Abstract
The C5a receptor is expressed by a variety of cell types. These studies demonstrate by immunohistochemistry that the receptor is present on the surface of proximal and distal tubular epithelial cells from normal kidney. In addition, the receptor was detected on transitional epithelial cells of the ureter and bladder. Primary proximal tubular cultures and a proximal tubular cell line both also expressed the C5a receptor, as demonstrated by immunofluorescence and by FACS analysis. The presence of mRNA encoding the receptor was confirmed by reverse transcriptase-polymerase chain reaction analysis. As opposed to its effect on glomerular mesangial cells, the receptor did not mediate a proliferative response by the proximal tubular cells. C5a also did not enhance the synthesis/secretion of transforming growth factor-beta 1, monocyte chemoattractant protein-1, platelet-derived growth factor-AB or tumour necrosis factor-alpha by cultured proximal tubular cells. Therefore, although the C5a receptor clearly is expressed by proximal tubular cells, clarification of its functional relevance on this cell type awaits further studies.
Keywords: complement, renal proximal tubules, C5a receptor
Introduction
The C5a receptor (CD88), a member of the rhodopsin family of seven transmembrane-spanning G-protein-linked receptors, is expressed by a wide variety of cell types [1–5]. Because the more obvious functions of C5a are directed toward leucocyte activation and enhancement of vascular permeability [6–11], initial assumptions were that the receptor was expressed primarily by bone marrow-derived cells and smooth muscle cells. However, in addition to neutrophils, monocyte/macrophages, eosinophils, mast cells and dendritic cells, the receptor is expressed by vascular smooth muscle cells, hepatocytes, bronchial and alveolar epithelial cells, astrocytes, microglia, neurones, endothelial cells, and renal mesangial cells [3,4,12–16].
The biological roles of the receptor in these different locations remain incompletely defined, although virtually all the activities so far described contribute to the inflammatory response. In hepatocytes, ligation of the receptor induces the synthesis of several acute inflammatory proteins, including complement factor B, C3, α1-antitrypsin, and α1-antichymotrypsin [3,13,14]. C5a induces secretion of IL-1, IL-6, IL-8 and tumour necrosis factor-alpha (TNF-α) in monocyte/macrophages, and induces expression of tissue factor and P-selectin on endothelial cells [12,17–21]. Cultured glomerular mesangial cells respond to stimulation with C5a by increased production of platelet-derived growth factor-AB (PDGF-AB) and monocyte chemoattractant protein-1 (MCP-1), increased c-jun and c-fos mRNA expression, AP-1 and CREB activation, and cellular proliferation [15,16]. In vivo, C5a also up-regulates IL-6 and lung vascular P-selectin [21,22], mediates the pulmonary symptoms resulting from complement activation on artificial surfaces such as haemodialysis membranes [23,24], and mediates damage in immune complex pulmonary injury [25]. In addition to these roles in the mediation of damage, the C5a receptor also has protective effects, as illustrated by the increased susceptibility of C5a receptor knockout mice to pulmonary bacterial infection [26].
Renal proximal tubular epithelial cells (PTEC) synthesize and secrete a number of cytokines, chemokines, growth factors and complement components [27–34]. Modulation of the expression of some of these mediators is involved in the progression of glomerular disease and in the renal response to other insults such as tubular toxins and ischaemia [27,28]. In these studies we have analysed expression of the C5a receptor by PTEC both in vivo and in cultured cells.
Materials and methods
Proximal tubular cell characterization and culture conditions
Primary human PTEC were purchased from Clonetics (San Diego, CA). The cells were characterized by the supplier morphologically and by the expression of γ glutamyl transferase. The cells were cultured at 37°C, 5% CO2, in renal epithelial cell growth medium (Clonetics) containing hydrocortisone 0·5 μg/ml, human epithelial growth factor 10 ng/ml, fetal bovine serum (FBS; 0·5%), epinephrine 0·5 μg/ml, and triiodothyronine 6·5 ng/ml (Clonetics). PTEC were used in these studies between passages 4 and 10. At the time of final passage, the cells were re-characterized to confirm phenotype stability. Using immunofluorescence, the cells were negative for von Willebrand factor (vWF) VIII, showed minimal staining for desmin, and were positive for cytokeratin, actin, alkaline phosphatase and γ glutamyl transferase. The human PTEC cell line, HK-2, was obtained from ATCC (Rockville, MD).
Immunohistochemistry
Paraffin-embedded human kidney tissue was obtained from the Pathology Core Laboratory, Jewish Hospital (St Louis, MO). All tissues were obtained from autopsy samples of patients who had died from unrelated illnesses and showed no signs of kidney disease. Tissue sections were prepared for immunoperoxidase staining as described by Botney et al. [35]. Endogenous peroxidase was blocked with 0·3% (v/v) H2O2 in methanol for 20 min at room temperature. Non-specific immunoglobulin binding sites were blocked with normal rabbit serum. Sections were subsequently incubated for 1 h at 37°C with rabbit anti-human C5a receptor antiserum (1:300 dilution) that was prepared by immunization of rabbits with a peptide consisting of amino acid residues 7–24 of the first extracellular domain of the C5a receptor [3]. Preimmune rabbit serum served as a negative control. Sections were then incubated for 20 min with affinity-purified, biotin-conjugated goat anti-rabbit IgG (1:1600 dilution; Vector Labs, Burlingame, CA), washed, and incubated for 20 min with horseradish peroxidase–streptavidin (1:4000 dilution; Vector Labs). Immunoglobulin complexes then were visualized by incubation with 3,3′-diaminobenzidine (0·5 mg/ml in 50 mm Tris–HCl pH 7·4, 0·3% H2O2, and 0·4% NiCl). Sections were washed, dehydrated, mounted in Permount and examined by light microscopy.
For immunofluorescence, cultured PTEC were seeded on glass coverslips. After 4 days the coverslips were washed twice with PBS, fixed for 5 min in cold 100% acetone, and air-dried for 5 min. The coverslips then were washed twice with PBS and incubated for 1 h in PBS, 5% non-fat dry milk, and 3% bovine serum albumin (BSA). IgG from the above described anti-C5aR antiserum [3] or from control rabbit anti-haemoglobin antiserum was purified using a HiTrap protein-G column (Pharmacia, Piscataway, NJ) and reconstituted to equivalent concentration with PBS. Coverslips were incubated with primary antibodies diluted 1:15 with PBS, 5% non-fat dry milk, and 3% BSA for 2 h, and then washed three times with PBS, 1% Triton X-100, and 0·2% Tween-20. This was followed by incubation with fluorescein-labelled goat anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:20 with PBS, 5% non-fat dry milk, and 3% BSA. The coverslips were again washed three times with PBS, 1% Triton X-100, and 0·2% Tween-20, and counterstained with propidium iodide. Photomicrographs then were obtained using identical exposure times.
FACS analysis
PTEC (70–80% confluent; approximately 1 week after passage) were detached from culture plates with collagenase/EDTA, washed with ice-cold PBS, and stained with a murine FITC-conjugated anti-C5a receptor MoAb that recognized the extracellular peptide corresponding to residues 1–31 (clone W17/1; RDI, Flanders, NJ), or a FITC-conjugated isotype-matched (IgG1) control antibody (Caltag Labs, Burlingame, CA). FACS analysis was performed with a FACScan instrument (Becton Dickinson).
Reverse transcriptase-polymerase chain reaction
Total mRNA was obtained from cultured U937 cells, normal human liver and cultured PTEC using Rneasy (Qiagen, Santa Clarita, CA). Reverse transcription was performed with Superscript II (Gibco BRL, Gaithersburg, MD) using 50–100 ng total RNA as template. The first strand synthesis was followed by polymerase chain reaction (PCR) amplification using C5a receptor-specific primers. The forward primer corresponded to nucleotides 17–36 (ATACCACCCCTGATTATGG), and the reverse primer to nucleotides 206–225 (GAACCAGATGGCATTGATGG) (GeneBank accession no. X58674). The segment amplified spanned the estimated 9-kb intronic sequence. Amplification was performed using Taq DNA polymerase (Gibco BRL) under the following conditions: denaturation 94°C for 3 min, 35 cycles of 94°C for 1 min, 60°C for 90 s, and 70°C for 2 min, and extension at 70°C for 5 min. The reverse transcriptase (RT)-PCR product (10 μl) from the PTEC, control RT-PCR products (10 μl), and a 25-bp ladder (Gibco BRL) were resolved on a 1% SeaKem agarose gel.
Induction of proliferation
PTEC were seeded at a density of 1000 cells/200 μl in 96-well plates. Two days after passage, cells were growth arrested by incubation in medium in the absence of serum and growth factors for 12 h. The medium was then removed and fresh medium added, either alone or containing recombinant C5a (rC5a) (50, 100, 200 and 400 nm) (Sigma, St Louis, MO). Quadruplicate samples, C5a-treated and untreated controls, were harvested after incubation at 37°C for 48 h and 72 h and proliferation was quantified using the Neutral Red Dye Bioassay kit (Clonetics). Dye uptake was quantified spectrophotometrically at 570 nm. 3H-thymidine uptake was performed as previously described using cultured PTEC that had been growth-arrested and treated as described above [36].
ELISAs for cytokines and growth factors
PTEC cultured in six-well plates were grown to 70% confluence. Following culture for 12 h in supplement-free medium, as above, the medium was removed and replaced with fresh medium, either alone or containing recombinant C5a. For determination of dose–response, rC5a was added to achieve final concentrations of 50, 100 and 200 nm, in addition to control samples lacking rC5a. The supernatants from these assays were removed at 48 h and 72 h post-incubation. Assays were performed in duplicate. Time course analysis was performed in triplicate, with a final rC5a concentration of 50, 100 and 200 nm; control samples were incubated without rC5a. Supernatants were removed at 24, 48 and 72 h. All samples were stored at −80°C until the assays were performed. ELISAs were performed for MCP-1, transforming growth factor-beta (TGF-β), TNF-α and PDGF-AB (R&D Systems, Minneapolis, MN). All samples and standards were run in duplicate according to the manufacturer's instructions, and sample values determined by regression analysis from generated standard curves.
Results
C5a receptor expression by genitourinary tract tissues
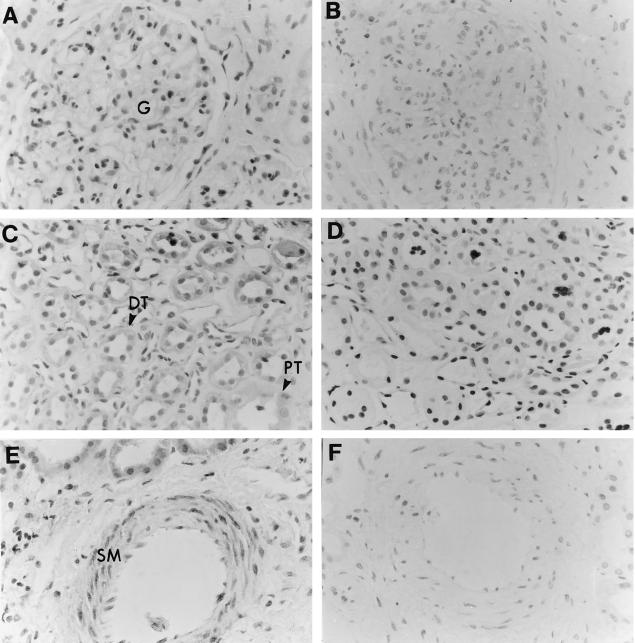
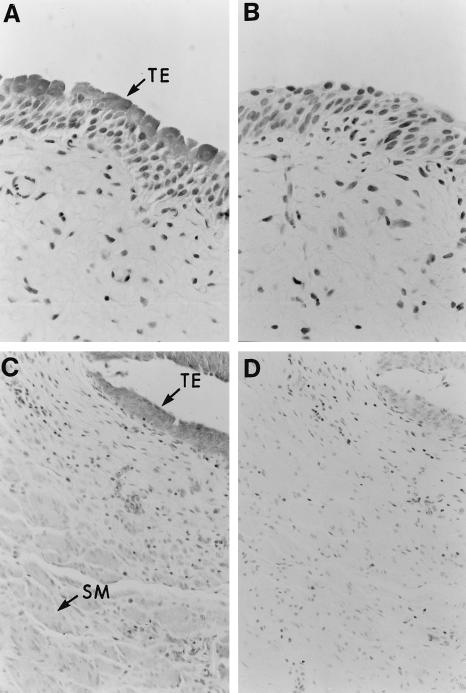
Immunohistochemical analysis of normal human kidney revealed prominent C5a receptor expression by both proximal and distal renal tubular epithelial cells (Fig. 1C). Vascular smooth muscle cells were also stained by antibody to the receptor (Fig. 1E). Although glomerular mesangial cells in culture express the C5a receptor [15,16], little expression was observed in mesangial cells in normal renal tissue (Fig. 1A). No staining of any cell type was observed with preimmune serum. The transitional epithelium of both the ureter and bladder also stained strongly for the C5a receptor (Fig. 2A,C). Weaker, but clearly positive, staining for the receptor was observed within the smooth muscle of both tissues (Fig. 2C).
Fig. 1.
Expression of the C5a receptor by human renal tissue. (A,C,E) Human kidney paraffin-embedded section stained with immunoperoxidase-labelled rabbit anti-C5a receptor antiserum as described in Materials and Methods. (A) Photomicrograph of a section demonstrating the lack of staining within the glomerular tuft (G). (C) Photomicrograph of a kidney section showing C5a receptor expression within the proximal (PT) and distal (DT) renal tubular epithelium. (E) Staining for the C5a receptor within the smooth muscle (SM) of a renal blood vessel. (B,D,F) Background immunostaining using rabbit preimmune serum.
Fig. 2.
Expression of the C5a receptor by human ureter and urinary bladder. (A,C) Paraffin-embedded sections of lower urinary tract stained with immunoperoxidase-labelled rabbit anti-C5a receptor antiserum. TE indicates the positive staining for the C5a receptor in the transitional epithelium of the ureter and urinary bladder. Although not as intense as seen in the transitional epithelium, positive staining for the C5a receptor was also apparent within the smooth muscle of the ureter and bladder wall. (B,D) Background immunoperoxidase staining using rabbit preimmune serum.
The C5a receptor is expressed by cultured proximal tubular epithelial cells
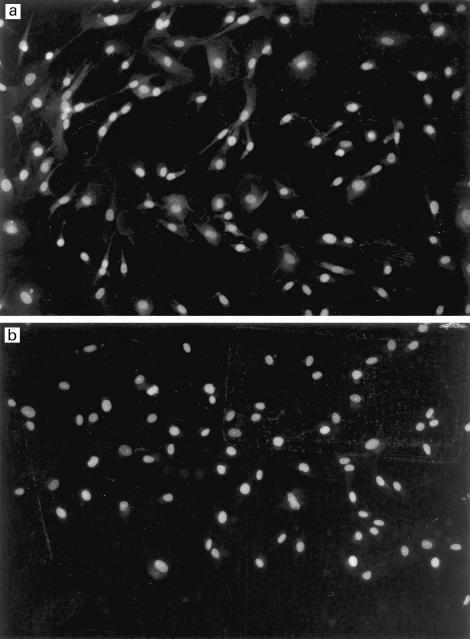
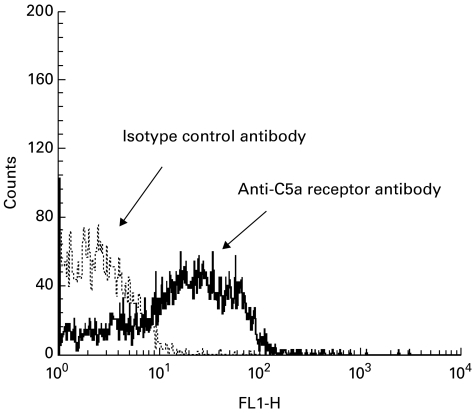
Immunofluorescent staining of the cultured proximal tubular epithelial cells with the rabbit anti-C5a receptor antibody demonstrated a strong cell surface label for the C5a receptor (Fig. 3a). No fluorescence was observed in the samples stained with control anti-haemoglobin antibody (Fig. 3b). FACS analysis with the anti-C5a receptor MoAb confirmed the cell surface expression of the receptor, with a seven-fold increase in FITC staining intensity compared with isotype-matched FITC-labelled control antibody (Fig. 4). Positive staining for the receptor was also observed on the surface of the human proximal tubular cell line, HK-2 (data not shown). The C5a receptor therefore appears to be expressed on the surface of proximal tubular epithelial cells both in vivo and in vitro.
Fig. 3.
Expression of the C5a receptor by primary cultures of human proximal tubular epithelial cells (PTEC). (a) Cell surface immunofluorescence using rabbit anti-C5a receptor antiserum. (b) Negative control using rabbit anti-haemoglobin antiserum.
Fig. 4.
FACS analysis of primary cultures of human proximal tubular epithelial cells (PTEC). The C5a receptor was detected with FITC-labelled murine MoAb to the C5a receptor in comparison with fluorescein-labelled isotype-matched control antibody.
Expression of C5a receptor mRNA
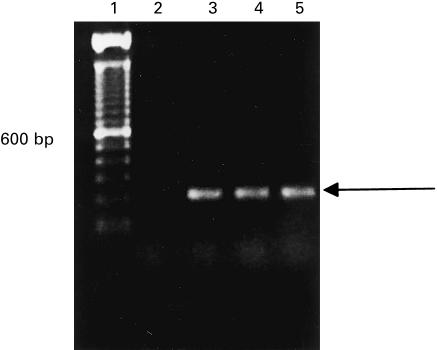
RT-PCR of mRNA isolated from the cultured proximal tubular epithelial cells produced the expected 209-bp product, as did RT-PCR using positive control mRNA from cultured U937 cells (Fig. 5). No larger PCR product was observed, which excludes the PCR amplification of genomic DNA contaminants.
Fig. 5.
Reverse transcriptase-polymerase chain reaction (RT-PCR) identification of C5a receptor mRNA from cultured human proximal tubular epithelial cells (PTEC). mRNA extracted from the PTEC was amplified by RT-PCR using the C5a receptor-specific primers, as described in Materials and Methods. A single band consistent with the expected size of 209 bp was observed on agarose gel electrophoresis with ethidium bromide staining. Lane 1, 100-bp ladder marker; lane 2, negative control; lane 3, PTEC; lane 4, PTEC; lane 5, human liver.
The effect of C5a on PTEC proliferation
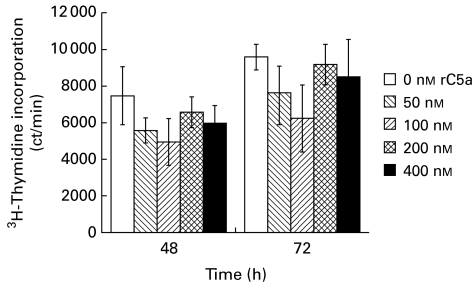
Previous data indicated that treatment of cultured mesangial cells with C5a enhanced expression of c-jun and c-fos [15] and resulted in induction of proliferation [16]. Serum-starved cultured proximal tubular epithelial cells therefore were treated with recombinant C5a (50–400 nm), and proliferation was quantified by both neutral red dye and 3H-thymidine uptake. In a number of experiments, no enhanced proliferation was observed in response to C5a treatment (Fig. 6). Identical experiments also were performed with PTEC that had not been growth-arrested. These cells also did not proliferate in response to C5a (data not shown).
Fig. 6.
Uptake of 3H-thymidine by cultured proximal tubular epithelial cells (PTEC) following treatment with C5a. Cells were plated and 3 days later were treated with C5a (50 nm, 100 nm, 200 nm, and 400 nm). Control cultures were treated with medium alone. 3H-thymidine uptake was quantified at 48 h and 72 h after C5a treatment. Treatment with C5a was not mitogenic.
The effect of C5a on secretion of chemokines and growth factors by cultured proximal tubular cells
Both TGF-β1 and MCP-1 were readily detected in culture supernatants from untreated proximal tubular epithelial cells (242 pg/ml and 6980 pg/ml, respectively) that were cultured in the presence of fetal calf serum and growth factors (hydrocortisone, human epithelial growth factor, epinephrine and triiodothyronine). Treatment of the cultured cells with C5a (50 nm) had little or no effect on the level of TGF-β1 (300 pg/ml following C5a treatment). Although several experiments suggested a possible slight increase in the level of MCP-1 (to as much as 8500 pg/ml after C5a treatment), the increase did not reach statistical significance. Neither PDGF-AB nor TNF-α were detected in untreated or treated culture supernatants.
Discussion
These studies demonstrate that the C5a receptor is expressed by PTEC and by transitional epithelium of the ureter and bladder. The combination of the immunohistochemical data on both tissue sections and cultured cells (Figs 1, 2 and 3), the FACS analysis (Fig. 4) and the RT-PCR data leave little doubt that the receptor is expressed by these epithelial cells both in vivo and in vitro. These data expand the growing list of tissues that express the C5a receptor. These include cells of virtually all derivations, including bone marrow-derived cells, smooth muscle cells, hepatocytes, endothelial cells, bronchial and alveolar epithelial cells and cells of the central nervous system [3,4,12–16]. PTEC are the second cell type within the kidney that has been shown to express the C5a receptor [15,16]. The level of expression appears to be much higher in epithelial cells (both PTEC and transitional epithelial cells) than in mesangial cells, which express the receptor at high levels in culture but at much lower levels in vivo [16].
In a number of cell types the biologic role of the receptor seems relatively straightforward, while in others, including both PTEC and mesangial cells, its function remains poorly defined. In general, all the described activities resulting from C5a receptor ligation function to promote the inflammatory response. For example, in granulocytes, C5a mediates chemotaxis, phagocytosis, release of active oxygen intermediates, release of proteolytic enzymes, and increased expression of adhesion molecules (particularly CD11b/CD18) [9,37–40]. Similarly, it induces increased expression of P-selectin and tissue factor on endothelial cells [12] in culture and of P-selectin on lung vascular tissue in vivo [12,18,19,21]. In a variety of cell types, ligation of the C5a receptor induces the secretion and/or the synthesis of a variety of cytokines, chemokines and growth factors, including IL-1, IL-6, TNF-α, IL-8, MCP-1, macrophage inflammatory protein (MIP)-2, MIP-1α and β, cytokine-induced neutrophil chemoattractant, and PDGF [16–20,41]. C5a induces the typical acute inflammatory changes in synthesis of plasma proteins by hepatocytes [3,13,14]. In addition, in mesangial cells, C5a treatment induces synthesis of c-jun and c-fos, resulting in a proliferative response, which is a common response of mesangial cells in glomerular diseases [15,16].
The studies described here have not yet defined an activity that results from C5a interaction with its receptor on the surface of PTEC. Ligation of the receptor clearly does not result in a proliferative response, as was observed with mesangial cells [16]. Furthermore, there was no significant induction of the limited number of cytokines, chemokines and growth factors that we have analysed (TNF-α, MCP-1, TGF-β, and PDGF). These four were selected for analysis based on several criteria. First, each has previously been shown to be synthesized by PTEC [29,42]. Second, as described above, TNF-α, PDGF and MCP-1 (in addition to several other chemokines) have all previously been shown to be induced by C5a [16,19,20,41]. Third, TGF-β is induced in PTEC during the development of the chronic interstitial changes with fibrosis that occur during the course of chronic glomerulonephritides. We reasoned that if complement activation might be involved in the generation of these changes then C5a might induce TGF-β. However, it appears from the data shown here that C5a, by itself, does not induce the synthesis of TGF-β. However, as shown by Czermak et al., at least in the case of several rat chemokines (MIP-2, MIP-1α, MIP-1β, cytokine-induced neutrophil chemoattractant, and MCP-1), C5a may function primarily to enhance synthesis induced by other factors [41]. It is possible that the conditions required by these primary PTEC cultures (hydrocortisone, epithelial growth factor, FBS, epinephrine, and triiodothyronine) for long-term maintenance result in maximal chemokine and growth factor synthesis. If this is the case, the addition of C5a would not result in further enhancement of synthesis. Unfortunately it was not possible to maintain these cells for prolonged periods in culture in the absence of these supplements.
It is likely that complement activation takes place within the interstitial tissue of the kidney and not simply within the glomerulus. This suggestion is supported both by the observations here that PTEC express the C5a receptor, and by previous studies that have demonstrated that PTEC in vivo express mRNA and protein for several complement components, including C3, C4, factor B and C5 [31,32]. In addition, PTEC in vitro have been shown to synthesize C3 and C4 [33,43]. In other cell types it has been shown that synthesis of these complement proteins is induced by a variety of cytokines. Several cytokines, including IL-1 and TNF-α, are up-regulated in glomeruli and/or in infiltrating monocyte/macrophages during immune complex glomerulonephritides [44–51]. These data also support the hypothesis that complement activation might take place during the development of the interstitial changes that accompany chronic glomerulonephritis. The observation that C5a levels are elevated in the urine of patients with renal allograft rejection in the absence of evidence for intravascular complement activation also suggests that interstitial complement activation may take place [52].
Mice with deletion of the C5a receptor gene via homologous recombination are unable to clear intrapulmonary-instilled Pseudomonas aeruginosa, even though they develop a marked intrapulmonary neutrophil influx [26]. The receptor therefore appears to play a major role in protection of the mucosa of the lung from bacterial infection. These data suggest the possibility that the C5a receptor may play a similar role in protection of the urinary tract.
In conclusion, from the data presented here and from previous data it is now clear that the C5a receptor is expressed within the kidney by both mesangial and proximal tubular epithelial cells. Furthermore, the receptor is expressed by distal tubular cells and the transitional epithelium of the ureter and bladder. In mesangial cells the receptor appears to mediate a proliferative response, while in PTEC, as in many other tissues, its function remains incompletely defined.
Acknowledgments
Supported by USPHS grants AI25011 (R.A.W.), HD22082 (A.E.D.), HD33727 (A.E.D.) and a grant from the Kidney Foundation of Greater Cincinnati (R.Z.).
References
- 1.Gerard C, Gerard NP. C5a anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 2.Wetsel RA. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr Opin Immunol. 1995;7:48–53. doi: 10.1016/0952-7915(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 3.Haviland DL, McCoy RL, Whitehead WT, et al. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861–9. [PubMed] [Google Scholar]
- 4.Lacy M, Jones J, Whittemore SR, et al. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes. J Neuroimmunol. 1995;61:71–78. doi: 10.1016/0165-5728(95)00075-d. [DOI] [PubMed] [Google Scholar]
- 5.Wetsel RA. Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cells. Immunol Letters. 1995;44:183–7. doi: 10.1016/0165-2478(94)00212-a. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein IM. Complement: biologically active products. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: basic principles and clinical correlates. New York: Raven Press, Ltd; 1992. pp. 63–80. [Google Scholar]
- 7.Hugli TE, Morgan EL. Mechanisms of leukocyte regulation by complement-derived factors Contemporary topics in immunobiology. New York: Plenum Press; 1984. pp. 109–17. [DOI] [PubMed] [Google Scholar]
- 8.Morgan EL. Modulation of the immune response by anaphylatoxins. Compl Inflamm. 1986;3:128–36. doi: 10.1159/000467890. [DOI] [PubMed] [Google Scholar]
- 9.Shin HS, Snyderman R, Friedman E, et al. Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science. 1968;162:361–53. doi: 10.1126/science.162.3851.361. [DOI] [PubMed] [Google Scholar]
- 10.Cochrane CG, Muller-Eberhard HJ. The derivative of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968;127:371–86. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson AR, Hugli TE, Muller-Eberhard HJ. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975;28:1067–74. [PMC free article] [PubMed] [Google Scholar]
- 12.Foreman KE, Vaporciyan AA, Bonish BK, et al. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–55. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCoy R, Haviland DL, Molmenti EP, Ziambaras T, Wetsel DH, Perlmutter DH. N-formylpeptide and complement C5a receptors are expressed in liver cells and mediate hepatic acute phase gene regulation. J Exp Med. 1995;182:207–17. doi: 10.1084/jem.182.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchner RR, Hugli TE, Ember JA, Morgan EL. Expression of functional receptors for human C5a anaphylatoxin (CD88) on the human hepatocellular carcinoma cell line HepG2. Stimulation of acute-phase protein-specific mRNA and protein synthesis by human C5a anaphylatoxin. J Immunol. 1995;155:308–15. [PubMed] [Google Scholar]
- 15.Wilmer WA, Kaumaya PT, Ember JA, Cosio FG. Receptors for the anaphylatoxin C5a (CD88) on human mesangial cells. J Immunol. 1998;160:5646–52. [PubMed] [Google Scholar]
- 16.Braun M, Davis AE., III Cultured human glomerular mesangial cells express the C5a receptor. Kidney Int. 1998;54:1542–9. doi: 10.1046/j.1523-1755.1998.00155.x. [DOI] [PubMed] [Google Scholar]
- 17.Okusawa S, Dinarello CA, Yancey KB, et al. C5a induction of human interleukin-1 synergistic effect with endotoxin or interferon-gamma. J Immunol. 1987;139:2635–40. [PubMed] [Google Scholar]
- 18.Scholz W, McClurg MR, Cardenas GJ, et al. C5a mediated release of interleukin-6 by human monocytes. Clin Immunol Immunopathol. 1990;57:297–307. doi: 10.1016/0090-1229(90)90043-p. [DOI] [PubMed] [Google Scholar]
- 19.Ember JA, Sanderson SD, Hugli TE, Morgan EL. Induction of IL-8 synthesis by human C5a anaphylatoxin. Am J Pathol. 1994;144:393–404. [PMC free article] [PubMed] [Google Scholar]
- 20.Okusawa S, Yancey YB, Meer JWvd, et al. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro: comparison with secretion of interleukin-1b and interleukin-1a. J Exp Med. 1988;168:443–8. doi: 10.1084/jem.168.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulligan MS, Schmid E, Till GO, et al. C5a-dependent up-regulation in vivo of lung vascular P-selectin. J Immunol. 1997;158:1857–61. [PubMed] [Google Scholar]
- 22.Hopken U, Mohr M, Struber A, et al. Inhibition of interleukin-6 synthesis in an animal model of septic shock by anti-C5a monoclonal antibodies. Eur J Immunol. 1996;26:1103–9. doi: 10.1002/eji.1830260522. [DOI] [PubMed] [Google Scholar]
- 23.Marchant A, Tielemans C, Husson C, et al. Cuprophane haemodialysis induces upregulation of LPS receptor (CD14) on monocytes: role of complement activation. Nephrol Dial Transplant. 1996;11:657–62. doi: 10.1093/oxfordjournals.ndt.a027355. [DOI] [PubMed] [Google Scholar]
- 24.Combe C, Pourtein M, Precigout VD, et al. Granulocyte activation and adhesion molecules during hemodialysis with cuprophane and high-flux biocompatible membranes. Am J Kidney Dis. 1994;24:437–42. doi: 10.1016/s0272-6386(12)80900-0. [DOI] [PubMed] [Google Scholar]
- 25.Mulligan MS, Schmid E, Beck-Schimmer B, et al. Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest. 1996;98:503–12. doi: 10.1172/JCI118818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 27.Fine LG, Norman JT, Ong A. Cell–cell cross-talk in the pathogenesis of renal interstitial fibrosis. Kidney Int. 1995;47(Suppl.):S48–S50. [PubMed] [Google Scholar]
- 28.Ong ACM, Fine LG. Tubular-derived growth factors and cytokines in the pathogenesis of tubulointerstitial fibrosis: implications for human renal disease progression. Am J Kid Dis. 1994;23:205–9. doi: 10.1016/s0272-6386(12)80973-5. [DOI] [PubMed] [Google Scholar]
- 29.Prodjosudjadi W, Gerritsma JSJ, Klar-Mohamad N, et al. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48:1477–86. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- 30.Tang WW, Qi M, Warren JS, Van GY. Chemokine expression in experimental tubulointerstitial nephritis. J Immunol. 1997;159:870–6. [PubMed] [Google Scholar]
- 31.Welch TR, Beischel LS, Frenzke M, Witte D. Regulated expression of complement factor B in the human kidney. Kidney Int. 1996;50:521–5. doi: 10.1038/ki.1996.344. [DOI] [PubMed] [Google Scholar]
- 32.Welch TR, Beischel LS, Witte DP. Differential expression of complement C3 and C4 in the human kidney. J Clin Invest. 1993;92:1451–8. doi: 10.1172/JCI116722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooimans RA, Stegmann APA, Dorp WTv, et al. Interleukin 2 mediates stimulation of complement C3 biosynthesis in human proximal tubular epithelial cells. J Clin Invest. 1991;88:379–84. doi: 10.1172/JCI115314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feucht HE, Zwirner J, Bevec D, et al. Biosynthesis of complement C4 messenger RNA in normal human kidney. Nephron. 1989;53:338–42. doi: 10.1159/000185778. [DOI] [PubMed] [Google Scholar]
- 35.Botney MD, Kaiser LR, Cooper JD, Mecham RP, Parghi D, Parks WC. Extracellular matrix protein gene expression in atherosclerotic hypertensive pulmonary arteries. Am J Pathol. 1992;140:357–64. [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf G, Haberstroh U, Neilson EG. Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol. 1992;140:95–107. [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez HN, Henson PM, Otani A, Hugli TE. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978;120:109–15. [PubMed] [Google Scholar]
- 38.Sacks T, Moldow CF, Craddock PR, Bowers TK, Jacob HS. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978;61:1161–7. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber C, Kitayama J, Springer TA. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996;93:10939–44. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molad Y, Haines KA, Anderson DC, et al. Immunocomplexes stimulate different signalling events to chemoattractants in the neutrophil and regulate l-selectin and beta 2-integrin expression differently. Biochem J. 1994;299:881–7. doi: 10.1042/bj2990881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czermak BJ, Sarma V, Bless NM, et al. In vitro and in vivo dependency of chemokine generation on C5a and TNA-α. J Immunol. 1999;162:2321–5. [PubMed] [Google Scholar]
- 42.Kujubu DA, Fine LG. Physiology and cell biology update: polypeptide growth factors and their relation to renal disease. Am J Kidney Dis. 1989;14:61–73. doi: 10.1016/s0272-6386(89)80096-4. [DOI] [PubMed] [Google Scholar]
- 43.Seelen MAJ, Brooimans RA, van der Woude FJ, et al. IFN-γ mediates stimulation of complement C4 biosynthesis in human proximal tubular epithelial cells. Kidney Int. 1993;44:50–57. doi: 10.1038/ki.1993.212. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto K, Hatano M. Production of interleukin-1 in glomerular cell cultures from rats with nephrotoxic serum nephritis. Clin Exp Immunol. 1989;75:123–8. [PMC free article] [PubMed] [Google Scholar]
- 45.Noronha IL, Kruger C, Andrassy K, et al. In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int. 1993;43:682–92. doi: 10.1038/ki.1993.98. [DOI] [PubMed] [Google Scholar]
- 46.Waldherr R, Noronha IL, Niemir Z, et al. Expression of cytokines and growth factors in human glomerulonephritis. Pediatr Nephrol. 1993;7:471–8. doi: 10.1007/BF00857578. [DOI] [PubMed] [Google Scholar]
- 47.Takemura T, Yoshioka K, Murakami K, et al. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994;424:459–64. doi: 10.1007/BF00191429. [DOI] [PubMed] [Google Scholar]
- 48.Akai Y, Iwano M, Kitamura Y, et al. Intraglomerular expressions of IL-1 alpha and platelet-derived growth factor (PDGF-B) mRNA in experimental immune complex-mediated glomerulonephritis. Clin Exp Immunol. 1994;95:29–34. doi: 10.1111/j.1365-2249.1994.tb06010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tam FW, Smith J, Cashman SJ, et al. Glomerular expression of interleukin-1 receptor antagonist and interleukin-1 beta genes in antibody-mediated glomerulonephritis. Am J Pathol. 1994;145:126–36. [PMC free article] [PubMed] [Google Scholar]
- 50.Niemir ZI, Stein H, Dworacki G, et al. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritis. Kidney Int. 1997;52:393–403. doi: 10.1038/ki.1997.346. [DOI] [PubMed] [Google Scholar]
- 51.Sterzl RB, Schulze-Lohoff E, Marx M. Cytokines and mesangial cells. Kidney Int. 1993;39:S26–S31. [PubMed] [Google Scholar]
- 52.Muller TF, Kraus M, Neumann C, Lange H. Detection of renal allograft rejection by complement components C5a and TCC in plasma and urine. J Lab Clin Med. 1997;129:62–71. doi: 10.1016/s0022-2143(97)90162-1. [DOI] [PubMed] [Google Scholar]