Abstract
Unlike other agents associated with drug-induced lupus, the isoprenoid alkane pristane induces autoantibodies pathognomonic of lupus, including anti-Sm, anti-dsDNA, and anti-ribosomal P in BALB/c and SJL/J mice. The susceptibility of other strains of mice to pristane-induced lupus is unknown and is the focus of the present study. Anti-nRNP/Sm, anti-Su, and anti-ribosomal P autoantibodies were produced by most strains of mice surveyed within several months of pristane treatment, although there was marked interstrain variability in their frequencies, levels, and times of onset. In sharp contrast, the production of autoantibodies against the double-stranded RNA binding proteins NF45/NF90/p110 was restricted to B6 and B10.S mice. We conclude that pristane selectively induces lupus-specific autoantibodies in virtually any strain of mouse regardless of its genetic background. However, H-2-linked as well as non-H2 genes influenced the expression of individual autoantibody markers. The widespread susceptibility of pristane-treated mice to lupus autoantibody production and the relatively small effect of MHC are unique features of this chemically induced lupus syndrome, with potential implications for understanding the pathogenesis of autoantibodies in idiopathic human systemic lupus erythematosus.
Keywords: systemic, lupus erythematosus, pristane, autoantibodies, animal models
Introduction
The production of autoantibodies is a central event in the pathogenesis of systemic lupus erythematosus (SLE) [1,2]. The strong association of anti-Sm, anti-dsDNA, and anti-ribosomal P antibodies with SLE but not with other diseases has been attributed to MHC differences or other genetic polymorphisms that determine autoantibody specificity. Autoantibody production triggered by chemicals or drugs [3–5] also is regulated at least in part by the genetic background [6–10]. For example, mercuric chloride, gold, and d-penicillamine induce anti-fibrillarin antibodies in H-2s mice but not in mice of other MHC haplotypes [8,11]. Similarly, the ability of procainamide to induce anti-chromatin autoantibodies is strongly influenced by genetic differences in drug metabolism and possibly MHC haplotype [6,12]. Interestingly, lupus-specific anti-Sm, anti-dsDNA, and anti-ribosomal P antibodies are not induced by procainamide or other drugs regardless of the genetic background, and renal involvement is rare [3].
Unlike other forms of chemically induced lupus, the isoprenoid alkane pristane (2, 6, 10, 14 tetramethylpentadecane) induces a wide spectrum of lupus-specific autoantibodies in BALB/c and SJL/J mice, including anti-Sm, anti-dsDNA, and anti-ribosomal P, as well as immune complex-mediated glomerulonephritis [13–15]. The present study examines whether the induction of lupus-related autoantibodies by pristane is restricted to these two strains. In contrast to autoantibody production induced by HgCl2, procainamide, or other drugs, the data indicate that pristane is capable of inducing the production of lupus autoantibodies on virtually any genetic background.
Materials and methods
Treatment of mice
Four-week-old female SJL/J, A.SW, B10.S, C57Bl/10 (B10), BALB/c ByJ, and BALB.K mice were purchased from Jackson Laboratory (Bar Harbor, ME), and housed in a conventional animal facility. C57Bl/6J (B6), B6H2k, and B6CH2bm12 mice were originally purchased from Jackson Laboratory and maintained in the University of North Carolina animal facility. DBA/1 and DBA/2 mice were maintained at Wayne State University's animal facility. Six A.SW mice were maintained at the University of North Carolina animal facility and the remainder at Rochester General Hospital. At 10 weeks of age, mice received a single i.p. injection of 0·5 ml pristane (Aldrich Chemical Co., St Louis, MO) or an equal volume of sterile PBS. Sera were collected from either the tail vein or the orbital sinus before injection, at 2 and 4 weeks afterwards, and at 1-month intervals thereafter. Urine samples were tested monthly for protein concentration using Albustix reagent strips (Miles Labs, Elkhart, IN).
Radiolabelling and immunoprecipitation
Autoantibodies to cellular proteins in murine sera were analysed by immunoprecipitation of 35S-radiolabelled K562 cell extract using 4 μl serum per sample as described [13,16]. Specificity was confirmed using human reference sera containing anti-nRNP, Sm, Su, ribosomal P, or NF45/NF90 autoantibodies.
Anti-ribosomal P peptide ELISA
A carboxyl-terminal 22 amino acid peptide carrying the major epitope of ribosomal P0 recognized by human autoimmune sera was synthesized by FMOC chemistry using a Rainin Symphony/Multiplex peptide synthesizer, and purified by reverse phase high performance liquid chromatography (HPLC) [15]. Microtitre plate wells (Maxisorp Immunoplate; Nunc, Naperville, IL) were coated with 50 μl of peptide (2 μg/ml) in 20 mm Tris–HCl, pH 8 at 4°C for 16 h. The wells were washed once with NET/NP40 (150 mm NaCl, 2 mm EDTA, 20 mm Tris pH 7·5, 0·3% Nonidet-P40) and blocked with 0·5% bovine serum albumin (BSA) in NET/NP40 for 1 h at 22°C. They were then incubated with 100 μl of 1:500 mouse serum in blocking buffer for 2 h. Wells were washed, incubated with 100 μl of 1:1000 alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology, Birmingham, AL) for 2 h and developed with p-nitrophenyl phosphate substrate. Optical density (OD) was determined at 405 nm using an ELISA plate reader (Molecular Devices, Menlo Park, CA).
Subclass ELISA for anti-P peptide antibodies
For comparing the subclasses of anti-P antibodies, part of the microtitre plate was coated with 3 μg/ml goat anti-mouse κ/λ light chain antibodies to generate standard curves with immunoglobulin standards (IgG1, IgG2a, IgG2b, and IgG3 myeloma proteins from Southern Biotechnology, each at 100 μg/ml). After blocking, the wells were incubated with 100 μl of myeloma protein standards (wells coated with anti-mouse κ/λ light chain antibodies) or with a 1:1000 dilution of mouse serum (wells coated with P peptide) for 16 h at 4°C. Alkaline phosphatase-conjugated goat anti-mouse antibodies (IgG1, IgG2a, IgG2b, or IgG3 specific; Southern Biotechnology) were then added as above. The ODs of immunoglobulin standards at 100, 50, 25, 10, 5, 2·5, 1, and 0·5 ng/ml were defined as 1000, 500, 250, 100, 50, 25, 10, and 5 units, respectively. Units of subclass-specific anti-P peptide immunoglobulin bound were calculated based on the standard curves using a four-parameter logistic equation as part of the Softmax ELISA plate reader software interface.
Results
Autoantibody production induced by drugs or chemicals such as procainamide, hydralazine, and mercuric chloride is restricted to a narrow range of autoantigens. Susceptibility to both autoantibody production and the development of symptomatic disease is under the influence of MHC and other genetic factors. In contrast, the spectrum of autoantibodies induced by pristane is considerably broader than that induced by other chemicals or drugs [13–15]. We address here the question of whether susceptibility to pristane-induced lupus, like susceptibility to other forms of chemically induced autoimmunity, is restricted to particular strains.
Frequency of pristane-induced autoantibodies in mice with different MHC haplotypes
The frequencies of anti-ribosomal P, anti-nRNP/Sm, and anti-Su autoantibodies were determined by immunoprecipitation of radiolabelled K562 extract using sera from A.SW (H-2s), B6 (H-2b), BALB/c ByJ (H-2d), and DBA/1 (H-2q) mice. As shown in Table 1, anti-nRNP/Sm and anti-Su autoantibodies were produced by all four strains, whereas anti-ribosomal P antibodies were produced by A.SW and B6 mice but not by BALB/c or DBA/1. Although the numbers were smaller, the H-2k strains B6.H2k (n = 7) and BALB/K (n = 6) also did not produce anti-ribosomal P following pristane treatment, but C3H mice produced anti-ribosomal P antibodies frequently after pristane injection (M. Satoh, unpublished data), suggesting that H-2k mice are capable of responding. Like anti-ribosomal P, the induction of autoantibodies to the double-stranded RNA binding proteins NF45/NF90, p130, p110, and p80 [16] was restricted to certain strains. Of the 11 strains tested, only B6 and B10.S mice produced these autoantibodies following pristane treatment (Table 2).
Table 1.
Frequency of autoantibodies to ribosomal P, nRNP/Sm, and Su
| H-2 | N | Ribosomal P (%)* | RNP/Sm (%)* | Su (%)* | |
|---|---|---|---|---|---|
| A.SW | s | 26 | 231 | 542 | 585,6 |
| B6 | b | 45 | 16 | 24234 | 18589 |
| BALB/c ByJ | d | 20 | 01 | 554 | 4578 |
| DBA/1 | q | 18 | 0 | 833 | 94679 |
Immunoprecipitation assay using sera obtained 6 months after injecting pristane 0·5 ml intraperitoneally.
P = 0·0287, Fisher's exact test
P = 0·0197, Fisher's exact test
P < 0·0001, Fisher's exact test
P = 0·0236, Fisher's exact test
P = 0·0029, Fisher's exact test
P = 0·0059, Fisher's exact test
P = 0·0014, Fisher's exact test
P = 0·0322, Fisher's exact test
P < 0·0001, Fisher's exact test.
Table 2.
Frequency of autoantibodies to NF45/NF90, p130, p110, and p80 in mice treated with pristane
| Strain* | H-2 | n† | NF45/NF90 (%) | P130 (%) | P110 (%) | P80 (%) |
|---|---|---|---|---|---|---|
| SJL/J | s | 8 | 0 | 0 | 0 | 0 |
| A.SW | s | 6 | 0 | 0 | 0 | 0 |
| B10.S | s | 6 | 33 | 33 | 33 | 33 |
| B10 | b | 7 | 0 | 0 | 0 | 0 |
| B6 (Jackson Lab) | b | 17 | 23 | 23 | 23 | 23 |
| B6 (Charles River) | b | 14 | 36 | 36 | 36 | 36 |
The following additional strains did not produce anti-NF45/NF90, p130, p110, and p80 autoantibodies: BALB.B (n = 5), B6CH2bm12 (n = 19), B6.H2k (n = 9), BALB.K (n = 6), BALB/c ByJ (n = 20), DBA/1 (n = 18).
All mice were female except for six males included in the B6 (Jackson) and 15 males included in B6CH2bm12 group. Mice received pristane at 12–14 weeks of age and autoantibodies were analysed 6 months afterwards by immunoprecipitation.
As shown in Table 1, autoantibody frequencies varied significantly: anti-ribosomal P ranged from 0% to 23%, anti-nRNP/Sm from 24% to 83%, and anti-Su from 18% to 94%, suggesting that the ability to produce anti-nRNP/Sm and anti-Su following pristane treatment is not restricted to particular strains. In contrast, H-2s, H-2b, and some H-2k mice produced anti-ribosomal P autoantibodies, whereas H-2d, H-2q mice did not, suggesting that MHC-linked genes influence their production to some degree. The production of anti-NF90/NF45, p130, p110, and p80 autoantibodies also was highly restricted, but the role of MHC haplotype was less clear: B10.S mice produced these autoantibodies, whereas two additional H-2s strains (SJL/J and A.SW) did not. Similarly, B6 mice from two different vendors produced these autoantibodies, whereas other H-2b strains (B10 and BALB.B) did not.
Autoantibody frequencies in H-2s mice
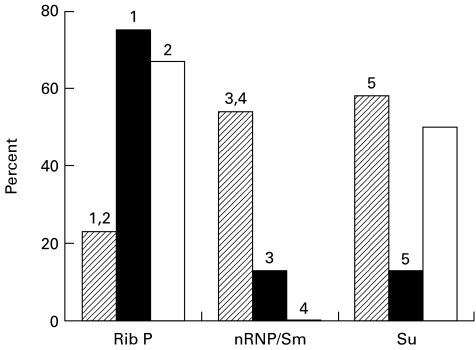
To define further the influence of the MHC haplotype in pristane-induced autoantibody production, the frequencies of anti-ribosomal P, anti-nRNP/Sm, and anti-Su in A.SW, SJL, and B10.S mice (all H-2s) were compared. As shown in Fig. 1, the frequencies of anti-ribosomal P autoantibodies in SJL/J and B10.S were 75% and 67%, respectively, versus 23% in A.SW (P = 0·0127 for SJL/J and P = 0·0599 for B10.S versus A.SW; Fisher's exact test). In contrast, the frequency of anti-nRNP/Sm was higher in A.SW (54%) than in SJL/J (13%) or B10.S (0%) (P = 0·529 for SJL/J and P = 0·0238 for B10.S versus A.SW; Fisher's exact test). Anti-Su antibodies were produced by A.SW mice at a frequency of 58% compared with 13% in SJL/J mice (P = 0·0425; Fisher's exact test). The frequency in B10.S (50%) was not significantly different than that in A.SW. These data strongly suggest that differences in the genetic background outside of the MHC play a critical role in determining autoantibody frequency.
Fig. 1.
Frequency of antiribosomal P, anti-nRNP/Sm, and anti-Su antibodies in H-2s mice. A.SW (n = 26), SJL/J (n = 8) and B10.S (n = 6) mice were injected with pristane (0·5 ml intraperitoneally) and autoantibodies were determined by immunoprecipitation assay. The percentage of positive sera is indicated for each autoantibody. Frequencies in SJL/J (▪) and B10.S (□) sera were compared with the frequency in A.SW sera (hatched) by Fisher's exact test. 1A.SW versus SJL, P = 0·0127; 2A.SW versus B10.S, P = 0·0599; 3A.SW versus SJL, P = 0·0529; 4A.SW versus B10.S, P = 0·0238; 5A.SW versus SJL, P = 0·0425.
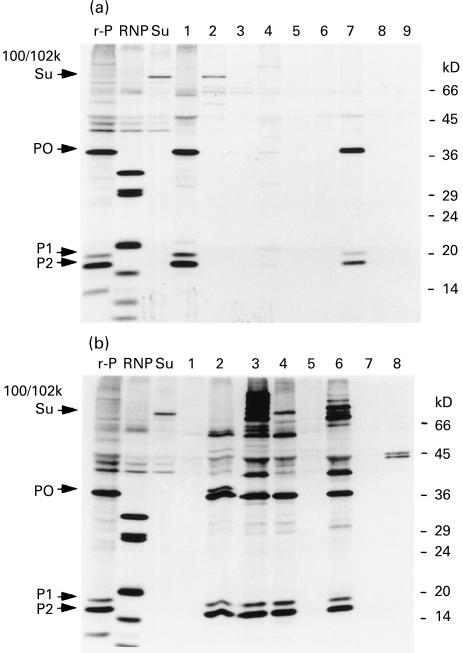
The importance of non-MHC genes in determining autoantibody frequency also was suggested by the autoantibody profiles of B10 (H-2b) versus B10.S (H-2s) mice. Anti-ribosomal P was the most prominent autoantibody specificity detected by immunoprecipitating sera from these strains (Fig. 2a,b, respectively). The characteristic P0, P1 and P2 bands were detected in immunoprecipitates of three of seven B10 sera (43%) and four of six B10.S sera (67%).
Fig. 2.
Immunoprecipitation using sera from pristane-treated B10 and B10.S mice. Radiolabelled K562 extract was immunoprecipitated using sera from pristane-treated mice or with prototype human sera with anti-ribosomal P (P0, P1, and P2, lane r-P), anti-nRNP/Sm (lane RNP), or anti-Su (100/102 kD proteins, lane Su). (a) Immunoprecipitation with B10 sera. Lanes 1–7, B10 mice treated 6 months earlier with pristane; lanes 8,9, B10 mice treated 6 months earlier with PBS. (b) Immunoprecipitation with B10.S sera. Lanes 1–6, B10.S mice treated with pristane 6 months earlier; lanes 7,8, B10.S mice treated 6 months earlier with PBS. Positions of molecular weight markers (in kD) are indicated on the right.
Antibodies to ribosomal P peptide
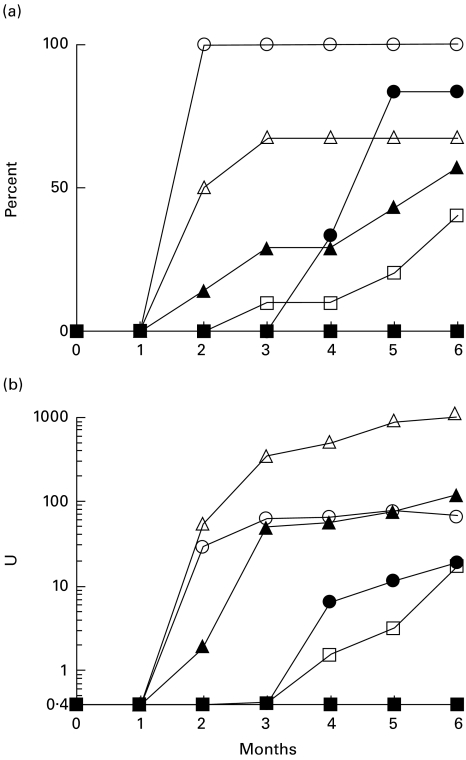
A sensitive C-terminal peptide-based ELISA has been used previously to measure anti-ribosomal P autoantibodies [17]. It has been shown that this region, which is shared by the P0, P1, and P2 proteins, bears the immunodominant antigenic determinant recognized by human and murine anti-ribosomal P autoantibodies. The onset of the anti-P response was examined in SJL/J, A.SW, B10.S (H-2s), B10, B6 (H-2b), and BALB/c (H-2d) mice 2, 4 and 6 months after pristane treatment by ELISA (Fig. 3a,b,c, respectively). All eight SJL/J mice treated with pristane developed anti-P peptide antibodies as early as 2 months after pristane injection. Half of the B10.S mice (three of six) also had anti-P antibodies at 2 months. However, the development of anti-P antibodies in A.SW, B10, and B6 mice was delayed until 4–6 months after pristane injection, even though these strains exhibited a comparable frequency of anti-P to that of B10.S at 6 months. None of the BALB/c mice produced anti-P antibodies during the 6 months following pristane treatment.
Fig. 3.
Anti-P peptide ELISA. Ten-week-old SJL/J, A.SW, B10.S (H-2s), B10, B6 (H-2b), and BALB/c (H-2d) received a single i.p. injection of pristane and sera were tested by anti-P peptide ELISA 2, 4 and 6 months later (a,b,c, respectively). OD405nm was converted to units using a standard curve generated by anti-ribosomal P reference serum. ○, PBS-injected mice; •, pristane-injected mice; +, mice that died between 4 and 6 months (results from the final bleed are shown). (c) Anti-P peptide antibody levels in the positive sera were compared using the Mann–Whitney test. 1B10.S versus A.SW, P = 0·0159; 2SJL/J versus A.SW, P = 0·0480; 3B10.S versus SJL, P = 0·0061; 4B10.S versus B10, P = 0·0571.
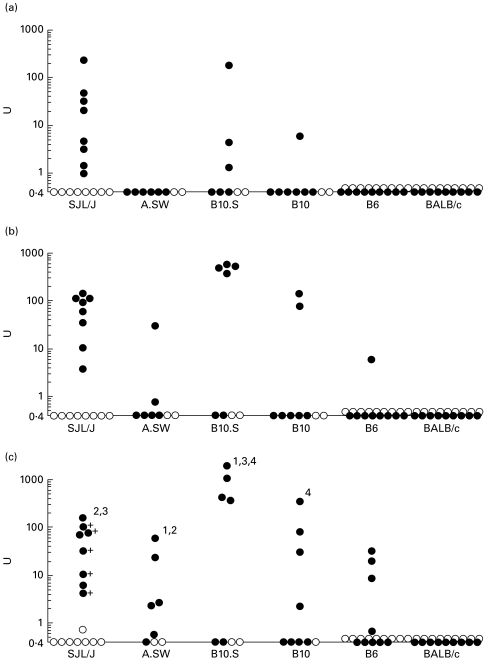
The possibility that some strains develop anti-P peptide antibodies at a high frequency whereas others show higher levels but a lower frequency was investigated (Fig. 4a,b, respectively). All SJL/J mice treated with pristane developed anti-P peptide antibodies at 2 months (Fig. 4a). B10.S (H-2s) mice also developed anti-ribosomal P antibodies 2–3 months after pristane treatment, but only in four of six mice. In A.SW (H-2s), the onset of anti-P was dramatic, but delayed approx. 2 months compared with SJL/J and B10.S. In contrast, the onset of anti-P was more gradual in the H-2b strains B10 and B6, with some mice developing anti-P antibodies as late as 5–6 months after pristane treatment.
Fig. 4.
Frequency and levels of anti-ribosomal P antibodies. (a) Onset of anti-ribosomal P antibody production. Sera obtained at monthly intervals from pristane-treated SJL/J (○), A.SW (•), B10.S (Δ), B10 (▴), B6 (□), and BALB/c (▪) mice were tested for anti-ribosomal P peptide antibodies by ELISA. The percentage of positive sera over time is shown. (b) Mean anti-ribosomal P antibody levels over time. Sera obtained monthly from pristane-treated SJL/J, A.SW, B10.S, B10, and B6 mice were anti-ribosomal P peptide antibodies by ELISA. The OD405nm was converted to units (U). The mean anti-P peptide activity (units) in positive sera over time is shown
Although the frequency was lower, the mean level of anti-P in positive B10.S mice was 10-fold higher than in SJL/J (Fig. 3b,c, note log scale). Despite the high frequency of anti-P antibodies in A.SW (five of six mice), the mean level of anti-P peptide was approx. 50-fold lower than in B10.S and approx. 10-fold lower than in SJL/J or B10. This suggests that H-2s is associated with a higher probability of developing anti-ribosomal P (Fig. 4a), whereas non-MHC background genes derived from B10 may be more important for the production of high levels of these autoantibodies.
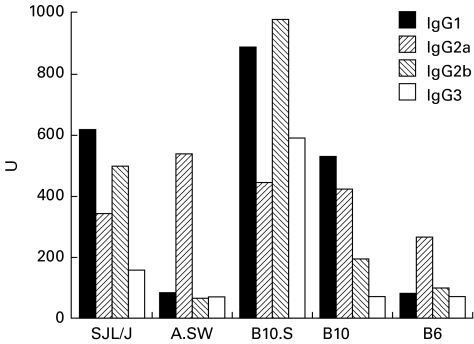
IgG2a anti-P antibodies were detected at similar levels (approx. 300–550 U) in all positive strains (Fig. 5). This subclass predominated in A.SW and B6 mice, which showed lower levels of anti-P than B10.S, B10, or SJL/J (Fig. 4b). The strains with high levels of anti-P were characterized by the development of anti-P antibodies of other subclasses in addition to IgG2a: IgG1 and IgG2b in SJL/J; IgG1, IgG2b, and IgG3 in B10.S; and IgG1 in B10.
Fig. 5.
IgG subclasses of anti-P antibodies. IgG subclasses of the anti-P antibodies were analysed by ELISA. IgG2a anti-P antibodies were detected at similar levels (approx. 300–550 U) in all anti-P-positive strains and were the predominant subclass in A.SW and B6 mice, which produced only low levels of anti-P. The strains with the highest anti-P antibody levels were characterized by significant production of other IgG subclasses: IgG1 and 2b in SJL/J; IgG1, 2b, and 3 in B10.S; and IgG1 in B10.
Environmental effects
Although the frequency of autoantibody production is affected by housing conditions [18], pristane induced autoantibodies in mice at three different facilities (University of North Carolina, Wayne State University, and Rochester General Hospital). Moreover, a similar spectrum of autoantibodies was seen in B6 mice from Charles River and from Jackson Laboratory (Table 2). Thus, the ability of pristane to induce lupus autoantibodies was not unique to a particular animal facility or supplier.
Discussion
The C19 isoprenoid alkane pristane induces plasmacytomas [19] and an erosive arthritis resembling rheumatoid arthritis [20,21]. These effects are highly strain-specific, occurring in BALB/c and DBA/1, but not in B6, B10, SJL/J or most other strains [20,22,23]. In contrast, the present study shows that pristane induces lupus autoantibodies (anti-nRNP/Sm, ribosomal P, and Su) in most, if not all, strains regardless of genetic background. On the other hand, autoantibodies against dsRNA binding proteins were more restricted, being detected only in B6 and B10.S mice, but not other strains (Table 2). The latter are linked in humans with overlap syndromes rather than with typical SLE [16,24]. Interestingly, autoantibodies were not induced by pristane in all mice of a given strain, though the frequencies generally were higher than the 25% reported for anti-Sm in MRL/lpr mice [25,26]. It has been suggested that autoantibody production in MRL/lpr mice involves stochastic events [26]. This may be true of pristane-induced lupus as well.
Although the induction of autoantibodies was largely independent of background, the specificities, levels, and frequencies of these autoantibodies appeared to be modulated by both H-2-linked as well as non-H-2 background genes. With the exception of arthritis in the DBA/1 mice and plasmacytomas in BALB/c ByJ, none of the mice surveyed developed joint disease or plasmacytomas after pristane injection, whereas autoimmunity developed in all strains. Thus, autoantibodies, plasmacytomas, and arthritis induced by pristane arise through at least partially independent mechanisms.
Pristane is unique among chemical triggers of lupus-like disease
Numerous chemicals and drugs capable of triggering either autoantibody production or a lupus-like syndrome have been identified [3–5]. However, none of them reproduces the spectrum of autoantibodies seen in human SLE. The latter is associated with certain ‘marker’ autoantibodies, including anti-Sm, anti-dsDNA, and anti-ribosomal P as well as a series of lupus-associated autoantibodies that includes anti-nRNP, anti-ssDNA/histone/chromatin, anti-Ro (SS-A), anti-La (SS-B), anti-Su, and autoantibodies against double-stranded RNA binding proteins [27]. Drugs, such as procainamide, hydralazine, quinidine, chlorpromazine, methyldopa, and isoniazid induce a highly restricted response directed exclusively against chromatin antigens (ssDNA, histones, and histone–DNA complexes) [3,4]. These agents do not induce either lupus marker autoantibodies (anti-Sm,-dsDNA,-ribosomal P) or lupus-associated autoantibodies (e.g. anti-Su,-nRNP), in outbred human populations. Similarly, mercury and other metals induce a highly restricted autoantibody response against the U3 ribonucleoprotein (fibrillarin) in mice of the H-2s haplotype [5,7,8].
Pristane is unique in its ability to induce in non-autoimmune prone mice a broad range of autoantibodies specific for or associated with lupus [13–16,28]. Most of these autoantibodies were inducible in a variety of different strains of mice. Strikingly absent in pristane-treated mice were anti-Ro (SS-A) and anti-La (SS-B) antibodies. The reason for their absence is not known, but is consistent with previous data suggesting that they also are unusual in mice with spontaneous lupus-like syndromes. In contrast, they can be induced readily by immunization [29], suggesting that mice are capable of producing them.
Role of the genetic background
Although the genetic background does not appear to be a major factor in determining susceptibility to autoantibody induction by pristane, there are important genetic effects on the phenotype of pristane-induced lupus. Anti-ribosomal P autoantibodies were produced by all of the H-2s and H-2b strains tested, but were not produced by H-2d or H-2q mice.
In contrast to anti-ribosomal P, the response to NF90/NF45, p130, p110, and p80 may reflect the influence of non-H-2-linked genes, since B10 and B10.S mice were responders, whereas other H-2b and H-2s strains were not (Table 2). It is likely that the frequency of anti-nRNP/Sm and anti-Su antibodies was affected by non-H-2-linked background genes, as well (Fig. 1). Taken together, these observations suggest that both MHC and non-MHC linked genes influence the ‘autoantibody phenotype’ of pristane-treated mice, even though the ability to induce lupus-associated autoantibodies was relatively strain-independent.
In conclusion, we have shown that most if not all ‘normal’ (non-autoimmune prone) strains of mice are susceptible to the induction of lupus-specific autoantibodies by pristane. Although susceptibility to autoantibody induction is widespread (i.e. not strongly dependent on genetic background), the autoantibody repertoire is shaped by both MHC and non-MHC linked genes. The relative unimportance of the MHC haplotype in determining whether a particular strain can respond to an autoantigen following pristane injection was unexpected. This raises the possibility that other factors, such as which cytokines are induced preferentially by pristane treatment in a given strain, play an important role in determining the targets of the autoimmune process.
Acknowledgments
We thank Dr David Klapper (UNC/PMBB Micro Protein Chemistry Facility) for synthesizing and purifying the P-peptide, Dr Kimberly J. Hamilton for assistance with analysis of the SJL/J mice, and Dr Evelyn Hess for helpful discussions. This work was supported by research grants from the United States Public Health Service (R01-AR44731, AI44074, and T32-AR7416). H.B.R. is an Arthritis Foundation Postdoctoral Fellow.
References
- 1.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 2.Fritzler MJ, Andrade L. Antibodies to nonhistone antigens in systemic lupus erythematosus. In: Lahita RG, editor. Systemic lupus erythematosus. San Diego: Academic Press; 1999. [Google Scholar]
- 3.Mongey AB, Hess EV. The potential role of environmental agents in systemic lupus erythematosus and associated disorders. In: Wallace DJ, Hahn BH, editors. Dubois' lupus erythematosus. Philadelphia: Lea and Febiger; 1993. [Google Scholar]
- 4.Burlingame RW, Rubin RL. Drug-induced anti-histone autoantibodies display two patterns of reactivity with substructures of chromatin. J Clin Invest. 1991;88:680–90. doi: 10.1172/JCI115353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griem P, Gleichmann E. Metal ion induced autoimmunity. Curr Opin Immunol. 1995;7:831–8. doi: 10.1016/0952-7915(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 6.Woosley RL, Drayer DE, Reidenberg MM, Nies AS, Carr K, Oates JA. Effect of acetylator phenotype on the rate at which procainamide induces antinuclear antibodies and the lupus syndrome. N Engl J Med. 1978;298:1157–9. doi: 10.1056/NEJM197805252982101. [DOI] [PubMed] [Google Scholar]
- 7.Hultman P, Ganowiak K, Turley SJ, Pollard KM. Genetic susceptibility to silver-induced anti-fibrillarin autoantibodies in mice. Clin Immunol Immunopathol. 1995;77:291–7. doi: 10.1006/clin.1995.1155. [DOI] [PubMed] [Google Scholar]
- 8.Hultman P, Bell LJ, Enestrom S, Pollard KM. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin Immunol Immunopathol. 1992;65:98–109. doi: 10.1016/0090-1229(92)90212-7. [DOI] [PubMed] [Google Scholar]
- 9.Speirs C, Fielder AH, Chapel H, Davey NJ, Batchelor JR. Complement system protein C4 and susceptibility to hydralazine-induced systemic lupus erythematosus. Lancet. 1989;1:922–4. doi: 10.1016/s0140-6736(89)92506-3. [DOI] [PubMed] [Google Scholar]
- 10.Batchelor JR, Welsh KI, Tinoco RM, et al. Hydralazine-induced systemic lupus erythematosus: influence of HLA-DR and sex on susceptibility. Lancet. 1980;1:1107–9. doi: 10.1016/s0140-6736(80)91554-8. [DOI] [PubMed] [Google Scholar]
- 11.Robinson CJG, Balazs T, Egorov IK. Mercuric chloride-, gold sodium thiomalate-, and d-penicillamine-induced antinuclear antibodies in mice. Toxicol Appl Pharmacol. 1996;86:159–69. doi: 10.1016/0041-008x(86)90046-3. [DOI] [PubMed] [Google Scholar]
- 12.Adams LE, Balakrishnan K, Roberts SM, et al. Genetic, immunologic and biotransformation studies of patients on procainamide. Lupus. 1993;2:89–98. doi: 10.1177/096120339300200205. [DOI] [PubMed] [Google Scholar]
- 13.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341–6. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh M, Kumar A, Kanwar YS, Reeves WH. Antinuclear antibody production and immune complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci USA. 1995;92:10934–8. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh M, Hamilton KJ, Ajmani AK, et al. Autoantibodies to ribosomal P antigens with immune complex glomerulonephritis in SJL mice treated with pristane. J Immunol. 1996;157:3200–6. [PubMed] [Google Scholar]
- 16.Satoh M, Shaheen VM, Kao PN, et al. Autoantibodies define a family of proteins with conserved double-stranded RNA binding domains as well as DNA-binding activity. J Biol Chem. 1999;274:34598–604. doi: 10.1074/jbc.274.49.34598. [DOI] [PubMed] [Google Scholar]
- 17.Elkon KB, Bonfa E, Brot N. Antiribosomal antibodies in systemic lupus erythematosus. Rheum Dis Clin North Am. 1992;18:377–90. [PubMed] [Google Scholar]
- 18.Hamilton KJ, Satoh M, Swartz J, Richards HB, Reeves WH. Influence of microbial stimulation on hypergammaglobulinemia and autoantibody production in pristane-induced lupus. Clin Immunol Immunopathol. 1998;86:271–9. doi: 10.1006/clin.1997.4481. [DOI] [PubMed] [Google Scholar]
- 19.Anderson PN, Potter M. Induction of plasma cell tumours in BALB/c mice with 2,6,10,14-tetramethylpentadecane (pristane) Nature (Lond) 1969;222:994–5. doi: 10.1038/222994a0. [DOI] [PubMed] [Google Scholar]
- 20.Potter M, Wax JS. Genetics of susceptibility to pristane-induced plasmacytomas in BALB/cAn: reduced susceptibility in BALB/cJ with a brief description of pristane-induced arthritis. J Immunol. 1981;127:1591–5. [PubMed] [Google Scholar]
- 21.Wooley PH, Seibold JR, Whalen JD, Chapdelaine JM. Pristane-induced arthritis. The immunologic and genetic features of an experimental murine model of autoimmune disease. Arthritis Rheum. 1989;32:1022–30. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- 22.Wooley PH. Immunogenetics of animal models of rheumatoid arthritis. In: Henderson B, Edwards JCW, Pettipher ER, editors. Mechanisms and models in rheumatoid arthritis. London: Academic Press; 1995. [Google Scholar]
- 23.Potter M, Wax JS, Blankenhorn E. BALB/c subline differences in susceptibility to plasmacytoma induction. Curr Top Microbiol Immunol. 1985;122:234–41. doi: 10.1007/978-3-642-70740-7_33. [DOI] [PubMed] [Google Scholar]
- 24.Labrador M, Alguero A, Diaz C, et al. Antibodies against a novel nucleolar and cytoplasmic antigen (p105-p42) present in the sera of patifents with a subset of rheumatoid arthritis (RA) with signs of scleroderma. Clin Exp Immunol. 1998;114:301–10. doi: 10.1046/j.1365-2249.1998.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–69. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 26.Bernard NF, Eisenberg RA, Cohen PL. H-2 linked Ir gene control of the T cell recognition of the Sm nuclear autoantigen and the aberrant response of autoimmune MRL/Mp-+/+ mice. J Immunol. 1985;134:3812–8. [PubMed] [Google Scholar]
- 27.Tan EM. Autoantibodies to nuclear antigens (ANA): their biology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- 28.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. IL-6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–90. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshmukh US, Lewis JE, Gaskin F, et al. Immune responses to Ro60 and its peptides in mice. I. The nature of the immunogen and endogenous autoantigen determine the specificities of the induced autoantibodies. J Exp Med. 1999;189:531–40. doi: 10.1084/jem.189.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]