Abstract
Chlamydia pneumoniae infection is associated with atherosclerosis and the organism has been identified in arterial lesions. To determine whether T lymphocyte-mediated immune responses to Chlamydia antigens within plaque could contribute to pathogenesis, we have derived T cell lines from atherosclerotic plaques of 32 patients. Culture with IL-2 alone proved insufficient for cellular activation and expansion, but additional stimulation with phytohaemagglutinin (PHA) or recall antigens allowed consistent establishment of T cell lines. Furthermore, in cultures of approx. 500 tissue fragments, Chlamydia organisms proved as effective as other recall antigens in producing outgrowth of arterial T cells (20–25% wells produced T cell lines). Testing the antigen responsiveness of T cell lines showed that those derived using Chlamydia organisms were more likely to respond to Chlamydia (5/29+) than those isolated using other stimuli (6/69+ for PHA; 5/57+ for PPD and tetanus toxoid (TT)). However, lines responsive to each of the recall antigens were observed. Using recombinant Chlamydia antigens, some Chlamydia-specific T cell lines were shown to respond to OMP2 and/or hsp60. Those recognizing Chlamydia hsp60 did not cross-react with human hsp60, but human hsp60-responsive lines were also observed. Thus, atherosclerotic plaque tissue contains a variety of memory T lymphocytes, and amongst these are cells capable of recognizing Chlamydia antigens. In a C. pneumoniae-infected plaque, such T cells may be activated by local antigen and could contribute to the inflammatory process in the arterial wall through CD40 ligand expression and cytokine secretion.
Keywords: atherosclerosis, T lymphocytes, Chlamydia, immunology, hsp60
Introduction
Increasing evidence has been presented suggesting a role for infection with Chlamydia pneumoniae (Cpn) in the pathogenesis of atherosclerosis. Initial observations of an increased prevalence of antibodies to Cpn in patients with coronary or carotid arterial disease [1–3], have been followed by demonstration of the organism within atherosclerotic plaque tissue, firstly by using polymerase chain reaction (PCR) methods [4–6] and more recently by culture [7,8]. The organism has the ability to infect several relevant cells including vascular endothelium [9], macrophages [10,11] and arterial smooth muscle cells [12]. There is also evidence that it can induce foam cell formation in macrophages [13], and in certain animal models of atherosclerosis Cpn infection has been shown to exacerbate disease [14,15].
Nevertheless, in humans the precise role of the organism in pathogenesis remains unclear. Macrophages, which have encountered and been infected with Cpn in the respiratory tract, may later be recruited to arterial lesions, or direct infection of plaque cells by circulating Cpn could also occur. However, in both cases it is possible that the organism is essentially a ‘passenger’ within the plaque with no active role in pathogenesis [16]. On the other hand, Cpn antigens could provoke an immune response which would contribute to the inflammatory nature of the atherosclerotic lesion. Chlamydiae are intracellular organisms, and elicit predominantly T cell-mediated immune responses [17–19]. Atherosclerotic plaque tissue is also rich in T cells, and it has recently been shown that interference with a cell surface molecule, CD40 ligand, expressed by activated T cells reduces experimental atherosclerosis [20]. Thus, it can be postulated that a proportion of plaque T lymphocytes is specific for Chlamydia antigens presented by cells within the plaque. Similar arguments have been advanced in favour of T cell responses to other antigens, such as oxidized low-density lipoproteins (LDL) [21], or hsp60 [22,23].
This study was designed to investigate the hypothesis that T cell responses to Chlamydia antigens might contribute to the pathogenesis of atherosclerosis by generating T cell lines from arteriosclerotic plaque tissue, and determining whether any of them were able to recognize Chlamydia antigens. We now report the establishment of such T cell lines. A proportion of the lines responded to Chlamydia antigens including hsp60. We also show however, that plaque-derived T cell lines could also recognize other recall antigens (e.g. tetanus toxoid (TT) and PPD). Thus it would appear that memory T cells can be recruited non-specifically to arterial plaques, where their local activation will depend on availability of specific antigen. In the case of Cpn-specific T cells, antigen may be present locally in Cpn-infected plaque cells.
Materials and methods
Antigens
Chlamydia trachomatis (Ct), serovar L2, was grown and titrated in HeLa cells as previously described [24]. Organisms were stored at −20°C.
Recombinant human, Ct, and Cpn hsp60, and OMP2 from Ct and Cpn were produced from DNA by PCR amplification and cloning into a pQE60 expression vector (Qiagen, Chatsworth, CA) which was used to transform Escherichia coli (M15 cells). This enabled the expression of recombinant proteins with a C-terminal histidine tag. Bacterial lysates containing the relevant antigen were sonicated and centrifuged six times in buffer (pH 7·8, 50 mm Na-phosphate, 30 mm NaCl) and solubilized in 6 m urea. Antigens were then purified by elution from nickel-containing His-Trap columns (Pharmacia, St Albans, UK) using 300 mm imidazole. All antigens were used at a final concentration of 10 μg/ml, previously shown to be optimal for T cell stimulation.
Establishment of T cell lines from atheroma-derived lymphocytes
Carotid endarterectomy material was obtained from a total of 32 patients. Initially material from plaque, wall and core sections of the lesion (as previously defined [25]) was treated separately; in each case the tissue was washed in PBS followed by RPMI (Gibco BRL, Paisley, UK) to remove any residual contaminating peripheral blood cells, dissected into approximately 1-mm2 pieces and maintained in 2-ml cultures in RPMI supplemented with 5% human AB + serum and 1× antibiotic/antimycotic solution (Sigma, Poole, UK) for 4–6 weeks. Dissected material was cultured in the presence and absence of 10–100 U/ml IL-2 and the emergence and expansion of T cells assessed microscopically. In subsequent experiments, since no difference between plaque and wall tissue had been observed (see Results), these regions of the lesion were used together. In this second protocol, replicate 1-mm2 pieces of lesional tissue were cultured in RPMI as before with the addition of IL-2 plus periodic stimulation with either 2 μg/ml phytohaemagglutinin (PHA; Murex Diagnostics, Dagenham, UK), 10 μg/ml TT (Statens Seruminstitut, Copenhagen, Denmark), 10 μg/ml PPD of Mycobacteriam bovis (Evans Medical, Liverpool, UK), or Ct (5 × 108 organisms/ml). To compare the effect of each antigenic stimulus tissue from each patient was divided between 48 2-ml cultures, with 12 cultures stimulated with each of the antigens or mitogen tested.
Proliferation assays
T cell lines derived from endarterectomy specimens were assayed for antigen specificity by proliferation assay. Cells were harvested from the 2-ml culture wells, washed three times in PBS and then seeded at 1–5 × 105/ml in 0·2-ml round-bottomed tissue culture plates with 2 × 105/ml irradiated autologous peripheral blood mononuclear cells (PBMC), as antigen-presenting cells (APC), and antigen or mitogen. Proliferative responses were determined by addition of 1 μCi/well 3H-thymidine (Amersham, Aylesbury, UK) during the last 6 h of a 3-day culture period. Cells were harvested and thymidine incorporation determined by scintillation counting. Following expansion of T cell lines in vitro using PHA (see below), antigen specificity was re-evaluated by proliferation as above. In all cases, the background 3H-thymidine incorporation by irradiated PBMC ± antigen, and by T cells cultured alone, was also measured. T cell lines were classified as antigen-responsive if the mean ct/min in response to APC plus antigen was significantly different (P < 0·05) from the mean ct/min in responses to APC alone and the stimulation index (SI) ((ct/min in response to APC + antigen)/(ct/min in response to APC alone)) was > 2.
Re-stimulation of lines
T cell lines demonstrating responses to antigen in the initial assay were re-stimulated and expanded in vitro by culture with PHA (2 μg/ml), IL-2 and 2 × 105 allogeneic irradiated PBMC. Fifteen to 20 days later the specificity of T cell lines was reassessed by proliferation assay.
Flow cytometry
T cell lines obtained from atheromatous plaque or wall were stained with antibodies to CD4 or CD8 (Dako A/S, Glostrup, Denmark), and T cell receptor (TCR) αβ or γδ (Becton Dickinson, Mountain View, CA), or appropriate isotype controls, all diluted to appropriate concentrations in 0·1% albumin, 0·01% NaN3. Cells were fixed (2% formaldehyde) and analysed on a Becton Dickinson flow cytometer.
PBMC responses to recall antigens
Peripheral blood was obtained at the same time as endarterectomy. PBMC were isolated by density centrifugation over Ficoll–Hypaque (Pharmacia). Proliferative responses to Ct elementary bodies (EB), Ct hsp60, TT (Statens Seruminstitut), PPD (Evans Medical) and PHA were evaluated using a 5-day 3H-thymidine incorporation assay (5 × 106 cells/ml) as described above. Remaining cells were cryopreserved for use as APC.
Micro-immunofluorescence
The presence of antibodies to Ct and Cpn in patients' plasma was determined by micro-immunofluorescence (MIF) (Labsystems, Helsinki, Finland). Serial dilutions of 1:32–1:512 were used, with values > 1/32 being designated positive.
Statistical analysis
Differences in mean proliferative responses were assessed by Student's t-test. The significance of differences in the proportions of lines obtained using a particular stimulus or with responses to a given antigen was assessed using R×C tests of independence, using both the G-test initially (including William's correction for small sample size), and then pairwise comparisons made by χ2.
Results
Culture with PHA and IL-2, but not IL-2 alone, allows establishment of T cell lines from atherosclerotic plaque
A total of 32 carotid endarterectomy samples was obtained, subdivided, and cultured in vitro as described in Materials and Methods. Analysis of the first 18 samples demonstrated that culture with IL-2 alone (a range of 10–100 U/ml was tried) failed to allow the emergence of T cells from the tissue regardless of the duration of culture or the concentration of IL-2 used (data not shown). However, when PHA was used as a non-specific stimulus for T cells in addition to IL-2, T cells emerged from the tissue and were expanded as T cell lines. The time required for the emergence of T cells was 4–6 weeks, substantially longer than that required for isolating T cell lines from other tissues such as synovium [26]. T cells could be isolated equally well from areas of biopsy previously designated [25] as wall and plaque, but not from the core.
Flow cytometric analysis of nine biopsy-derived T cell lines demonstrated the presence of both CD4+ and CD8+ cells. Ratios of CD4+ and CD8+ T cells within the lines ranged from 67% CD4+:29% CD8+ to 35% CD4+:62% CD8+. Mean values were 40·7% CD4+, 52·8% CD8+, with the majority of both subsets (88–93%) bearing the αβ TCR rather than γδ TCR (4–7%).
It would appear therefore that IL-2 alone is insufficient for the generation of T cell lines from plaque material, and that a stimulus which is able to activate T cells either by engaging the antigen-specific TCR or, in the case of PHA, CD2/CD3, is needed for successful outgrowth of T cells from the atheromatous lesion material.
Stimulation with recall antigens, including Chlamydia, also allows the isolation of T cells lines from atherosclerotic lesions
Since the non-specific T cell stimulus, PHA, allowed the isolation of T cell lines, stimulation of cultures with the recall antigens PPD and TT, for which most normal individuals have T cell memory, was also tested using tissue from a further 14 patients. In view of the possibility that Chlamydia-specific T cells might be amongst those present in atheromatous tissue, we also tested the effect of Chlamydia antigens in the form of Ct elementary bodies. Ct was used rather than Cpn because it was readily available at high titre. As discussed below, Ct and Cpn have a number of highly homologous antigens, and we have previously identified T cells which recognize both Ct and Cpn antigens [27]. Evidence of previous infection with Chlamydia in the patients from which endarterectomy material was used in these studies was evaluated by: (i) PBMC proliferative responses to Ct elementary bodies and to Ct hsp60, and (ii) antibody titres to Ct and Cpn as determined by MIF. Details are given in Table 1; 8/11 had antibodies to Cpn compared with 1/11 who had antibodies to Ct. Seven of 13 showed significant PBMC proliferative responses to Ct and 6/13 to Ct hsp60. Three of 13 showed a response to both antigens.
Table 1.
Chlamydia infection status of patients used in this study
| Age (years)/ | Proliferative responses (SI) of PBMC to | Titres of antibodies to | |||
|---|---|---|---|---|---|
| Patient ID | sex | Ct EB | Ct hsp60 | Cpn | Ct |
| WD | 77 M | 1·02 | 5·6 | – | – |
| 484 | 88 M | ND | ND | ND | ND |
| SZ | 75 M | 2·2 | 7 | 128 | – |
| TR | 64 M | 6·6 | 3·2 | – | – |
| MI | 78 M | 1·2 | 0·75 | 512 | – |
| HP | 55 M | 6·8 | 2 | 128 | – |
| 7464 | 73 M | 3·2 | 6·4 | 128 | – |
| PN | 76 F | 1·5 | 6·9 | ND | ND |
| RK | 76 M | 2·9 | 2·5 | 512 | – |
| FR | 64 F | 79 | 56 | 32 | 32 |
| 195 | 67 M | 3·3 | 2 | 512 | – |
| TR | 71 M | 3·6 | 2·1 | 128 | – |
| ST | 79 M | 27·5 | 2·8 | ND | ND |
| 5868 | 74 M | 1·5 | 2·5 | – | – |
Peripheral blood mononuclear cell (PBMC) proliferative responses to Chlamydia trachomatis (Ct) elementary bodies (EB) and Ct hsp60. Serum antibody titres to Chlamydia pneumoniae (Cpn) and Ct, as determined by micro-immunofluorescence assay (MIF). Results are represented as reciprocal titres with a titre < 1/32 being negative (–). ND, Patients not tested; SI, stimulation index.
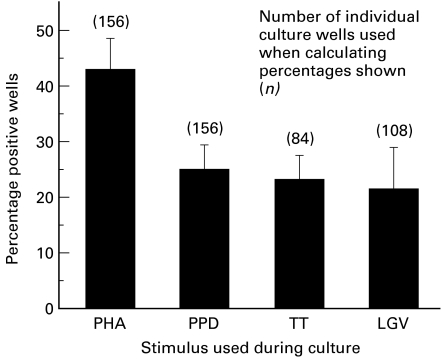
Forty-eight arterial cultures were set up from each patient, using each of the three recall antigens and PHA. As shown in Fig. 1, the recall antigens PPD and TT were both effective in allowing T cell lines to be established, although both stimuli were less efficient than PHA. Outgrowth of a T cell line was considered to have occurred if upon subsequent testing the T cell line showed a proliferative response to PHA of > 10 000 ct/min (see below). Likewise, T cell lines were readily obtained from cultures which included Ct as a stimulatory antigen. Comparison of the frequencies with which T cell lines were established confirmed that PHA was significantly more effective than any of the recall antigens (P < 0·05). However, there was no significant difference between any of the recall antigens used, and each was markedly more efficient than IL-2 alone.
Fig. 1.
Production of T cell lines from endarterectomy plaque material following mitogenic or antigenic stimulation. Plaques from patients were divided into 48 replicate cultures and 12 wells stimulated with either phytohaemagglutinin (PHA), PPD, tetanus toxoid (TT) or Chlamydia trachomatis (Ct) plus IL-2. Wells in which outgrowth of T cells was visible microscopically were assayed for T cell proliferative responses to antigen or mitogen in the presence of irradiated autologous peripheral blood mononuclear cells (PBMC). Wells in which T cells gave a proliferative response > 10 000 ct/min following PHA restimulation were scored positive as having produced T cell lines. Results show the proportion of wells (overall mean values) giving rise to T cell lines for each of the stimuli used in the initial cultures. Although PHA was significantly better at allowing establishment of T cell lines from biopsy material (P > 0·05), there were no significant differences between the three recall antigens in their ability to produce T cell lines (P < 0·05).
Therefore, although antigenic (or mitogenic) stimulation was required for the successful generation of T cell lines, Chlamydia antigens were as effective as other recall antigens in achieving this.
Culture with Chlamydia antigens preferentially produces T cell lines which show responsiveness to Chlamydia on secondary testing
The atheromatous lesion-derived T cell lines were tested in proliferation assay for their ability to respond to the original antigen used to stimulate T cell outgrowth and the other recall antigens. Responses to PHA were used as a positive control. The results are summarized in Table 2.
Table 2.
Percentages of lines reactive to PPD, tetanus toxoid (TT), and Chlamydia trachomatis (Ct) produced from cultures stimulated with either antigen or mitogen, from a total of 14 patients
| Stimulus used during culture | Number of PPD-reactive lines produced | Number of TT-reactive lines produced | Number of Ct-reactive lines produced | Number of lines unresponsive to antigens tested |
|---|---|---|---|---|
| PHA | 20 | 9 | 6 | 38 |
| PPD | 7 | 7 | 1 | 28 |
| TT | 4 | 6 | 4 | 8 |
| Ct | 2 | 2 | 8 | 28 |
The number of patients producing at least one line specific for PPD was 13, for TT was eight and for Ct was 10. Lines capable of responding to more than one antigen were also observed. PHA, Phytohaemagglutinin.
Activation of all atheroma-derived T cells with the non-specific mitogen PHA generated T cells lines some of which were able to respond to each of the three recall antigens tested. Responses to PPD were most common, followed by TT-responsive lines, with a lower percentage of Ct-responsive lines. Using PPD as the antigenic stimulus, the proportions of lines obtained which recognized PPD and/or TT were similar, but again, a significantly smaller proportion of lines responded to Chlamydia (P < 0·05). A smaller number of T cell lines from tissue stimulated with TT was available for testing in proliferation assays but there were no significant differences in the proportions responding to each of the recall antigens. Strikingly however, when Chlamydia was used as an antigenic stimulus the resulting T cell lines were much more likely to recognize Chlamydia than either of the other recall antigens (P > 0·05). In addition, a variable proportion of the T cell lines produced with each of the stimuli had no response to any of the antigens tested. Amongst the antigen-responsive lines the SIs recorded in response to each of the recall antigens were similar: the mean response to Ct was 4·2 (range 2·1–16·0), to TT 5·3 (range 2·1–39·0), and to PPD 5·8 (range 2·1–56·0).
Further investigation of atheroma-derived T cell lines which showed evidence of antigen responsiveness on first testing
T cell lines from atheroma tissue which responded to either PPD, TT or Ct were expanded by restimulation with PHA, IL-2 and allogeneic irradiated PBMC. PHA was chosen because of limited availability of autologous APC from the patients. In a proportion of T cell lines, the original antigen response pattern was no longer evident following this non-specific restimulation and expansion, whilst in a few other cases, responses to a different recall antigen emerged. However, in most cases the original specificity (for PPD, TT or Ct) was maintained.
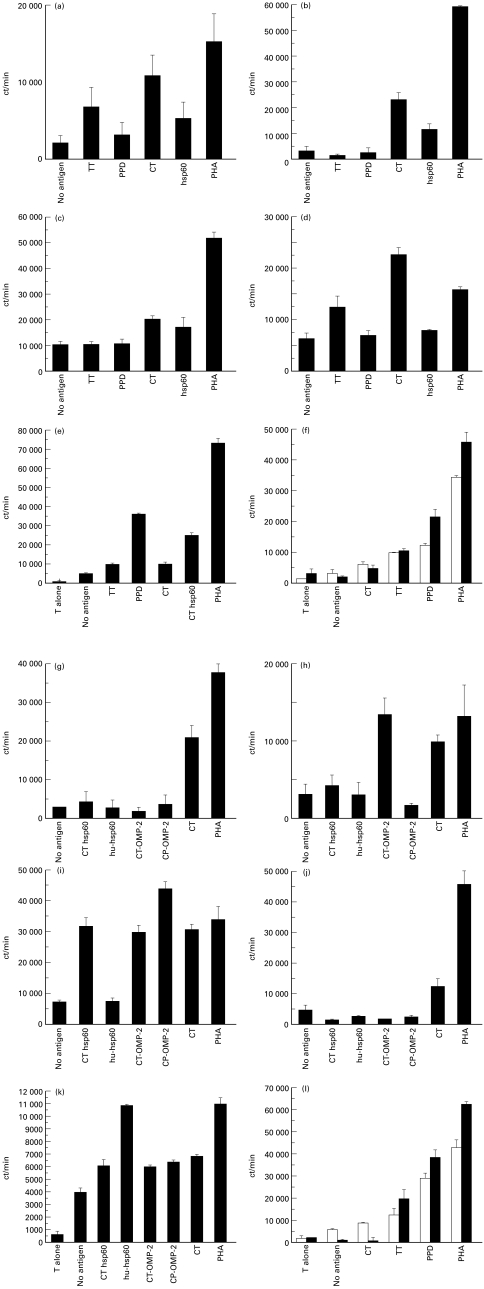
For T cell lines maintaining responses to Chlamydia it was possible to investigate which antigens were recognized, using recombinant antigens from C. trachomatis or C. pneumoniae. Examples of four T cell lines derived from a single patient (identified as HP in Table 1) are shown in Fig. 2. Proliferative responses from an initial screen are shown in panels a–d (left), and the proliferative responses following expansion in the corresponding panels g–j (right). Two of the four lines (a,g and d,j) maintained Ct responsiveness although they failed to respond to any of the recombinant antigens tested. One line (c,i) recognized both Ct OMP2 and Ct hsp60. This line was also able to recognize the corresponding Cpn antigens. However, the response to Chlamydia hsp60 did not cross-react with human hsp60. The fourth line (b,h) recognized Ct OMP2 but not Cpn OMP2.
Fig. 2.
Proliferative responses of T cell lines following initial isolation, and following in vitro expansion. The ability of T cell lines cultured from endarterectomy material to respond to PPD, tetanus toxoid (TT) and Chlamydia trachomatis (Ct) was evaluated by proliferation assay. Examples from this initial screen are shown in (a–e). Lines that showed a response to chlamydial antigens were expanded through non-specific (phytohaemagglutinin (PHA)) re-stimulation and subsequently re-tested to (i) ensure chlamydial specificity was maintained, and (ii) evaluate defined antigenic responses using recombinant antigens from Ct and Chlamydia pneumoniae (Cpn). Human hsp60 prepared in the same Escherichia coli expression vector was used as a control. These results for each line are shown in the adjacent panel, i.e. (g–k). PPD and TT lines were also expanded by PHA restimulation. Specificity could also be maintained and enhanced as shown by the examples in (f,l). □, The initial screen; ▪, the secondary screen. Results are depicted as ct/min and represent triplicate means with standard error bars.
Amongst other cell lines tested one line (e,k) responded to human hsp60 (patient ID 7464, Table 1); although on initial testing a marginal response to Ct was evident, the response to Ct and Ct or Cpn recombinant antigens on retesting was not significantly higher than the response to APC alone. The reactivity to human hsp60 in this case appeared to be independent of a response to Chlamydia hsp60. Lastly, maintenance of the same pattern of antigen responsiveness after non-specific expansion with PHA was not unique to Chlamydia-responsive lines. Similar results were observed following expansion of both TT- and PPD-responsive lines (examples from patient ID 5868), as shown (f,l).
Discussion
In this study we have shown that it is possible to derive T cell lines from atherosclerotic plaque tissue removed at carotid endarterectomy, although obviously this represents a late-stage lesion. Several features of the protocol required for isolation are worthy of comment. First, using IL-2 alone T cell lines were rarely established. This contrasts with our experience of culturing synovium, colonic epithelium and sural nerve tissue where IL-2 alone was sufficient to ensure T cell outgrowth. It does however, support previous findings where endarterectomy-derived T cells required stimulation with anti-CD3 plus IL-2 [21,28]. This suggests that the majority of the cells in the plaque are not responsive to IL-2, and presumably in a resting state having down-regulated IL-2 receptor expression. The second feature noted was that prolonged culture (4–6 weeks) was required to establish lines; this is reminiscent of the time required to establish T cell lines from sural nerve and quite different from the few days required for synovium. This timing may reflect the fact that the T cells are embedded deep in the organized plaque tissue or have specific attachments to other cells or the extracellular matrix. It is possible that prior digestion of the plaque (e.g. with collagenase) might allow T cells to be isolated more readily, and might also reveal a small population of IL-2-responsive cells which otherwise does not expand sufficiently to emerge from the tissue.
In contrast with the results obtained using IL-2 alone, T cell lines were regularly established using stimuli which act on TCR, including the mitogen PHA and common recall antigens (PPD and TT). Although the use of these stimuli necessarily imposes a selection pressure and cannot reveal anything about the properties of plaque T cells which are unable to proliferate in vitro, it represents a reasonable strategy for determining whether Chlamydia-specific T cells are part of the plaque population, and if so, whether T cells specific for other common recall antigens can also be detected. In contrast, PHA represents a universal stimulus to T cells and, in the presence of IL-2, is able to activate even anergic cells. The results obtained would be consistent with a requirement for activation of T cells in situ, subsequent expression of IL-2 receptors and outgrowth of T cell lines in the presence of added IL-2 and perhaps other cytokines made by the stimulated T cells. Amongst the antigens which allowed T cell isolation was C. trachomatis (Ct). This organism was used because it is more readily grown in vitro than C. pneumoniae, but it shares a set of highly homologous antigens with Cpn, including hsp60, where we have shown directly that a T cell epitope in hsp60 recognized by Ct-specific T cells is completely conserved in Cpn [27]. Further more, in a recent study [29] published whilst this manuscript was in preparation, Chlamydia-responsive T cell lines were established from aortic aneurysm (AA) tissue. Six out of eight of these lines that showed a response to Cpn also cross-reacted with Ct.
Our results show that certain plaque-derived T cell lines are able to recognize Chlamydia antigens. However, we also clearly show that the same T cell lines can recognize other recall antigens such as PPD and TT. This recognition of PPD was also observed in many of the AA-derived T cell lines mentioned above [29]. This is turn suggests that memory T cells of many specificities can be recruited to the inflammatory environment of the arterial plaque. There was not an exact correlation between the antigen used in the generation of the T cell line and its specificity on subsequent testing. This might be expected if stimulation of PPD-specific T cells in the plaque by culture in vitro with PPD results in cytokine production which either activates, or improves the survival of bystander T cells of other specificities such as Chlamydia or TT. Such activation/survival promotion would not occur with IL-2 alone (as shown above), but would require the support of activated APC allowing interactions between costimulatory molecules such as CD40 or CD80/86 on the APC and their counterparts on T cells. These cells would then be expanded during the secondary in vitro stimulation with PHA, and could come to dominate a particular line. Stimulation of plaque tissue with PHA produced T cell lines responsive to each of the three antigens tested in significantly different proportions. This could reflect the frequency of T cells with a particular antigen specificity in the plaque itself, and would imply a lower proportion of Ct-specific T cells. This idea is supported by the lower percentage of Ct-responsive lines produced following stimulation with PPD. Again in line with this notion, a lower number of Ct-specific T cells in the plaque would give rise to less bystander activation and therefore less outgrowth of T cells with other antigenic specificities. This would account for the enrichment of Chlamydia-reactive lines amongst those obtained using Ct.
Given that memory T cells of any specificity can be recruited to the arterial plaque, the mere isolation of Chlamydia-specific T cells does not in itself implicate them in the pathogenesis of atherosclerosis. However, unlike antigens such as PPD and TT, there is evidence that Chlamydia antigen is present in the plaque, since the organism can be isolated from the tissue. Therefore, following recruitment to the lesion, which might indeed be non-specific, Chlamydia-specific T cells might be activated by presentation of specific antigen. To prove this point it would be necessary to show that the frequency of Chlamydia-specific T cells is substantially higher in plaque relative to peripheral blood, and different from the relative frequencies of other memory T cells in plaque and peripheral blood, since memory cells may be preferentially recruited to sites of inflammation [30,31]. This may be achieved if TCR used by Chlamydia-specific T cells are characterized and the relative frequency of these TCR in unmanipulated plaque tissue and peripheral blood from the same patient can be determined. Although previous studies of TCR have suggested that T cells in atheromatous plaque tissue are polyclonal [28,32], expansions of Chlamydia-specific T cells could still be identified and quantified by TCR analysis [33].
Alternatively, it might be possible to demonstrate that Chlamydia-specific T cells are in a different activation state to those which recognize other recall antigens. However, our failure to isolate T cell lines using IL-2 alone suggests that few of the cells in plaque are activated. Also, in an atherosclerotic lesion which evolves over a large number of years (and in tissue which is obtained in the late stages of this process), it is possible that evidence of prior activation by local antigen which originally contributed to pathology may no longer be detectable. We did not analyse cytokine production by our antigen-responsive T cell lines, since the long period of in vitro culture is likely to modify this substantially; in future studies it may be possible to identify cytokines being made by T cells in plaque which express a Chlamydia-specific TCR prior to expansion in vitro, e.g. by in situ hybridization with a TCR CDR3-specific probe combined with intracellular staining for cytokine. However, this work would build on prior in vitro characterization of the expanded antigen-specific T cells from part of the plaque tissue, followed by immunohistology studies on frozen sections from adjacent tissue.
We have isolated a significant number of Chlamydia-reactive lines that maintained this specificity through non-specific PHA expansion, and similar results were obtained with PPD- and TT-reactive lines. This has allowed us to proceed beyond other studies with plaque-derived T cell lines and to analyse more specifically the Chlamydia antigens recognized by plaque-derived T cells using recombinant antigens from both Cpn and Ct. Our hypothesis that stimulation in vitro with Ct might allow the isolation of Cpn-reactive T cells was supported by the isolation of a T cell line with responses to both Cpn and Ct OMP2, and indeed a preferential response to the Cpn protein. Although recombinant antigen preparations contain low levels of contamination by lipopolysaccharide (LPS; < 0·2 endotoxin units (EU) /μg protein), the responses could not be attributed to this since T cell lines distinguished clearly between the different recombinant antigens. We have also shown an example of a T cell line specific for Ct OMP-2 which does not cross-react with Cpn OMP-2. This would be most easily explained if the patient had previously encountered Ct and had T cell memory for its antigens; in these circumstances Ct-specific memory T cells could be recruited to the plaque as easily as memory T cells of any other specificity. However, there is no confirmatory evidence that this patient was previously infected by Ct, and the findings do not imply that Ct infection induced the atherosclerosis. The Ct MIF result was negative, but antibody responses to Ct may decline with age and become undetectable by MIF. In addition, we cannot exclude the possibility that more extensive analysis of the response to Ct OMP2 would show some degree of cross-reactivity with Cpn OMP2, or that its primary specificity is for another antigen with cross-reactivity confined to Ct OMP2.
Amongst the Ct antigens to which we showed reactivity is hsp60. There is a significant body of work implicating immune responses to bacterial hsp60 in a number of forms of inflammation [34,35], and evidence for such a mechanism in atherosclerosis has been obtained from both clinical studies and experimental models [36–39]. One issue is whether pathological responses to hsp60 are primarily directed towards bacteria-specific epitopes, or whether the response can also be directed against self hsp60, since there is substantial amino acid sequence similarity between bacterial and mammalian hsp60. In addition, Chlamydia hsp60 has been shown to co-localize with human hsp60 in plaque-derived material and share properties such as increasing expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in infected endothelial cells and production of tumour necrosis factor-alpha (TNF-α) and IL-6 from smooth muscle cells [40]. The responses of our lines to Ct hsp60 were shown to be Chlamydia-specific by their lack or cross-reactivity with human hsp60 prepared in the same E. coli expression vector system. We have however also produced T cells lines showing responses to human hsp60 from endarterectomy material. In this case there was also reactivity to irradiated autologous APC alone, i.e. in the absence of added antigen. This may not be surprising, since such cells will express hsp60 and may be able to present it to T cells, albeit at a suboptimal level which is enhanced by addition of exogenous antigen.
Further characterization of the T cell lines described in this study by cloning and epitope mapping is now underway. This will establish the degree of conservation of epitopes in Cpn- and Ct-derived proteins and, where the epitopes show some variation in amino acid sequence, whether the T cells show preferential responses to the Cpn epitope. Likewise, epitopes recognized in human hsp60 need to be defined to determine whether they are in regions of the hsp60 molecule which are generally conserved between humans and bacteria. In view of the recent demonstration of cross-reactivity between a T cell-recognized epitope in Chlamydia OMP2 and cardiac myosin [41], characterization of the response to OMP2 will also be of interest.
Acknowledgments
This work was funded by grants from the British Heart Foundation and the Arthritis Research Campaign.
References
- 1.Thom D, Grayston J, Siscovick D, Wang S, Weiss N, Daling J. Association of prior infection with Chlamydia pneumoniae and angiographically demonstrated coronary artery disease. JAMA. 1992;268:68–72. [PubMed] [Google Scholar]
- 2.Melnick S, Sharar E, Folsom A, Grayston J, Sorlie P, Wang S, Szklo M. Past infection with Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Am J Med. 1993;95:499–504. doi: 10.1016/0002-9343(93)90332-j. [DOI] [PubMed] [Google Scholar]
- 3.Patel P, Mendall M, Carrington D, Strachan D, Leatham E, Molineaux N, Levy J. Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. Brit Med J. 1995;311:711–4. doi: 10.1136/bmj.311.7007.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo C, Shor A, Campbell L, Fukushi H, Patton D, Grayston J. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–9. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 5.Kuo C, Grayston J, Campbell L, Goo Y, Wissler R, Benditt E. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old) Proc Natl Acad Sci USA. 1995;92:6911–4. doi: 10.1073/pnas.92.15.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasi F, Denti F, Erba M, et al. Detection of Chlamydia pneumoniae but not Helicobacter pylori in atherosclerotic plaques of aortic aneurysms. J Clin Microbiol. 1996;34:2766–9. doi: 10.1128/jcm.34.11.2766-2769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson LA, Campbell LA, Kuo CC, Rodriguez DI, Lee A, Grayston JT. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–5. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez JA, Ahkee S, Summersgill JT, et al. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–82. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Fryer RH, Schwobe EP, Woods ML, Rodgers GM. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Invest Med. 1997;45:168–74. [PubMed] [Google Scholar]
- 10.Kaukorantatolvanen SSE, Teppo AM, Laitinen K, Saikku P, Linnavuori K, Leinonen M. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb Pathog. 1996;21:215–21. doi: 10.1006/mpat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 11.Gaydos C, Summersgill J, Sahney N, Ramirez J, Quinn T. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–20. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoebel E, Vijayagopal P, Figueroa JE, Martin DH. In vitro infection of smooth muscle cells by Chlamydia pneumoniae. Infect Immun. 1997;65:503–6. doi: 10.1128/iai.65.2.503-506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalayoglu MV, Byrne GI. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177:725–9. doi: 10.1086/514241. [DOI] [PubMed] [Google Scholar]
- 14.Muhlestein JB, Anderson JL, Hammond EH, Zhao LP, Trehan S, Schwobe EP, Carlquist JF. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–6. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- 15.Laitinen K, Laurila A, Pyhala L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–5. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capron L. Chlamydia in coronary plaques—hidden culprit or harmless hobo? Nature Med. 1996;2:856–7. doi: 10.1038/nm0896-856. [DOI] [PubMed] [Google Scholar]
- 17.Johansson M, Schon K, Ward M, Lycke N. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-gamma: is this true for humans? Scand J Immunol. 1997;46:546–52. doi: 10.1046/j.1365-3083.1997.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 18.Hassell AB, Reynolds DJ, Deacon M, Gaston JSH, Pearce JH. Identification of T-cell stimulatory antigens of Chlamydia trachomatis using synovial fluid-derived T-cell clones. Immunology. 1993;79:513–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Halme S, Vonhertzen L, Bloigu A, Kaprio J, Koskenvuo M, Leinonen M, Saikku P, Surcel HM. Chlamydia pneumoniae-specific cell-mediated and humoral immunity in healthy people. Scand J Immunol. 1998;47:517–20. doi: 10.1046/j.1365-3083.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- 20.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–3. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 21.Stemme S, Faber B, Holm J, Wiklund O, Witztum J, Hansson G. T lymphocytes from human atherosclerotic plaques recognize oxidised low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–7. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Q, Dietrich H, Steiner H, Gown A, Schoel B, Mikuz G, Kaufmann S, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- 23.Wick G, Schett G, Amberger A, Kleindienst R, Xu QB. Is atherosclerosis an immunologically mediated disease? Immunol Today. 1995;16:27–33. doi: 10.1016/0167-5699(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 24.Kuo C-C, Wang S-P, Grayston J. Growth of trachoma organism in HeLa 229 cell culture. In: Hobson D, Holmes K, editors. Nongonococcal urethritis and related infections. Washington DC: American Society for Microbiology; 1977. pp. 328–36. [Google Scholar]
- 25.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson G. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1985;6:131–8. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 26.Viner N, Bailey L, Life P, Bacon P, Gaston J. Isolation of Yersinia specific T cell clones from the synovial membrane and synovial fluid of a patient with reactive arthritis. Arthritis Rheum. 1991;34:1151–7. doi: 10.1002/art.1780340911. [DOI] [PubMed] [Google Scholar]
- 27.Deane K, Jecock R, Pearce J, Gaston J. Identification and characterization of a DR4-restricted T cell epitope within chlamydia hsp60. Clin Exp Immunol. 1997;109:439–45. doi: 10.1046/j.1365-2249.1997.4711371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stemme S, Rymo L, Hansson G. Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest. 1991;65:654–9. [PubMed] [Google Scholar]
- 29.Halme S, Juvonen T, Laurila A, Morosin M, Saikku P, Surcel H-M. Chlamydia pneumoniae reactive T lymphocytes in the walls of abdominalaortic aneurysms. Eur J Clin Invest. 1999;29:546–52. doi: 10.1046/j.1365-2362.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 30.Pitzalis C, Kingsley G, Covelli M, Meliconi R, Markey A, Panayi G. Selective migration of the human helper-inducer memory T cell subset—confirmation by in vivo cellular kinetic studies. Eur J Immunol. 1991;21:369–76. doi: 10.1002/eji.1830210218. [DOI] [PubMed] [Google Scholar]
- 31.Galea P, Brezinschek R, Lipsky P, Oppenheimer-Marks N. Phenotypic characterization of CD4-/αβ TCR+ and γδ TCR+ T cells with a transendothelial migratory capacity. J Immunol. 1994;153:529–41. [PubMed] [Google Scholar]
- 32.Oksenberg JR, Stavri GT, Jeong MC, Garovoy N, Salisbury JR, Erusalimsky JD. Analysis of the T-cell receptor repertoire in human atherosclerosis. Cardiovasc Res. 1997;36:256–67. doi: 10.1016/s0008-6363(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 33.Bieganowska KD, Ausubel LJ, Modabber Y, Slovik E, Messersmith W, Hafler DA. Direct ex vivo analysis of activated, Fas-sensitive autoreactive T cells in human autoimmune disease. J Exp Med. 1997;185:1585–94. doi: 10.1084/jem.185.9.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaston JSH. Heat shock proteins and arthritis—new readers start here. Autoimmunity. 1997;26:33–42. doi: 10.3109/08916939709009548. [DOI] [PubMed] [Google Scholar]
- 35.van Eden W, Hogervorst E, Wauben M, vanderZee R, Boog C. Heat shock proteins as antigens in autoimmunity. Biochem Soc Trans. 1991;19:171–5. doi: 10.1042/bst0190171. [DOI] [PubMed] [Google Scholar]
- 36.Xu QB, Willeit J, Marosi M, et al. Association of serum antibodies to heat-shock protein-65 with carotid atherosclerosis. Lancet. 1993;341:255–9. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- 37.Xu Q, Wick G. Stress proteins in atherogenesis. In: van Eden W, Young D, editors. Stress proteins in medicine. New York: Marcel Dekker; 1996. pp. 445–64. [Google Scholar]
- 38.Xu Q, Kleindienst R, Waitz W, Dietrich H, Wick G. Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions in rabbits specifically responding to heat shock protein 65. J Clin Invest. 1993;91:2693. doi: 10.1172/JCI116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu QB, Kleindienst R, Schett G, Waitz W, Jindal S, Gupta RS, Dietrich H, Wick G. Regression of arteriosclerotic lesions induced by immunization with heat shock protein 65-containing material in normocholesterolemic, but not hypercholesterolemic, rabbits. Atherosclerosis. 1996;123:145–55. doi: 10.1016/0021-9150(96)05800-5. [DOI] [PubMed] [Google Scholar]
- 40.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein localises in human atheroma and regulates macrophage tumour necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–7. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 41.Bachmeier K, Neu N, delaMaza L, Pal S, Hessel A, Penninger J. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–9. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]