due almost exclusively to a non-specific component of response, which is in the sharp contrast to the response of their BALB/c counterparts (Fig. 5b).
Fig. 5.
Lymph nodes cells from BALB/c but not from B6 Pseudomonas-immune mice developed into a stable T cell line following repeated cycles of rest and stimulation with heat-killed Pseudomonas (a), and displayed exclusively Pseudomonas-specific proliferative response (b). Data are presented as mean ct/min. Comparison between Pseudomonas-stimulated and non-stimulated cultures (b) was performed at the first stimulation cycle. The results of one out of four similar independent experiments are presented; error bars represent s.d.
Cytokine production
Th1/Th2 cytokine profiles were evaluated in supernatants of poly and monoclonal T cell cultures of BALB/c and B6 origin. Lymph node cells isolated 7 days after immunization produced IL-12 but not IFN-γ or IL-4, indicating that they were obtained at a very early phase of the response. IL-12 production in cultures of cells from BALB/c mice was fully dependent upon in vitro simulation with antigen. In two independent experiments, IL-12 levels ranged from 880 to 1040 pg/ml and 80 to 120 pg/ml in stimulated and control cultures, respectively. In contrast, IL-12 levels in B6 cultures were 1720–2460 pg/ml in stimulated cultures versus 760–880 pg/ml in control cultures. Thus, the bulk IL-12 production paralleled the clonal proliferative response.
There were no differences in cytokine profiles between T cell clones from lymph nodes of BALB/c and B6. All BALB/c and B6 clones developed from lymph nodes produced IFN-γ, with a range of 10–100 pg/ml for individual clones. In addition, about half of the clones from either strain produced significant amounts of IL-4 (range 250–2000 pg/ml). Thus, T cell clones from lymph node cells of either strain were of either Th1 or Th0 type. However, among T cell clones of lung origin a clear bias toward a Th2-like response was found in BALB/c mice as opposed to a Th1-like response in B6 mice. As shown in Table 1, both the antigen-specific (1G10) and the non-specific (1C7) B6 lung T cell clones produced IFN-γ. Consistent with the observation of high non-specific proliferation, clone 1C7 produced high levels of IFN-γ in the absence of antigenic stimulation. In contrast, all four lung T cell clones from BALB/c mice produced IL-4 and IL-10, but not IFN-γ. The bacteria non-specific clone, 2F3, produced high amounts of IL-4 and IL-10 in the absence of antigen.
Table 1.
Th1/Th2-like dichotomy between T cell clones from lungs of B6 and BALB/c mice*
| Mouse strain | T clone | PA-specific proliferation† | IFN-γ | IL-4 | IL-10 |
|---|---|---|---|---|---|
| B6 | 1G10 | + | 125 (14) | 0 (0) | 0 (0) |
| 1C7 | − | 73 (146) | 0 (0) | 0 (0) | |
| BALB/c | 1D8 | + | 0 (0) | 52 (0) | 2100 (0) |
| 1H4 | + | 0 (0) | 385 (117) | 1140 (0) | |
| 1D1 | + | 0 (0) | 75 (0) | 2300 (0) | |
| 2F3 | − | 0 (0) | 2700 (830) | 4370 (1140) |
Concentrations of factors are expressed in pg/ml. Figures in parentheses, concentrations in control (Pseudomonas aeruginosa-non-stimulated) cultures. Cytokine contents in two independently obtained sets of supernatants of each clone were measured by ELISA in three two-fold serial dilutions and are expressed as arithmetic means (s.d. of < 10% among triplicate determinations multiplied by the titre). The results of one experiment are displayed. Positive results obtained in two independent experiments differed by < 30%. 0, Concentration lower than the sensitivity of the ELISA. Note that Ps. aeruginosa-non-specific clones 1C7 and 2F3 produced significant amounts of, respectively, IFN-γ and IL-4/IL-10 in the absence of antigen.
See Ps. aeruginosa titration curves in Fig. 4.
Discussion
Evidence from experimental studies in mice [2–4] and in humans [5–13] suggests an important role for T cells in the development of immunity to Ps. aeruginosa. Earlier we demonstrated that differences among inbred mouse strains in the level of resistance to chronic bronchopulmonary Pseudomonas infection correlated with the level of T cell responses in vivo and in vitro ([14,16] and unpublished observations). In the present study we established T cell clones from lymph nodes and lungs of resistant BALB/c and susceptible B6 mice following local immunization with heat-killed bacteria in order to understand further the role of T cells in immunity to Pseudomonas.
Previous studies by our laboratory [21] and others [22,23] demonstrated a low frequency of lung T cell clonal growth. We were surprised however to observe a similar situation with lymph node cells from heat-killed Pseudomonas-immunized mice. We reasoned that under limiting dilution conditions, where only a few T cells are present, proliferation may be totally abrogated by mediators such as prostaglandins or nitric oxide (NO), which are known to suppress T cell proliferation and production of the T cell growth factor, IL-2 [24–27]. Prostaglandin-mediated suppression of lung T cell proliferation to Pseudomonas was observed in our earlier study [14]; indeed, conventional growth of T cell clones was observed when indomethacin was added to culture medium.
Despite similar cloning efficiencies of T cells from lymph nodes of BALB/c and B6 mice, there were clear differences in the antigen specificity and genetic restriction of expanded T cell clones from the two mouse strains. Whereas all lymph node T cell clones from BALB/c mice were antigen-specific and MHC class II-restricted, a large proportion of B6 clones proliferated vigorously in the absence of antigen. The role of non-specific inflammation in pathogenesis of Pseudomonas-triggered disease is well established [1], but this concerns mainly neutrophil-mediated reactions. To our knowledge, this is the first report on the induction of a substantial non-specific component within the spectrum of T cell responses that develop following immunization of mice with this bacterium. The possible role of these T cells in pathogenic and/or defensive Pseudomonas-provoked inflammation remains to be evaluated. Since non-specific T clones possess a strong proliferative capacity (Fig. 3) and actively produce cytokines in a strain-specific manner (Table 1), they might contribute to either proinflammatory, IFN-γ-dependent or anti-inflammatory, IL-10-dependent responses of the host to the lung colonization with Pseudomonas. It was shown for certain types of lung pathology, e.g. lung transplantation and cancer, that IFN-γ produced by T cells, along with macrophage-produced prostaglandins, may promote the fibrosis of lung tissue [15]. This observation is consistent with our data on much more severe lung pathology in Ps. aeruginosa-infected B6 compared with BALB/c mice [16,17]. Further elucidation of the role of non-specific T cell responses induced by Pseudomonas is in progress, and comparison of several H-2 congenic mouse strains has demonstrated that this type of response develops predominantly in mice carrying H-2b haplotype, irrespective of the genetic background (N. Kobets, unpublished observations).
In addition, lymph node T cells from immune BALB/c mice in bulk cultures, subjected to repeated stimulation with APC and antigen, readily developed into a stable, polyclonal T cell line. In contrast, T cells from B6 mice did not survive repeated stimulation in polyclonal cultures, although they were successfully cloned under limiting dilution conditions. There are several possible explanations for this finding. First, this discrepancy may be explained by technical differences in the maintenance of poly and monoclonal cultures: polyclonal cultures were maintained without the addition of exogenous cytokines. This implies that T cells from BALB/c mice do not require exogenous growth factors, while T cells from their B6 counterparts may be dependent. Second, among non-specific T cells, which are abundant in polyclonal B6 cultures, there could be autoreactive clones that inhibit the growth of Pseudomonas-specific T cell clones and then cease to proliferate themselves. Since autoreactive populations are apparently lacking among BALB/c T cells, antigen-specific T clones accumulate in these cultures and eventually form a stable line.
When the responses of T cell clones prepared from the lungs of Pseudomonas-resistant and susceptible mice were compared, important interstrain differences were observed. First, the frequency of T cell clones initially activated in the interstitial lung tissue following intratracheal immunization with heat-killed bacteria was many times higher in BALB/c compared with B6 mice. This may be due to marked differences between these mouse strains in the magnitude of lung T cell responses [14,16]. Second, lung T cell clones from the two mouse strains, unlike T cells from lymph nodes, differed in their cytokine profiles. Lung T cell clones from B6 mice, which do not efficiently clear bronchopulmonary Pseudomonas infection and experience predominantly neutrophilic inflammation and lung tissue damage [14–16], were Th1-like and produced IFN-γ, a potent activator of macrophages for bactericidal activity. In contrast, clones from the lungs of BALB/c mice, which efficiently clear the infection and experience less severe tissue-damaging inflammation, consisting of predominately macrophages, were Th2-like and produced IL-4 and IL-10 but not IFN-γ. These data are consistent with recent observations that transgenic mice expressing IL-4 in respiratory epithelial cells, as well as conventional mice which received IL-4 intranasally, combat Pseudomonas infection much more efficiently than the control animals [28].
Previous studies have demonstrated a role in protection against lung Pseudomonas infection for IFN-γ production by CD4+ T cells from orally immunized rats [29], as well as an anti-inflammatory effect of exogenous IFN-γ in the rat model of chronic bronchopulmonary infection [30]. However, our results parallel several lines of evidence suggesting an important role for IL-10 in protecting peripheral organs from tissue damage. Groux et al. [31] recently proposed an immunoregulatory role for a novel subset of CD4+ T cells, designated T regulatory 1 (Tr1) cells, apparent during chronic activation, which produce high levels of IL-10 and suppress antigen-specific immune responses and down-regulate pathological immune responses in vivo. Furthermore, we observed an unusual predominance of IL-10-producing T cell clones in the lungs of mice chronically infected with Mycobacterium tuberculosis [21]. Recent studies in mice during acute pneumonia or following intraperitoneal Pseudomonas infection provide evidence for a protective role of IL-10 versus the disease-promoting effect of the proinflammatory cytokine, IFN-γ [32,33]. Importantly, statistically significant differences in the levels of several cytokines, including IL-10, were found between CF patients with Pseudomonas infection and healthy subjects, whereas non-infected CF patients did not differ from healthy controls [34].
Taken together, our results suggest that differences between T cell clones derived from Pseudomonas-resistant and -susceptible mice in terms of their antigen specificity and profile of cytokine production, in particular IL-10 production by lung-derived clones, may contribute to control of infection versus tissue damage in the lung. Studies are in progress in our laboratories using mice deficient in IL-10, either by treatment with a neutralizing anti-IL-10 MoAb or by targeted deletion of the IL-10 gene, to investigate further the in vivo role of IL-10 in resistance to bronchopulmonary Pseudomonas infection.
Acknowledgments
This work was supported by the grant No. 96-04-49543 from the Russian Foundation for Basic Research and by the Canadian Cystic Fibrosis Foundation.
References
- 1.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Ped Pulmon. 1997;24:137–42. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Pier GB, Markham RB. Induction in mice of cell-mediated immunity to Pseudomonas aeruginosa by high molecular weight polysaccharide and vinblastin. J Immunol. 1982;128:2121–9. [PubMed] [Google Scholar]
- 3.Powderly WG, Pier GB, Markham RB. T lymphocyte-mediated protection against Pseudomonas aeruginosa infection in granulocytopenic mice. J Clin Invest. 1986;78:375–80. doi: 10.1172/JCI112587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markham RB, Powderly WG. Exposure of mice to live Pseudomonas aeruginosa generates protective cell-mediated immunity in the absence of antibody response. J Immunol. 1988;140:4112–7. [PubMed] [Google Scholar]
- 5.Porwell WG, Gebel HM, Rodey GE, Markham RB. In vitro response of human T cells to Pseudomonas aeruginosa. Infect Immun. 1983;40:670–4. doi: 10.1128/iai.40.2.670-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parmerly MJ, Iglewski BH, Horvat RT. Identification of the principal T lymphocyte-stimulating antigens of Pseudomonas aeruginosa. J Exp Med. 1984;160:1338–49. doi: 10.1084/jem.160.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulmer AJ, Pryjma A, Tarnok Z, Ernst M, Flad HD. Inhibitory and stimulatory effects of Pseudomonas aeruginosa pyocyanine on human T and B lymphocytes and human monocytes. Infect Immun. 1990;58:808–15. doi: 10.1128/iai.58.3.808-815.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mody CH, Buser RM, Syme RM, Woods DE. Pseudomonas aeruginosa exoenzyme S induces proliferation of human T lymphocytes. Infect Immun. 1995;63:1800–5. doi: 10.1128/iai.63.5.1800-1805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen RU, Stern RC, Polmar SH. Cellular immunity to bacteria: impairment of in vitro lymphocyte response to Pseudomonas aeruginosa in cystic fibrosis patients. Infect Immun. 1977;18:735–40. doi: 10.1128/iai.18.3.735-740.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen RU, Stern RC, Polmar SH. Lymphocyte responsiveness to Pseudomonas aeruginosa in cystic fibrosis: relation to status of pulmonary disease in sibling pairs. J Pediatr. 1978;93:201–5. doi: 10.1016/s0022-3476(78)80496-x. [DOI] [PubMed] [Google Scholar]
- 11.Van Geffel R, Hubert E, Urbain J. Study of helper and suppressor T cells in cystic fibrosis. Immunol Letters. 1982;5:155–9. doi: 10.1016/0165-2478(82)90101-8. [DOI] [PubMed] [Google Scholar]
- 12.Knutsen AP, Slavin RG, Roodman KR, et al. Decreased T helper cell function in patients with cystic fibrosis. Intl Arch Allergy Appl Immunol. 1988;85:847–50. doi: 10.1159/000234504. [DOI] [PubMed] [Google Scholar]
- 13.Lahat N, Rivlin J, Iancu TC. Functional immunoregulatory T cell abnormalities in cystic fibrosis patients. J Clin Immunol. 1989;9:287–95. doi: 10.1007/BF00918660. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson MM, Kondratieva TK, Apt AS, et al. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1995;99:98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark JG, Madtes DK, Hackman RC, et al. Lung injury induced by alloreactive Th1 cells is characterized by host derived mononuclear cell inflammation and activation of alveolar macrophages. J Immunol. 1998;161:1913–20. [PubMed] [Google Scholar]
- 16.Tam M, Snipes GJ, Stevenson MM. Characterization of chronic bronchopulmonary Pseudomonas aeruginosa infection in resistant and susceptible inbred mouse strains. Am J Respir Cell Mol Biol. 1999;20:710–9. doi: 10.1165/ajrcmb.20.4.3223. [DOI] [PubMed] [Google Scholar]
- 17.Sapru K, Stotland PK, Stevenson MM. Quantitative and qualitative differences in bronchoalveolar inflammatory cells in Pseudomonas-resistant and susceptible mice. Clin Exp Immunol. 1999;115:103–9. doi: 10.1046/j.1365-2249.1999.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apt AS, Kramnik IB, Moroz AM. Regulation of T-cell proliferative responses by cells from solid lung tissue of M. tuberculosis infected mice. Immunol. 1991;73:173–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Holt PG, Robinson BWS, Reid M, et al. Extraction of immune and inflammatory cells from human lung parenchyma: evaluation of enzymatic digestion procedure. Clin Exp Immunol. 1986;66:188–200. [PMC free article] [PubMed] [Google Scholar]
- 20.Pichugin AV, Khaidukov SV, Moroz AM, Apt AS. Capacity of murine T cells to retain long-term responsiveness to mycobacterial antigens is controlled by H-2 complex. Clin Exp Immunol. 1998;111:316–24. doi: 10.1046/j.1365-2249.1998.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyadova I, Eremeev V, Majorov K, et al. An ex vivo study of T lymphocytes recovered from the lungs of I/St mice infected with and susceptible to Mycobacterium tuberculosis. Infect Immun. 1998;66:4981–8. doi: 10.1128/iai.66.10.4981-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt PG, Kees UR, Shon-Hegard MA, et al. Limiting dilution analysis of T cells extracted from solid human lung tissue: comparison of precursor frequencies for proliferative responses and lymphokine production between lung and blood T cells from individual donors. Immunol. 1988;64:649–54. [PMC free article] [PubMed] [Google Scholar]
- 23.Strickland D, Thepen T, Kees R, et al. Regulation of T cell function in lung tissue by pulmonary alveolar macrophages. Immunol. 1993;80:266–72. [PMC free article] [PubMed] [Google Scholar]
- 24.Chouaib S, Welte K, Mertelsmann R, Dupont P. Prostaglandin E2 acts at two district pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985;135:1172–9. [PubMed] [Google Scholar]
- 25.Holt PG, Oliver J, Bilyk N, et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills CD. Molecular basis of suppressor> macrophages. Arginine metabolism via nitric oxide synthetase pathway. J Immunol. 1991;146:2719–23. [PubMed] [Google Scholar]
- 27.Taylor-Robinson AW, Liew FY, Severn A, et al. Regulation of the immune response by nitric oxide differentially produced by Th1 and Th2 cells. Eur J Immunol. 1994;24:980–8. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 28.Jain-Vora S, LeVine AM, Chroneos Z, et al. Interleukin-4 enhances pulmonary clearance of Pseudomonas aeruginosa. Infect Immun. 1998;66:4229–36. doi: 10.1128/iai.66.9.4229-4236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunkley ML, Clancy RL, Cripps AS. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunol. 1994;83:362–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Johansen HK, Hougen HP, Rygaard J, Hoiby N. Interferon-gamma (IFN-γ) treatment decreased the inflammatory response in chronic Pseudomonas aeruginosa pneumonia in rats. Clin Exp Immunol. 1996;103:212–8. doi: 10.1046/j.1365-2249.1996.d01-618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groux H, O’Garra A, Bigler M, et al. A CD4+ T cell subset inhibits antigen-specific T cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 32.Sawa T, Corry DB, Gropper MA, et al. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol. 1997;159:2858–66. [PubMed] [Google Scholar]
- 33.Fruh R, Blum B, Mossman T, et al. Th1 cells trigger tumor necrosis factor-alpha mediated hypersensitivity to Pseudomonas aeruginosa after adoptive transfer into SCID mice. Infect Immun. 1995;63:1107–12. doi: 10.1128/iai.63.3.1107-1112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonfield TL, Panuska JR, Konstan MW, et al. Inflammatory cytokines in the cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]