Abstract
Staphylococcal superantigens have been implicated in the pathogenesis of atopic dermatitis (AD). This may occur through superantigenic activation of T lymphocytes and their subsequent induction of the skin homing receptor CLA on activated cells. We investigated the proliferative responses of peripheral blood mononuclear cells (PBMC) from 10 patients with an infective exacerbation of AD and six normal controls to the staphylococcal superantigens, staphylococcal enterotoxin A and B (SEA, SEB) and toxic shock syndrome toxin-1 (TSST-1), and the mitogens phytohaemagglutinin (PHA) and concanavalin A (Con A). We also assessed CLA and T cell receptor (TCR) Vβ-chain expression by immunofluorescence and flow cytometry before and after stimulation. PBMC from AD patients showed two-fold increased proliferation to SEA and SEB (P < 0·01) compared with normals, whereas the response to mitogenic stimulation was identical. Analysis of (TCR) Vβ-chain expression demonstrated increased use of superantigen-reactive Vβ families in freshly isolated PBMC in AD patients compared with controls. This pattern of Vβ-chain expression was only observed in the CLA+ but not the total population of T cells. Furthermore, there was a positive correlation between the enhanced PBMC proliferative response and increased expression of superantigen-reactive Vβ families in atopic patients. These data support the concept that superantigens are important in the pathogenesis of this common condition, and also provide evidence that the increased use of certain Vβ families in circulating, CLA+, skin homing lymphocytes is of functional significance.
Keywords: eczema, lymphocyte, skin-homing, superantigen
Introduction
Both lesional and clinically normal skin in patients with atopic dermatitis (AD) is frequently colonized by Staphylococcus aureus [1, 2]. Recently it has been reported that the majority of S. aureus strains isolated from atopic patients' skin produce superantigenic toxins such as staphylococcal enterotoxins A and B (SEA, SEB) or toxic shock syndrome toxin (TSST) [3,4]. There are several lines of evidence indicating that these toxins play an important pathogenic role in this disease. First, SEB when applied on both intact normal and intact atopic skin induces dermatitis [5]. Second, staphylococcal toxins have been shown in vitro to induce IgE production when cultured with peripheral blood mononuclear cells (PBMC) from patients with AD [6]. Third, 50% of patients have serum IgE specific for staphylococcal enterotoxins, and exhibit in vitro basophil histamine release on exposure to the relevant toxin [7]. Finally, increased incidence of newly diagnosed AD has been reported in patients following recovery from toxic shock syndrome [8].
Insight into the nature of the stimulus that causes accumulation of activated T cells in skin can be gained from analysis of the T cell receptor (TCR) distribution. Superantigens, unlike conventional antigens, activate T cells expressing certain TCR Vβ-chain families [9]. Consequently, a significant shift in the pattern of Vβ expression is expected to occur in T cells responding to such a stimulus. Increased usage of certain TCR Vβ-chain families has been reported in both the blood and skin of patients with AD, implying possible superantigen-driven expansion or selection [10–12]. T cells expressing the putative skin homing receptor CLA are believed to play an important role in inflammatory skin conditions such as AD. Lesions of AD are characterized by cellular infiltrates composed of CLA+ T cells [13]. Additionally it has been shown that the Vβ-chain restriction [11,12] and also proliferation to the house dust mite allergen [14] resides in the CLA+ population of circulating peripheral blood lymphocytes (PBL) in AD. Interestingly, superantigens up-regulate cell surface CLA expression on responding cells [15] and so T cells activated in this manner may show an increased tendency to home to skin. This provides a potential mechanism whereby bacterial superantigens may exacerbate cutaneous inflammation.
The objective of this study was to characterize further the role of staphylococcal superantigens in the pathogenesis of AD by assessing the proliferative responses of PBMC from patients with an exacerbation of AD and healthy controls to the superantigens SEA, SEB and toxic shock syndrome toxin (TSST) and determine whether this correlated with the Vβ profile of CLA+ PBL. Proliferation to the mitogens concanavalin A (Con A) and phytohaemagglutinin (PHA) was also assessed to determine whether any observed alteration in proliferative response was superantigen-specific. In addition, cell surface TCR Vβ-chain and CLA expression was analysed before and after stimulation to confirm that cells were responding in a superantigen, rather than antigen, dependent manner, and to determine whether PBL from atopic individuals show an increased tendency to develop a skin homing phenotype in response to superantigenic stimulation compared with normals. We used well characterized MoAbs to five TCR Vβ-chain families (Vβ2, Vβ3.1, Vβ5.1, Vβ5.2/3, and Vβ17). These Vβ-chains were chosen as they interact with the superantigens used in this study [9]; SEA stimulates Vβ5.2/3, SEB stimulates Vβ3.1 and Vβ17, and TSST stimulates Vβ2. Vβ5.1, which is unreactive with these superantigens, was included as a control.
PATIENTS AND METHODS
Patients and controls
Patients' consent and ethical approval was obtained prior to the study. Ten patients admitted to hospital with an acute exacerbation of AD (six males and four females, mean age 28·3 years) and six normal controls (four males and two females, mean age 31·5 years) were included. AD was diagnosed in accordance with the criteria of Hanifin & Rajka [16]. All AD patients had positive skin cultures for S. aureus at two or more cutaneous sites and had an eczema area and severity index (EASI) of > 10 (range 10·1–17·6, mean 12·8). All patients were receiving conventional topical therapy at the time of the study, including topical steroids, but had not been on systemic treatment or antibiotics for at least 3 months previously.
Reagents
The mitogens Con A and PHA and the toxins SEA, SEB and TSST were purchased from Sigma (Poole, UK). Antibodies used for immunofluorescence labelling and flow cytometric analysis are listed in Table 1. All anti-TCR Vβ antibodies were FITC-conjugated. The rat IgM anti-CLA MoAb, HECA-452, was detected using PE-conjugated goat anti-rat IgM (Sigma). Appropriate isotype controls were used for each assay.
Table 1.
Antibodies used
| Antibody | Isotype | Concentration (µg/5 × 105 cells) | Source |
|---|---|---|---|
| HECA-452 | Rat IgM | 1 | Gift from Dr L. J. Picker, University of Texas, Dallas, TX |
| Anti-Vβ2 | Mouse IgG1 | 1 | Immunotech, Luton, UK |
| Anti-Vβ3.1 | Mouse IgG1 | 0·4 | Serotec, Oxford UK |
| Anti-Vβ5.1 | Mouse IgG1 | 0·75 | Serotec |
| Anti-Vβ5.2/3 | Mouse IgG1 | 1 | Serotec |
| Anti-Vβ17 | Mouse IgG1 | 1 | Serotec |
| R4-22 | Rat IgM isotype standard | 1 | PharMingen, San Diego, CA |
| 679.1Mc7 | Mouse IgG1 isotype standard | 1 | Immunotech |
Proliferation assay
Blood (20 ml) was collected from each subject at the same time of day. PBMC were isolated by Lymphoprep (Nycomed, Oslo, Norway) density gradient centrifugation and washed twice in sterile PBS. Cells were then resuspended at 1 × 106 cells/ml in RPMI 1640 (Gibco, Paisley, UK) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin G, 100 μg/ml streptomycin, 25 μg/ml amphotericin B and 2 mm l-glutamine. One hundred microlitres or 1 × 105 cells/well of isolated PBMC were then transferred to 96-well flat-bottomed microtitre plates (Greiner, Stonehouse, UK), and cultured in the presence of Con A (5 μg/ml), PHA (1 μg/ml), SEA, SEB, TSST (all at 10 ng/ml) or culture medium alone. Three wells were included for each stimulant. Previous experiments had determined that these were the minimum concentrations of stimulant required to produce maximum proliferation. Cells were incubated at 37°C in humid air containing 5% CO2. After 72 h incubation, cells were pulse-labelled with 3H-thymidine (3H-TdR; 1 mCi/well; Amersham Int., Aylesbury, UK). Cells were harvested 16 h later on microglass filter strips using a cell harvester (Dynatech Autowash 2000) and incorporated radioactivity was determined by a liquid scintillation counter (Wallac Rackbeta using Wallac Betaplate scintillation fluid; Leicester, UK). Proliferation was expressed as the mean of the three counts.
Double immunofluorescence labelling
In all subjects PBMC were isolated as described and 2 × 106 cells/well cultured in 12-well cell culture clusters (Greiner) in the presence of mitogen or superantigen as outlined above. Cells were cultured for 72 h, washed three times in sterile PBS, resuspended in supplemented RPMI containing 20 U/ml rIL-2 (Sigma), and cultured for a further 24 h prior to immunofluorescence labelling. Cells were cultured with rIL-2 after initial stimulation to allow re-expression of the T cell receptor, which is internalized on superantigenic activation. Previous studies have shown that this does not affect CLA expression [15]. Cultured PBMC were then washed twice and resuspended at 5 × 106 cells/ml in PBS containing 0·1% azide and 0·1% bovine serum albumin (PBS−0·1% azide−0·1% BSA). Cells (100 μl; 5 × 105) were incubated at 4°C for 30 min with appropriate antibody, washed a further three times in cold PBS−0·1% azide−0·1% BSA and resuspended at 1 × 106 cells/ml in PBS containing 1% paraformaldehyde.
Flow cytometric analysis
Flow cytometric analysis was performed on a FACScan (Becton Dickinson, Oxford, UK) using LYSIS II software. Appropriate forward and 90° light scatter gates were used to select the resting lymphocyte and T cell blast populations accordingly. Fluorescent labelled positive cells were selected by setting gates with reference to relevant red and green negative controls. Positive cells were expressed as a percentage of total lymphocytes, obtained from a count of 10 000 cells.
Statistical analysis
Results are given as a mean percentage ± s.e.m. Statistical comparisons were made using the Mann–Whitney U-test. Correlation was assessed by calculating the Pearson correlation coefficient (r).
Results
Proliferation assay
PBMC from 10 patients with AD and six normal controls were stimulated with the mitogens Con A or PHA, the staphylococcal superantigens SEA, SEB or TSST, or culture medium alone. Proliferation was assessed by 3H-TdR incorporation and expressed as ct/min. The results are shown in Fig. 1.
Fig. 1.
Peripheral blood mononuclear cells (PBMC) from atopic dermatitis (AD; ▪) patients (n = 10) and normal controls (□; n = 6) were cultured for 3 days in the presence of superantigen, mitogen, or culture medium alone. Proliferation was assessed by 3H-thymidine incorporation and is expressed as mean + s.e.m. scintillation ct/min. Results show significantly increased proliferation to staphylococcal enterotoxin A (SEA) and B (SEB) (P < 0·01) and a trend towards increased proliferation to toxic shock syndrome toxin (TSST) in atopic patients compared with normals. Proliferation in response to concanavalin A (Con A) and phytohaemagglutinin (PHA) was identical between the two groups.
Patients with AD demonstrated a significantly greater proliferative response to SEA and SEB than normal controls (P < 0·01 for each). AD patients showed a trend towards increased proliferation to TSST compared with normals, although not achieving statistical significance (P = 0·13). There were no differences between AD patients and normals in the proliferative responses to Con A, PHA or in unstimulated cells.
Flow cytometric analysis
PBMC from AD patients and normal controls were assessed for cell surface CLA and Vβ-chain expression by two-channel flow cytometry before and after mitogen/superantigen stimulation.
There was a significant increase in Vβ2, Vβ5.3/3 and Vβ17-positive PBL within the CLA+ population of cells in AD patients compared with normals (Fig. 2a). However, there was no difference in the mean level of expression of these Vβ-chains in the total (CLA+ and CLA−) population of PBL between eczema patients and normals (Fig. 2b).
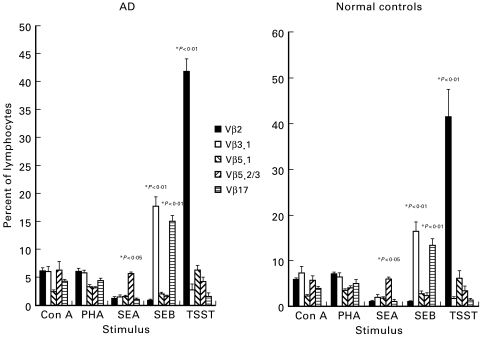
Fig. 2.
T cell receptor Vβ-chain expression was analysed on freshly isolated peripheral blood mononuclear cells (PBMC) from atopic dermatitis (AD) patients (n = 10) and normal controls (n = 6) for five Vβ-chain families by immunofluorescence and flow cytometry. Results are expressed as the mean + s.e.m. percentage of lymphocytes expressing a particular Vβ-chain. (a) Vβ expression in CLA+ PBMC in AD patients (▪) versus controls (□). (b) Vβ expression in total PBMC in AD patients (▪) versus controls (□). (c) Vβ expression in CLA+ (▪) versus CLA− (□) PBMC in AD patients. (d) Vβ expression in CLA+ (▪) versus CLA− (□) PBMC in controls.
Eczema patients showed a significant increase in Vβ2, Vβ5.3/3 and Vβ17 expression in CLA+ compared with CLA−PBL (Fig. 2c). Normal controls showed no differences in Vβ-chain expression between CLA+ and CLA− cells (Fig. 2d).
Unstimulated PBMC from AD patients demonstrated increased CLA expression (mean 16·5 ± 3·1% of lymphocytes) versus normal controls (mean 7·6 ± 0·6%; P < 0·01) (Fig. 3)
Fig. 3.
CLA expression on freshly isolated peripheral blood mononuclear cells (PBMC), and PBMC cultured for 3 days in the presence of superantigen or mitogen, was analysed by immunofluorescence and flow cytometry in patients with atopic dermatitis (AD; ▪) (n = 10) and healthy controls (□) (n = 6). Results are expressed as the mean + s.e.m. percentage of CLA+ PBMC. Freshly isolated PBMC from AD patients showed increased CLA expression compared with normals (16·5% versus 7·6%; P < 0·01). Staphylococcal enterotoxin A (SEA), B (SEB), toxic shock syndrome toxin (TSST), concanavalin A (Con A) and phytohaemagglutinin (PHA) all caused significant up-regulation of CLA expression compared with unstimulated cells, but there was no difference between atopics and normals.
The mitogens Con A and PHA and the superantigens SEA, SEB and TSST all caused significant up-regulation of CLA expression compared with unstimulated cells (Fig. 3). SEA, SEB, TSST and Con A induced CLA expression on between 40% and 50% of proliferating T cell blasts, whilst PHA induced CLA expression on approximately 25% of these cells. There were however, no differences in CLA expression in stimulated cells between AD patients and normals.
As would be predicted for superantigens, SEA, SEB and TSST all caused expansion of individual Vβ-chain families, whereas the mitogens Con A and PHA stimulated all Vβ families equally (Fig. 4). SEA caused expansion of Vβ5.2/3, SEB-stimulated cells predominantly expressed Vβ3.1 and Vβ17, and TSST caused marked expansion of Vβ2+ cells. These results are consistent with previous studies [9]. The profile of Vβ-chain expression in stimulated PBMC between AD patients and normals was, however, almost identical (Fig. 4).
Fig. 4.
T cell receptor Vβ-chain expression was analysed on peripheral blood mononuclear cells (PBMC) cultured for 3 days in the presence of superantigen or mitogen in atopic dermatitis (AD) patients (n = 10) and normal controls (n = 6). Results are expressed as the mean + s.e.m. percentage of lymphocytes expressing a particular Vβ-chain. Statistical comparisons are between the percentage of cells expressing each Vβ family following stimulation with the percentage prestimulation. Concanavalin A (Con A) and phytohaemagglutinin (PHA) stimulated all Vβ families equally, whereas the superantigens expanded individual Vβ-chain families. The profile of Vβ-chain expression between atopics and normals was identical.
Correlation between Vβ-chain expression and superantigen-induced proliferation
Superantigens stimulate T cells belonging to certain Vβ-chain families; TSST reacts with Vβ2, SEA with Vβ5.2/3 and SEB with Vβ3.1 and 17. A correlation was therefore made between the proliferative response to each superantigen and expression of the appropriate Vβ-chain in each atopic individual (Fig. 5).
Fig. 5.
A correlation was made between the proliferative response to each superantigen and expression of the corresponding superantigen-reactive Vβ-chain in each individual with atopic dermatitis (AD). The Pearson correlation coefficient (r) was calculated for each pair. (a) Toxic shock syndrome toxin (TSST) and Vβ2. (b) Staphylococcal enterotoxin B (SEB) and Vβ17. (c) SEB and Vβ3.1. (d) Staphylococcal enterotoxin A (SEA) and Vβ5.2/3.
There was a significant positive correlation between proliferation to TSST and Vβ2 expression (r = 0·85; P < 0·02), and SEB-induced proliferation and Vβ17 expression (r = 0·80; P < 0·05). There was a positive correlation for SEB/Vβ3.1 (r = 0·70) and SEA/Vβ5.2/3 (r = 0·37), but this did not reach statistical significance.
Discussion
In this study, PBMC from AD patients showed approximately two-fold increased proliferation to the staphylococcal superantigens SEA and SEB, and to a lesser extent TSST, but not to the mitogens Con A or PHA, when compared with healthy controls. Such enhanced superantigen-mediated proliferation has been reported in AD [17], but the mechanism for this observation has yet to be clarified. It may reflect a superantigen-specific process, or may be a consequence of more general immune activation and increased numbers of circulating memory cells in patients with active AD. Additionally, the hyper-responsiveness observed in this study might be a consequence of staphylococcal infection rather than AD per se, since our control population were healthy volunteers. Analysis of the TCR Vβ-chain repertoire of freshly isolated PBMC from AD patients demonstrated increased usage of superantigen-reactive Vβ-chains restricted to the CLA+ population of T cells only. Furthermore, there was a positive correlation between superantigen-induced proliferation and increased expression of corresponding superantigen-reactive Vβ-chains. Recent studies have demonstrated similar increased usage of superantigen-reactive Vβ-chains confined to the CLA+ subset of PBL in patients with AD [11,12]. That such increased Vβ expression correlates with enhanced staphylococcal toxin-induced proliferation indicates that this observation is likely to be of functional significance.
On the basis of these data we postulate that in vivo superantigen exposure generates a population of Vβ-restricted, CLA+ PBL, and that increased numbers of these superantigen-reactive cells are responsible for the observed increased in vitro PBMC proliferative response. It is less likely that this enhanced proliferative response is due to a state of generally increased immune activation in these patients, since proliferation to the mitogens Con A and PHA was the same as in healthy controls, although the use of mitogenic stimulation may have masked a subtle difference in immune activation that may have been revealed by the use of irrelevant antigenic stimulation as a control. It is possible that the increased proliferation in AD patients to these staphylococcal toxins results from circulating memory T cells generated from previous exposure responding in an antigen-, rather than superantigen-dependent manner. However, analysis of the TCR Vβ repertoire following the proliferation assay demonstrated that the staphylococcal toxins caused expansion of individual superantigen-reactive Vβ families, indicating that proliferation was caused by superantigenic stimulation.
Superantigens up-regulate expression of the skin homing receptor, CLA, on T cells in vitro [15]. One possible mechanism by which superantigens may cause exacerbations of AD is through generation of CLA+ skin homing lymphocytes. Indeed, this study has demonstrated significantly increased numbers of CLA+ circulating PBL in patients with AD compared with normals. If this finding reflects a superantigen-mediated process, it could be explained by the more frequent and heavier skin colonization by superantigen-producing S. aureus in AD compared with normals, coupled with increased penetration of bacterial toxin through inflamed skin. An alternative hypothesis is that PBL from atopic individuals show an increased tendency to express CLA in response to superantigen. This study has demonstrated that whilst the superantigens SEA, SEB and TSST are potent inducers of CLA expression on PBMC in culture, there is no difference in the magnitude of this response between atopics and normal controls. These data therefore provide no support for the above hypothesis, although such in vitro conditions may not necessarily predict in vivo responses. Additionally, our data indicate that the mitogen Con A induces CLA expression on proliferating T cell blasts at an equivalent level to that induced by superantigens (approximately 40% of responding cells), whilst PHA induces CLA expression on approximately 25% of these cells, representing three-fold increased expression compared with normal resting PBL. This demonstrates that superantigen-induced up-regulation of CLA expression is not a specific phenomenon as has been previously reported [18], but also occurs in response to mitogenic stimulation.
In conclusion, this study has demonstrated that PBMC from patients with an acute exacerbation of AD show enhanced proliferative responses to staphylococcal superantigens, and that these responses correlate with increased use of superantigen-reactive Vβ families confined to the CLA+ population of PBL. This provides further evidence not only for the role of bacterial superantigens in exacerbating AD, but also for the functional importance of circulating, CLA+, skin homing lymphocytes in this common condition.
Acknowledgments
This work was supported by the Special Trustees of St Thomas' Hospital.
References
- 1.Aly R, Maibach HI, Shinefield HR. Microbial flora of atopic dermatitis. Arch Dermatol. 1977;113:780–2. [PubMed] [Google Scholar]
- 2.Gloor M, Peters G, Stoika D. On the resident aerobic bacterial skin flora in unaffected skin of patients with atopic dermatitis and in healthy controls. Dermatologica. 1982;164:258–65. doi: 10.1159/000250099. [DOI] [PubMed] [Google Scholar]
- 3.McFadden JP, Noble WC, Camp RDR. Superantigenic exotoxin secreting potential of staphylococci isolated from atopic eczematous skin. Br J Dermatol. 1993;128:631–2. doi: 10.1111/j.1365-2133.1993.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 4.Bunikowski R, Mielke M, Skarabis H, et al. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J All Clin Immunol. 1999;103:119–24. doi: 10.1016/s0091-6749(99)70535-x. [DOI] [PubMed] [Google Scholar]
- 5.Strange P, Skov L, Lisby S, Nielsen PL, Baadsgaard O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Arch Dermatol. 1996;132:27–33. [PubMed] [Google Scholar]
- 6.Neuber K, Steinrucke K, Ring J. Staphylococcal enterotoxin B (SEB) affects in vitro IgE synthesis, IFNγ, IL-4 and IL-5 production in atopic eczema. Int Arch Allergy Immunol. 1995;107:179–82. doi: 10.1159/000236970. [DOI] [PubMed] [Google Scholar]
- 7.Leung DYM, Harbeck R, Bina P, et al. Presence of IgE antibodies to staphylococcal enterotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993;89:1374–80. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michie CA, Davis T. Atopic dermatitis and staphylococcal superantigens. Lancet. 1996;347:324. doi: 10.1016/s0140-6736(96)90498-5. [DOI] [PubMed] [Google Scholar]
- 9.Saloga J, Gelfand EW, Knop J. Superantigens. Exp Dermatol. 1996;5:65–71. doi: 10.1111/j.1600-0625.1996.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 10.Neuber K, Loliger C, Kohler I, Ring J. Preferential expression of T-cell receptor Vβ-chains in atopic eczema. Acta Derm Venereol. 1996;76:214–8. doi: 10.2340/0001555576214218. [DOI] [PubMed] [Google Scholar]
- 11.Strickland I, Hauk PJ, Trumble AE, Picker LJ, Leung DM. Evidence for superantigen involvement in skin homing of T cells in atopic dermatitis. J Invest Dermatol. 1999;112:249–53. doi: 10.1046/j.1523-1747.1999.00502.x. [DOI] [PubMed] [Google Scholar]
- 12.Torres MJ, Gonzalez FJ, Corzo JL, et al. Circulating CLA+ lymphocytes from children with atopic dermatitis contain an increased percentage of cells bearing staphylococcal-related T-cell receptor variable segments. Clin Exp Immunol. 1998;28:1264–72. doi: 10.1046/j.1365-2222.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 13.De Vries JM, Langeveld-Wildschut EG, van Reijsen FC, et al. Non-specific T cell homing during inflammation in atopic dermatitis: expression of cutaneous lymphocyte associated antigen and integrin αEβ7 on skin-infiltrating T cells. J All Clin Immunol. 1997;100:694–701. doi: 10.1016/s0091-6749(97)70175-1. [DOI] [PubMed] [Google Scholar]
- 14.Babi LFS, Picker LJ, Soler MTP, Drzimalla K, Flohr P, Blaser K, Hauser C. Circulating allergen reactive T cells from patients with atopic dermatitis and allergic contact dermatitis express the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen. J Exp Med. 1995;181:1935–40. doi: 10.1084/jem.181.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung DYM, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–53. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venerol. 1980;92:44–47. [Google Scholar]
- 17.Yudate T, Yamada H, Tezuka T. Role of staphylococcal enterotoxins in pathogenesis of atopic dermatitis: growth and expression of T cell receptor Vβ of peripheral blood mononuclear cells stimulated by enterotoxins A and B. J Derm Science. 1996;13:63–70. doi: 10.1016/0923-1811(95)00502-1. [DOI] [PubMed] [Google Scholar]
- 18.Zollner TM, Nuber V, Duijvestijn AM, Boehncke WH, Kaufmann R. Superantigens but not mitogens are capable of inducing upregulation of E-selectin ligands on human T lymphocytes. Exp Dermatol. 1997;6:161–6. doi: 10.1111/j.1600-0625.1997.tb00200.x. [DOI] [PubMed] [Google Scholar]