Abstract
It is suggested that CD30 and CD26 are surface molecules expressed on activated Th2 and Th1 cells, respectively. We examined plasma levels of soluble CD26 (sCD26) and sCD30 in patients with atopic dermatitis (AD) when their eruptions were aggravated and in non-atopic healthy controls, and then analysed the possible correlation between these values and the levels of several clinical markers. The plasma levels of both sCD30 and sCD26 were significantly higher in AD patients than in controls, both in exacerbation status and after conventional treatment. Multiple regression analyses showed that plasma sCD30 was a much better predictor of the levels of serum IgE, serum LDH and plasma sCD25, and the area and the score of AD eruption than sCD26, although elevated levels of both sCD30 and sCD26 are associated with these clinical predictors of AD. Importantly, sCD30 plasma levels decreased significantly in AD patients after conventional treatment, while no significant transition was noted in the concentration of sCD26. Moreover, a significant reduction of sCD30 levels was observed in the group of patients whose eruption score was reduced > 50%, whereas it was not in those < 50%. These findings provide evidence that the successful treatment of AD is associated with down-activation of Th2.
Keywords: soluble CD30, soluble CD26, atopic dermatitis, plasma
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease frequently seen in individuals with a genetic predisposition to increased IgE synthesis and IgE-mediated allergic reactions, i.e. allergic rhinitis or asthma [1–3]. A cytokine-mediated immune dysregulation, or in other words Th1/Th2 imbalance, to environmental allergens is thought to be an important pathogenic mechanism of the disease [1–4]. Th2 cells produce predominantly IL-4, IL-5 and IL-13, which promote IgE synthesis and eosinophilia and participate in humoral immunity [5]. These features suggest that Th2-type cytokines play a pathogenic role in allergic diseases. Indeed, predominance of Th2 cells from the blood and lesions of patients with AD has been demonstrated, as well as in other atopic disorders [6–10]. In contrast, non-atopic persons show mainly Th1 immunity characterized by production of interferon-gamma (IFN-γ), which inhibits the growth of Th2 cells [11]. A deficiency in the ability of atopic T cells to produce Th1 cytokines, but not Th2 cytokines, on stimulation is also documented [12]. It is therefore expected that successful treatment of AD would be accompanied by down-activation of Th2 cells, though little evidence has been presented about the transition of the Th1/Th2 balance in association with the course of AD.
The surface antigen CD30 molecule is a 120-kD membrane-bound glycoprotein belonging to the tumour necrosis factor (TNF) receptor superfamily, originally described as a marker for Hodgkin and Reed–Sternberg cells in Hodgkin's disease [13,14]. In addition, it has been shown that CD30 is an activation marker of T cell clones revealing a Th2-related cytokine production pattern [15,16]. The soluble form of the molecules (sCD30) is released into the bloodstream after cellular activation, and elevated serum levels of sCD30 has been reported in patients with AD [17–21]. However, little information is available concerning the possible correlation between the levels of sCD30 and several clinical markers that represent atopy and disease activity of AD: serum levels of lactate dehydrogenase (LDH) and IgE, blood eosinophil count and the eruption area and score. More importantly, there has been no study in which the transition of sCD30 values was examined in association with the course of AD, when the eruptions of AD are aggravated and improved.
Recently, it has been shown that surface expression of CD26, a glycoprotein dipeptidyl peptidase IV, correlates with the production of IFN-γ in CD4+ T cells in patents with granulomatous diseases and those with allergy against birch pollen [22,23]. Thus, it is suggested that high expression of CD26 would indicates a Th1-like immune reaction, although no information was found about the levels of its soluble form (sCD26) in plasma or serum of AD patients or other skin diseases.
In this study, we examined plasma levels of sCD26 and sCD30 in patients with exacerbated AD and non-atopic healthy controls, and then analysed the possible correlation between these values and the levels of several clinical markers. We also evaluated whether the values of sCD30 and sCD26 could change in association with improvement of the eruption in AD.
PATIENTS and METHODS
Patients
Eighty-eight patients with chronic (history of AD, median 22 years, range 6–35 years), moderate to severe AD diagnosed according to the criteria of Hanifin & Rajka [24] (age, median 25 years old, range 14–57 years) were selected for this study. Peripheral blood was obtained when their eruptions were exacerbated. The median serum IgE level of the patients was 4280 U/ml (range 20–57 900 U/ml; reference value < 380 U/ml). Patients were then treated with conventional therapy including systemic antihistamine and topical steroids. No patients had been treated with systemic steroids or immune suppressive agents. Plasma was obtained once more from 45 of these patients after treatment of AD for an average of 192 ± 113 days. The activity of AD was calculated using a modified scoring system of Costa et al. [25]. Our scoring method consisted of scoring types of lesions (erythema, exudation/crust, excoriation, lichenification, oedema, prurigo and dryness) in 22 anatomical areas: face, scalp, right and left sides of the chest, abdomen, back and buttocks, anterior and posterior aspects of both arms, thighs and lower legs. We estimated the clinical score at the sum of the number of types of lesions in all areas, giving a maximum score of 154. The values obtained with these scoring methods showed a significant correlation with those of Costa (r = 0·893, P < 0·0001). Healthy controls without any history of atopic diseases including AD, bronchial asthma or allergic rhinitis (n = 12, median serum IgE level 25 U/ml, range 1–183 U/ml) also participated in this study. Informed consent was obtained from all subjects or from their parents.
ELISA
Plasma levels of sCD30 and sCD26 (Bender Med System, Vienna, Austria) were measured with commercially available kits for sandwich ELISA, according to the manufacturer's instructions. The detection limit of the assay was estimated to be 6·3 U/ml for sCD30 and 39·0 ng/ml for sCD26. All plasma samples, stored at (80°C until use, were assayed in duplicate. Serum levels of soluble IL-2 receptor (sCD25) were also measured using ELISA (Yamanouchi Pharma, Tokyo, Japan).
Statistical analysis
Data were expressed as mean ± s.d. unless otherwise indicated. Mann–Whitney U-tests were performed for comparison analysis between patients with AD and control subjects. Linear regression analyses were carried out to determine a possible correlation between values of sCD30 and sCD26 and several clinical markers such as eruption score, total serum IgE levels, LDH levels, sCD25 levels and peripheral blood eosinophil counts using log-transformed values except for eruption areas and eruption scores. Multiple regression analyses were performed to analyse which value of sCD30 or sCD26 accounts for the levels of the other clinical predictors. Transition of log sCD30 and log sCD26 in AD patients in association with treatment was analysed using Student's paired t-test. All statistical analyses were assessed using StatView Version 5 (SAS Institute Inc., Cary, NC) on a Macintosh computer. P < 0·05 was regarded as significant.
Results
The plasma levels of sCD30 and sCD26 in patients with AD
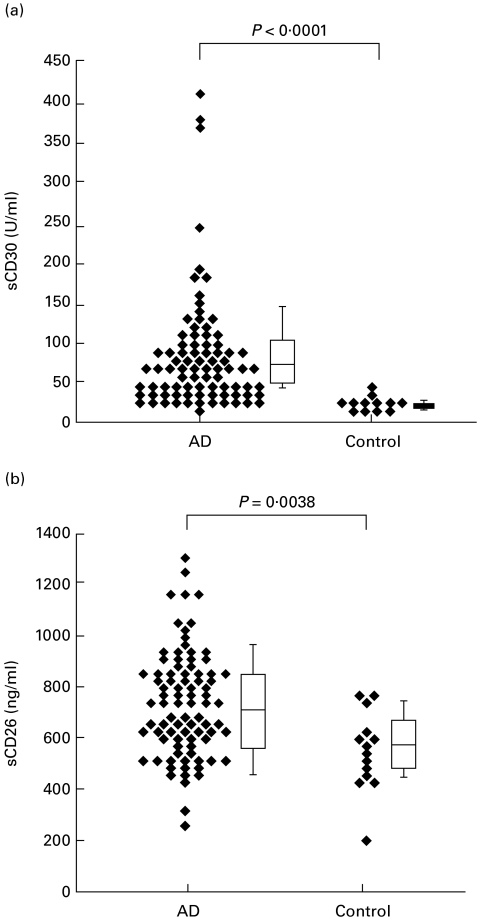
We examined the plasma levels of sCD30 and sCD26 in AD patients when their eruptions were aggravated. The plasma levels of sCD30 were significantly higher in AD patients (median 71·4 U/ml, range 33–400 U/ml) than in control subjects (median 33·3 U/ml, range 20–55 U/ml) (P < 0·0001), as previously reported (Fig. 1) [17–21]. Significantly higher levels of sCD26 were also noted in AD patients when their eruptions were in unfavourable conditions (median 710 ng/ml, range 257–1300 ng/ml) compared with non-atopic healthy controls (median 540 ng/ml, range 392–754 ng/ml) (P = 0·0038) (Fig. 1). The correlation between log sCD30 and log sCD26 was not significant (r = 0·378, P = 0·0549).
Fig. 1.
Plasma levels of sCD30 and sCD26 in patients with atopic dermatitis (AD) during exacerbation and normal healthy subjects. Concentration of plasma sCD30 (a) and sCD26 (b) was examined using ELISA.
Correlation between sCD30 and sCD26 concentration and other predictors of disease activity in AD
We next examined the association between the levels of log sCD30 or log sCD26 and the values of several clinical markers in AD patients when their eruptions were in aggravation (Table 1). The correlation between levels of log IgE and log sCD30 and between log IgE and log sCD26 was weak but significant. The logarithmic values of peripheral eosinophil counts (log Eos) and log LDH showed positive correlation both with log sCD30 and log sCD26. The levels of log sCD25 correlated significantly with log sCD30, while no correlation was found between log sCD25 and log sCD26. The values of patients' eruption area and eruption score also correlated significantly with both log sCD30 and log sCD26. These findings indicate that elevated levels of both sCD30 and sCD26 are associated with clinical markers of AD. Multiple regression analyses were then performed to evaluate which value of log sCD30 or log sCD26 accounts for other clinical markers in AD (Table 2). The contribution of log sCD30 and sCD26 to log IgE was small. Log LDH, log Eos, and log sCD25 were explained by log sCD30 and log sCD26. The area and score of the eruption were also accounted for by log sCD30 and log sCD26. The regression coefficients of sCD30 were higher than those of sCD26 for all clinical markers, suggesting sCD30 is more relevant to disease activity of AD than sCD26.
Table 1.
Correlation between sCD30 and sCD26 concentrations and other predictors of disease activity in atopic dermatitis (AD)
| Correlation analysis sCD30* | Correlation analysis sCD26* | ||||
|---|---|---|---|---|---|
| Median value (range) | r | P | r | P | |
| Serum IgE (U/ml) | 4455 (20–57 900) | 0·324 | 0·0351 | 0·277 | 0·0159 |
| Serum LDH (U/l) | 250 (136–648) | 0·729 | < 0·0001 | 0·508 | < 0·0001 |
| Eosinophil (/μl) | 581 (131–4759) | 0·714 | < 0·0001 | 0·397 | 0·0003 |
| Serum sCD25 (U/ml) | 552 (249–3230) | 0·879 | < 0·0001 | 0·272 | 0·2114 |
| Eruption area (%) | 31 (2–89) | 0·804 | < 0·0001 | 0·419 | < 0·0001 |
| Eruption score | 36 (4–77) | 0·586 | < 0·0001 | 0·277 | 0·0159 |
Reference values: serum IgE, < 380 U/ml; serum lactate dehydrogenase (LDH), 114–243 U/l; peripheral eosinophil counts, < 420/μl; serum sCD25, 246–742 U/ml.
The values except for eruption areas and eruption scores were log-transformed for linear regression analyses.
Table 2.
Multiple regression analyses to evaluate which values of sCD30 and sCD26 account for clinical markers in atopic dermatitis (AD)*
| Coefficient of determination | Regression coefficient sCD30 | Regression coefficient sCD26 | ||||
|---|---|---|---|---|---|---|
| r2 | P | r | P | r | P | |
| Serum IgE (U/ml) | 0·129 | 0·0061 | 0·255 | < 0·0001 | 0·168 | 0·1307 |
| Serum LDH (U/l) | 0·59 | 0·0005 | 0·625 | < 0·0001 | 0·263 | 0·0015 |
| Eosinophil (/μl) | 0·532 | < 0·0001 | 0·651 | < 0·0001 | 0·16 | 0·0659 |
| Soluble CD25 (U/ml) | 0·778 | < 0·0001 | 0·861 | < 0·0001 | 0·076 | 0·5016 |
| Eruption area (%) | 0·657 | < 0·0001 | 0·761 | < 0·0001 | 0·11 | 0·1307 |
| Eruption score | 0·345 | < 0·0001 | 0·546 | < 0·0001 | 0·0091 | 0·3702 |
Multiple regression analyses were carried out with each clinical marker as dependent variable and sCD30 and sCD26 values as independent variables.
Transition of plasma levels of sCD30 and sCD26 in association with the disease course in AD
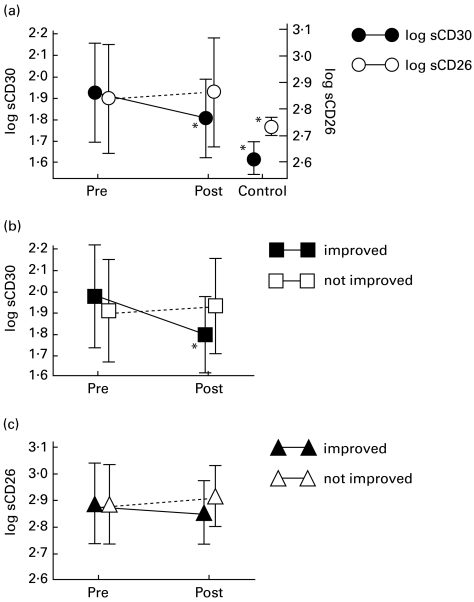
Finally, we analysed whether the values of sCD30 and sCD26 could change in association with the improvement of the eruption in AD. The median value of the eruption score was reduced from 35·5 to 25·0 after treatment. As shown in Fig. 2a, log sCD30 was significantly reduced in AD patients after conventional treatment (n = 45, P = 0·0032). However, no significant change in association with treatment was noted in the concentration of log sCD26 (P = 0·254). Both log sCD30 and log sCD26 levels after conventional therapy were still significantly higher than those of non-atopic controls (P = 0·0007 and 0·0074, respectively; unpaired t-test). Patients were then divided into two groups for further analysis, i.e. one group consisted of patients whose eruption score was reduced > 50% (n = 14) and the other consisted of those whose score was reduced < 50% (n = 31). The values of log sCD30 in the improved group were significantly reduced in association with treatment (P < 0·0001), while those in the unimproved group were not (P = 0·8340) (Fig. 2b). Neither the improved group (P = 0·0634) nor the unimproved one (P = 0·4757) showed significant change in log sCD26 (Fig. 2c).
Fig. 2.
Transition of plasma sCD30 and sCD26 levels in association with exacerbation followed by treatment in atopic dermatitis (AD) patients. (a) Plasma was obtained when the eruptions were aggravated (Pre) and after treatment (Post), then sCD30 and sCD26 levels were estimated using ELISA. These values during aggravation status and after treatment were compared using the paired t-test and those after treatment and non-atopic controls were compared using the unpaired t-test. Patients were divided into two groups; one consisted of patients whose eruption score was reduced > 50% (n = 14) and the other consisted of those whose score was reduced < 50% (n = 31), then the values of log sCD30 (b) and log sCD26 (c) during exacerbation and after treatment were compared using the paired t-test. *P < 0·05.
Discussion
We first examined which of Th1 or Th2 cells was involved in exacerbation of eruptions in chronic AD patients by examining plasma levels of sCD26 and sCD30, respectively. Hamid et al. suggested that the relative contribution of Th1- and Th2-type cytokines to the pathogenesis of AD depends on the duration of the eruption [2]. They demonstrated by in situ hybridization that, compared with normal control skin, acute skin lesions had significantly larger numbers of cells that were positive for IL-4, IL-5, and IL-13 mRNA, whereas they contained few IFN-γ mRNA-expressing cells [8,26]. When compared with acute AD, chronic AD skin lesions revealed significantly lower numbers of IL-4 and IL-13 mRNA-positive cells but increased numbers of IL-5 and IFN-γ-positive cells. Other observations revealed that increased in situ expression of the Th2-type cytokines IL-4 and IL-10 was always observed together with increased expression of the Th1-type cytokine IFN-γ within identical chronic skin lesions of the same patients [27,28]. These findings indicate that acute exacerbation of AD eruption is driven by Th2 cells, while maintenance of chronic lesions is associated with both Th1 and Th2 cells. Although we found significantly higher levels of both sCD30 and sCD26 in plasma obtained from AD patients in aggravated conditions compared with healthy non-atopic controls, multiple regression analysis showed that sCD30 levels accounted for sCD25 much better than sCD26. In addition, the plasma levels of sCD25, a representative marker for lymphocytic activation, correlated with those of sCD30 but not of sCD26 in AD patients in exacerbation. It is therefore suggested that both Th1 and Th2 cells, and particularly Th2 cells, are activated and most probably play pathogenic roles in AD patients when the eruptions are aggravated.
In the present study, plasma sCD30 levels exhibited a weak but significant correlation with serum IgE levels. Concerning IgE, some studies could not show correlation between serum IgE levels and sCD30 [17,19], while others could [18,21]. Serum IgE levels of the present AD patients were higher than those of patients in the studies showing no correlation between them, suggesting AD lesions were much more severe in the present patients. This may help explain the discrepancy in the results regarding the link between IgE and sCD30.
Eosinophilia is a well-known clinical feature representing disease activity of AD. Eosinophils play an important role in exacerbation of AD as effector cells by secreting cytotoxic granule proteins such as eosinophil cationic protein [29]. Currently, it is believed that the production and activation of eosinophils is augmented mainly by IL-5 and therefore Th2 cells should be a major subset of activated lymphocytes in AD patients with eosinophilia. Indeed, we showed that increased sCD30 accounted for eosinophilia much better than sCD26 by multiple regression analysis.
One of the major factors leading to exacerbation of AD eruption is increase of allergen exposure and increased number of antigen-specific IgE and T cells. Increase of IgE strongly up-regulates the expression of high-affinity IgE receptor (FcεRI) on mast cells and monocytes/macrophages [30–32]. IgE-dependent up-regulation of FcεRI expression strikingly augments the ability of mast cells to release histamine, leukotriene C4, IL-4 and IL-6 [30,31] and of monocytes to release TNF-α and several chemokines following FcεRI aggregation by multivalent allergens (N. Katoh & T. Bieber, unpublished observation). These events enhance allergic inflammation directly by mediator release and indirectly via activation of both Th1 and Th2 cells. Furthermore, elevated expression of FcεRI induces up-regulation of the capacity for IgE-mediated antigen presentation to T cells [33], which was shown to favour Th2 polarization by increasing prostaglandin E2 production, especially in AD subjects [34]. Taking these facts together, it is suggested that Th2 cells are predominantly involved in allergen-induced, IgE-mediated aggravation of AD, to which both Th cell subsets contribute. The present findings are consistent with this hypothesis; plasma sCD30 accounted for serum LDH, the area and the score of AD eruption when the AD lesions were exacerbated much better than sCD26, although both sCD30 and sCD26 were significantly correlated with them.
The most important finding of this study was that plasma sCD30 levels decreased significantly in AD patients after conventional treatment for exacerbation, while no significant transition was noted in the concentration of sCD26. Moreover, the significant reduction of sCD30 levels was observed in the improved group, whereas it was not in the unimproved group. It is therefore suggested that successful treatment of AD is associated with down-activation of Th2 cells. These findings further support the concept that Th2 cells play a predominant role in aggravation of AD. Until now, the precise mechanism by which activity of Th2 cells is down-regulated during successful treatment for acute aggravation was unknown. Recovering the skin barrier function associated with improvement of dermatitis reduces the patient's exposure to environmental allergens, which may lead to diminishing the IgE-mediated, Th2-favoured antigen presentation. Alternatively, it is possible that reduction of allergen exposure and treatment with topical corticosteroids would suppress a positive feedback mechanism for the development of Th2 response mediated by mast cells, e.g. corticosteroid inhibits IL-4 production by mast cells which results in suppressing autocrine survival of mast cells induced by FcεRI engagement by multivalent allergens [35]. Reduction of histamine release from mast cells accompanied by decreased allergen exposure also leads to restoring the capacity of monocytes to produce IL-12 [36]. On the other hand, it is implied that the failure to induce remission with treatments is related to a failure to down-activate both Th1 and Th2 cells.
In conclusion, the presenting findings are consistent with the hypothesis that the aggravation of AD is predominantly driven by activated Th2 cells, and improvement of the eruption is accompanied by down-activation of Th2 cells. Plasma sCD30 levels may be a useful circulating marker to monitor disease activity in AD.
Acknowledgments
We would like to thank Dr Kotaro Ozasa for his guidance with statistical analysis. This work was supported in part by a grant from the Japanese Ministry of Education, Science, Sports and Culture.
References
- 1.Leung DYM. Atopic dermatitis: the skin as a window into the pathogenesis of chronic allergic disease. J Allergy Clin Immunol. 1995;96:302–19. doi: 10.1016/s0091-6749(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 2.Leung DYM. Pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1999;104:S99–108. doi: 10.1016/s0091-6749(99)70051-5. [DOI] [PubMed] [Google Scholar]
- 3.Cooper KD. Atopic dermatitis: recent trends in pathogenesis and therapy. J Invest Dermatol. 1994;102:128–37. doi: 10.1111/1523-1747.ep12371746. [DOI] [PubMed] [Google Scholar]
- 4.Grewe M, Bruijnzeel-Koomen CAFM, Schöpf E, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–61. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 6.Kägi MK, Wüthrich B, Montano E, Barandun J, Blaser K, Walker C. Differential cytokine profiles in peripheral blood lymphocyte supernatants and skin biopsies from patients with different forms of atopic dermatitis, psoriasis and normal individuals. Int Arch Allergy Immunol. 1994;103:332–40. doi: 10.1159/000236651. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa M, Sugi N, Kawaguchi H, Ishii N, Nakajima H, Minami M. Predominance of type 2 cytokine-producing CD4+ and CD8+ cells in patients with atopic dermatitis. J Allergy Clin Immunol. 1997;99:673–82. doi: 10.1016/s0091-6749(97)70030-7. [DOI] [PubMed] [Google Scholar]
- 8.Hamid Q, Boguniewicz M, Leung DYM. Differential in situ cytokine expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thepen T, Langeveld-Wildschut EG, Bihari IC, et al. Biphasic response against aeroallergens in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol. 1997;97:828–37. doi: 10.1016/s0091-6749(96)80161-8. [DOI] [PubMed] [Google Scholar]
- 10.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 11.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 12.Jung T, Lack G, Schauer U, et al. Decreased frequency of interferon-γ- and interleukin-2-producing cells in patients with atopic disease measured at the single cell level. J Allergy Clin Immunol. 1995;96:515–27. doi: 10.1016/s0091-6749(95)70296-2. [DOI] [PubMed] [Google Scholar]
- 13.Dürkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor family that is characteristic for Hodgkin's disease. Cell. 1992;68:421–7. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 14.Schwab U, Stein H, Gerdes J, Lemke H, Kirchner H, Schaadt M. Production of a monoclonal antibody specific for Hodgkin and Sternberg–Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982;299:65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- 15.Del Prete G, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995;9:81–86. [PubMed] [Google Scholar]
- 16.Romagnani S, Del Prete G, Maggi E, Chilosi M, Caligaris-Cappio F, Pizzolo G. CD30 and type 2 T helper (Th2) responses. J Leuk Biol. 1995;57:726–30. doi: 10.1002/jlb.57.5.726. [DOI] [PubMed] [Google Scholar]
- 17.Bengtsson Å, Holm L, Bäck O, Fransson J, Scheynius A. Elevated serum levels of soluble CD30 in patients with atopic dermatitis (AD) Clin Exp Immunol. 1997;109:533–7. doi: 10.1046/j.1365-2249.1997.4731373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frezzolini A, Paradisi M, Ruffelli M, Cadoni S, De Pitá O. Soluble CD30 in pediatric patients with atopic dermatitis. Allergy. 1997;52:106–9. doi: 10.1111/j.1398-9995.1997.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 19.Dummer W, Bröcker EB, Bastian BC. Elevated serum levels of soluble CD30 are associated with atopic dermatitis, but not with respiratory atopic disorders and allergic contact dermatitis. Br J Dermatol. 1997;137:185–7. doi: 10.1046/j.1365-2133.1997.18031887.x. [DOI] [PubMed] [Google Scholar]
- 20.Caproni M, Bianchi B, D'Elios M, De Carli M, Amedei A, Fabbri P. In vivo relevance of CD30 in atopic dermatitis. Allergy. 1997;52:1063–70. doi: 10.1111/j.1398-9995.1997.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 21.Latza U, Davis S, Wilhelm D, McKnight B, Seyfarth M, Stein H. Soluble cytokine receptor CD30 in atopic disorders: a case control study. Clin Exp Allergy. 1999;29:97–104. doi: 10.1046/j.1365-2222.1999.00450.x. [DOI] [PubMed] [Google Scholar]
- 22.Scheel-Toelner D, Richter E, Toelner KM, et al. CD26 expression in leprosy and other granulomatous diseases correlates with the production of interferon-γ. Lab Invest. 1995;73:685–90. [PubMed] [Google Scholar]
- 23.Willheim M, Ebner C, Baier K, et al. Cell surface characterization of T lymphocytes and allergen-specific T cell clones: correlation of CD26 expression with TH1 subsets. J Allergy Clin Immunol. 1997;100:348–55. doi: 10.1016/s0091-6749(97)70248-3. [DOI] [PubMed] [Google Scholar]
- 24.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92(Suppl.):44–47. [Google Scholar]
- 25.Costa C, Rilliet A, Nicolet M, Saurat JH. Scoring atopic dermatitis: the simpler the better? Acta Derm Venereol (Stockh) 1989;69:41–45. [PubMed] [Google Scholar]
- 26.Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DYM. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–31. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- 27.Grewe M, Gyufko K, Schopf E, Krutman J. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994;343:25–26. doi: 10.1016/s0140-6736(94)90879-6. [DOI] [PubMed] [Google Scholar]
- 28.Ohmen JD, Hanifin JM, Nickoloff BJ, et al. Overexpression of IL-10 in atopic dermatitis. Contrasting cytokine patterns with delayed-type hypersensitivity reactions. J Immunol. 1995;154:1956–63. [PubMed] [Google Scholar]
- 29.Kapp A. The role of eosinophils in the pathogenesis of atopic dermatitis—eosinophil granule proteins as markers of disease activity. Allergy. 1993;48:1–5. doi: 10.1111/j.1398-9995.1993.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi M, Lantz CS, Oettgen HC, et al. IgE enhances mouse mast cell FcεRI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med. 1997;185:663–72. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi M, Sayama K, Yano K, et al. IgE enhances Fcε receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fcε receptor I expression and mediator release. J Immunol. 1999;162:5455–65. [PubMed] [Google Scholar]
- 32.Reishcl IG, Corvaïa N, Effenberger F, Wolff-Winiski B, Krömer E, Mudde GC. Function and regulation of FcεRI expression on monocytes from non-atopic donors. Clin Exp Allergy. 1996;26:630–41. [PubMed] [Google Scholar]
- 33.Jürgens M, Wollenberg A, Hanau D, de la Salle H, Bieber T. Activation of human epidermal Langerhans cells by engagement of the high affinity receptor for IgE, FcεRI. J Immunol. 1995;155:5184–9. [PubMed] [Google Scholar]
- 34.Kapsenberg ML, Hilkens CMU, Wierenga EA, Kalinski P. The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin Exp Allergy. 1999;29(Suppl. 2):33–36. [PubMed] [Google Scholar]
- 35.Yoshikawa H, Nakajima Y, Tasaka K. Glucocorticoid suppresses autocrine survival of mast cells by inhibiting IL-4 production and ICAM-1 expression. J Immunol. 1999;162:6162–70. [PubMed] [Google Scholar]
- 36.van der Pouw Kraan TC, Snijders A, Boeije LC, et al. Histamine inhibits the production of interleukin-12 through interaction with H2 receptor. J Clin Invest. 1998;102:1866–73. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]