Abstract
We set out to examine if the IgG-producing cells in the colonic mucosa in UC are committed to tropomyosin isoform 5 (hTM5), a putative autoantigen in UC. Lamina propria mononuclear cells (LPMC) were isolated from colonoscopic biopsy specimens from recto-sigmoid and proximal colon. Twenty-three patients with UC, eight with Crohn's colitis (CC), and 10 non-inflammatory bowel disease (non-IBD) controls were included. The ELISPOT assays were used to quantify lamina propria B cells producing total immunoglobulin (IgA, IgG, IgM), IgG, IgA, as well as IgG against hTM5 isoform. The median value of percentage of total IgG-producing lymphocytes was similar in UC (12%) and CC (11%), but was significantly (P < 0·0002) higher than non-IBD controls (6%). However, in UC, but not in CC and non-IBD, a large number of lamina propria B cells produced IgG against hTM5 (median values: UC 42%, CC 2·5%, non-IBD 0%). This difference in UC when compared with CC and non-IBD was highly significant (P < 0·00001). Twenty-one of 23 (91%) patients with UC had percentage of anti-hTM5 IgG-producing immunocytes more than 2 s.d. above the mean for non-UC patients. In UC but not in CC and non-IBD controls, the increased number of IgG-producing cells are largely committed to produce IgG against hTM5-related epitope(s).
Keywords: inflammatory bowel disease, autoantibody, tropomyosin, ulcerative colitis
Introduction
UC is a chronic inflammatory disease of the colon which may be perpetuated by autoimmune processes [1]. Although a variety of antibodies against epithelial cells or bacteria have been found in the serum of patients with UC, it is unclear whether these are epiphenomena or related to the disease process. A marked increase in the mucosal IgG-producing immunocytes [2] and IgG antibodies against unknown colonic antigens [3] and neutrophil antigens [4] has been reported in patients with UC. Mucosal IgG overproduction, particularly IgG1 [5], may be relevant in the pathogenesis of UC, as it may cause complement activation. However, the specific antigen(s) involved in this mucosal IgG immune recognition and the antigenic epitope recognized by the increased number of plasma cells seen in the UC colon, have not been clearly defined.
We earlier reported the presence of tissue-bound IgG antibody in the colonic mucosa of patients with UC [6] and identified a 40 000-D mol. wt colonic protein, p40, that reacts with the IgG eluted from UC colon mucosa [7]. Colon tissue-bound IgG from Crohn's disease (CD) involving the colon did not recognize p40, suggesting an autoantigenic role of p40 in UC [7]. Subsequently, p40 was purified to homogeneity and partial sequence analysis demonstrated that p40 belongs to the tropomyosin family [8]. Tropomyosins (TMs) are cytoskeletal microfilamental proteins present in all eukaryotic cells with organ-specific isoforms and multiple isoforms may be present in the same cell with distinct functions [9]. At least eight isoforms (molecular weights ranging from 30 000 to 40 000 D) are identified in human fibroblast cells [10]. Recently, we have reported that colonic epithelial cells contain mainly hTM isoform 5, followed by hTM4 [11]. TMs are capable of inducing significant immune responses related to allergy [12] and autoimmunity [13]. In a computer-based physical and chemical analysis of various human autoantigens, it was demonstrated that several sequences of TM residues were among the most potent autoantigens [13]. This suggests a strong autoantigenic role for TM peptide(s) in the autoimmune process.
To examine the immune response against hTMs by the mucosal immunocytes, we have estimated by ELISPOT assay the number of mucosal B lymphocytes committed to autoantibody production against hTM isoform 5 from the colonic mucosal biopsy specimens obtained during colonoscopy from patients with UC, Crohn's colitis (CC) and patients with diarrhoea without UC or CC (non-inflammatory bowel disease (non-IBD)). The clinical activity of the disease, duration of disease, treatment, and histological grades were correlated with the total number of IgG-producing cells and the hTM5-specific IgG-producing cells.
Materials and methods
Patients
During October 1997 to May 1998, 41 out-patients from the Robert Wood Johnson IBD clinic undergoing sigmoidoscopic or colonoscopic examinations for diarrhoeal symptoms or for IBD cancer surveillance were included in this study. A total of 23 patients with UC (11 female), eight with CC (three female) and 10 non-IBD control subjects (six female) were included. Of the eight patients with CC, five patients had active inflammation in the recto-sigmoid colon, and three had right-sided colitis. The study was approved by the Institutional Research Review Committee. The age of the patients with UC ranged from 23 to 70 years., with median age of 41·5 years. Twenty patients were symptomatic and three were in remission. The median age of the patients with CC was 27 years, ranging from 17 to 57 years. All of these eight patients were symptomatic. All of the UC patients were taking azulfidine or mesalamine. Three were taking prednisone (< 15 mg/day) and four were taking purinethol (50 mg/day in two, 75 mg/day in two). The CC patients were also taking mesalamines and two patients were taking purinethol (50 mg/day). None of the CC patients was taking steroids. The non-IBD control subjects included patients with diarrhoea due to lactose intolerance (n = 2), irritable bowel syndrome (n = 4), C. deficile (n = 1), yersinia (n = 1), ischaemic colitis (n = 1) and status post partial colon resection for colon cancer (n = 1). The patients with C. deficile and ischaemic colitis had inflammation in the sigmoid colon. The median age of this group was 40 years (range 29–65 years).
Tissue samples
During colonoscopy or sigmoidoscopy, the mucosa was graded as in complete remission or with mild or moderate activity. Since these were all out-patients, none of the patients had severe colitis. Six to eight biopsy specimens were obtained from the recto-sigmoid region (10–30 cm from the anus) from each of the 23 patients with UC, five with CC with recto-sigmoid involvement, and 10 non-IBD control subjects. In the remaining three patients with CC with right-sided colitis, the biopsy specimens were taken from the right colon at grossly inflamed sites and placed in calcium- and magnesium-free (CMF) Hanks' balanced salt solution (HBSS). In seven patients with UC, additional biopsy specimens were obtained from the proximal colon (> 30 cm from the anus to caecum). At least two biopsy specimens were sent from each site for routine histology. The biopsy specimens were graded histologically by a single pathologist (P.S.A.) who was unaware of the endoscopic findings, as well as the experimental data. The grading was as follows: grade 0, normal; grade 1, occasional cryptitis with increased inflammatory cells/mononuclear cells; grade II, cryptitis with crypt abscess; grade III, florid colitis. In CC, additional comment was made if there was granuloma.
Isolation of lamina propria mononuclear cells from biopsy specimens
The biopsy specimens (six to eight) were kept at 4°C and were processed within 1 h. Lamina propria mononuclear cells (LPMC) were isolated following established methods [14] using EDTA and collagenase V, followed by Percoll gradient separation. The yield of LPMC from the biopsy specimens ranged from 0·5 to 4 × 106 cells and the viability ranged from 85% to 95%.
ELISPOT assay
For the ELISPOT assay, multi-screen HA filtration microtitre plates (Millipore, Danbury, CT; Cat. no. MAHA S4510) were used. The wells in each plate were coated in triplicate with (i) anti-human immunoglobulin including IgA, IgG and IgM (both heavy and light chains; Jackson ImmunoResearch Laboratory, West Grove, PA) (5 μg/ml in PBS pH 7·4, 100 μl/well); (ii) goat anti-human IgG (Fc fragment-specific; Jackson ImmunoResearch Laboratory; 5 μg/ml in PBS, 100 μl/well); (iii) recombinantly synthesized human tropomyosin isoform 5 (hTM5; 5 μg/ml in carbonate buffer pH 9·6; 100 μl/well); or (iv) human albumin (5 μg/ml in carbonate buffer; 100 μl/well). The plate was left at 4°C overnight and the next day was washed with PBS five times, then blocked with 1% goat serum in HBSS (200 μl/well) at 37°C for 1 h.
After removing the blocking fluid, the LPMC were added in 100-μl aliquots (1000–100 000 cells/well), depending on the coating material and as determined from initial experiments in order to adjust the total number of spots (plaque-forming cells (PFC)) to be between 25 and 150 spots/well [15]. The plate was then incubated at 37°C for 14 h, washed five times with 0·05% Tween 20 in PBS, then once with PBS alone. The PFC were identified using alkaline phosphatase-conjugated goat anti-human immunoglobulin (IgA + IgG + IgM, both H + L chains; Jackson ImmunoResearch Laboratory) at a dilution of 1:5000 with 1% goat serum 100 μl/well, or goat anti-human IgG (FCγ fragment-specific; Jackson ImmunoResearch Laboratory; 1:5000 dilution, 100 μl/well). For the hTM5- and albumin-coated wells, alkaline phosphatase-labelled anti-human IgG (Fcγ fragment-specific as above) was used. For IgA-producing cells, alkaline phosphatase-labelled anti-human IgA (α-chain-specific; Jackson ImmunoResearch Laboratory) was used. IgM immunocytes were not estimated. The plates were incubated at 37°C for 2–3 h with the appropriate detecting antibody, washed with PBS three times followed by the addition of substrate 5-bromo-4 chloro-3 indolyl phosphate nitroblue tetrazolium (BCIP/NBT) substrate (Sigma Biochemical, St Louis, MO; 50 μl/well). The plate was kept in the dark for 2 h at room temperature for colour development, washed with PBS, and then viewed under a stereomicroscope to count the spots. The count for IgG or IgA is expressed as a percentage of the total number of immunoglobulin (IgA + IgG + IgM)-producing cells. The IgG-producing cells reactive against hTM5 were estimated after subtracting the human albumin control value and then expressed as a percentage of the total number of IgG immunocytes.
Statistical analysis
The results from both total IgG and hTM5-specific IgG-producing immunocytes were not normally distributed in all groups. Therefore, the non-parametrical Mann–Whitney U-test was used for statistical analysis of the data among groups, and results are expressed as means ± s.e.m. and median values in the text, table and figure.
Results
IgG-producing immunocytes
Table 1 shows the percentage of LPMC producing IgG in patients with UC, CC and non-IBD controls with statistical analysis. Seventy percent to 95% of the cells were IgA-producing cells, as would be expected. In the recto-sigmoid mucosa, the mean percentage ± s.e.m. of IgG-producing immunocytes in the LPMC from UC was 13·2 ± 1·4% (median 12%, range 6–32%); for CC 11·1 ± 1·3% (median 11%, range 6–16%), and for the non-IBD controls, the value was 6 ± 1% (median 6%, range 3–10%). The percentage of IgG-producing cells for both UC and CC was significantly higher compared with the non-IBD controls (P < 0·0002 and P < 0·008, respectively) (Table 1). However, the values for UC did not differ significantly from IgG-producing immunocytes found in CC.
Table 1.
Percentage of total IgG-producing immunocytes and anti-tropomyosin isoform 5 (hTM5) IgG-producing immunocytes quantified by ELISPOT assay in the colonic mucosal biopsy specimens in patients with ulcerative colitis (UC), Crohn's colitis (CC) and non-inflammatory bowel disease (non-IBD) controls
| UC (n = 23) | CC (n = 8*) | Non-IBD (n = 10) | ||
|---|---|---|---|---|
| Recto-sigmoid mucosa | ||||
| Percentage of total IgG | ||||
| B cells | Mean ± s.e.m. | 13·2 ± 1·4a | 11·1 ± 1·3b | 6 ± 1c |
| (median, range) | (12, 6–32) | (11, 8–16) | (6, 3–12) | |
| Percentage of anti-hTM5 | ||||
| IgG B cells | Mean ± s.e.m. | 40·1 ± 3·4d | 2·9 ± 1·2e | 4·1 ± 2·1f |
| (median, range) | (42, 8–75) | (2·5, 0–10) | (0, 0–18) | |
| Proximal colon mucosa | ||||
| Percentage of total IgG | ||||
| B cells | Mean ± s.e.m. | 12·6 ± 3·4g | – | – |
| (median, range) | (10, 3–27) | |||
| Percentage of anti-hTM5 | ||||
| IgG B cells | Mean ± s.e.m. | 20·7 ± 6·0h | – | – |
| (median, range) | (17, 0–50) |
Biopsy specimens from eight patients included grossly inflamed tissue, five from recto-sigmoid area and three from right colon.
aversusb, no significant difference,aversusc,– P < 0·0002
bversusc, P < 0·008,dversuse or f, P < 0·00001
gversusa, b or c, no significant difference,hversuse, P < 0·02, h versus f, P < 0·03.
dversus h, P < 0·05
IgG-producing immunocytes reactive against hTM5
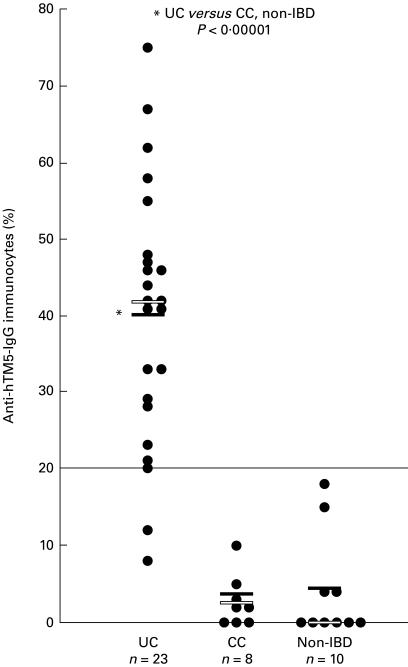
Of the IgG-producing B cells, the percentage of immunocytes committed against hTM5 isoform in the recto-sigmoid biopsy specimens from each of 41 subjects in the three groups of patients is shown in the scattergram(Fig 1). The mean percentage ± s.e.m. of anti-hTM5 IgG-producing immunocytes in UC was 40·1 ± 3·4% (median 42%, range 8–75%); in CC it was 2·9 ± 1·2% (median 2·5%, range 0–10%), and in non-IBD controls it was 4·1 ± 2·1% (median 0%, range 0–18%). Twenty-one of the 23 (91%) UC patients had anti-hTM5 IgG-producing immunocyte values at or above an arbitrary line of 20% (Fig. 1). This value of 20% is above the highest value seen in a single non-UC patient (18%, which was in a patient with ischaemic colitis) and is also 2 s.d. above the mean value of all of the non-UC patients. This difference in UC was statistically highly significant (P < 0·000 01) when compared with either CC or non-IBD groups (Table 1). Although the total number of IgG-producing immunocytes was similar in both UC and CC patients, anti-hTM5 IgG-producing immunocytes could not be detected at all in three of eight CC patients with active colitis and the values for the remaining five patients were < 10%. Similarly, in the non-IBD group, six of 10 did not react at all (Fig. 1). Indeed, both the patients with infectious colitis due to C. deficile and yersinia had zero anti-hTM5 IgG-producing immunocyte values. The values for the other four patients were 4%, 4%, 15% (a patient with irritable bowel syndrome) and 18% (the patient with ischaemic colitis).
Fig 1.
The percentage of B cells producing IgG against tropomyosin isoform 5 (hTM5) in the recto-sigmoid biopsy specimens from each of the 41 subjects including UC, Crohn's colitis (CC) and non-inflammatory bowel disease (non-IBD). The mean value for each group is shown by the solid horizontal bar and the median value by the open bar. The values for UC were significantly (P < 0·000 01) higher than both CC and non-IBD groups.
IgG-producing immunocytes in UC LPMC: relationship with histology
The recto-sigmoid biopsy specimens from the 23 UC patients showed histological grading of 0 in three patients, grade 1 in nine, grade II in nine, and only two were graded as III. For simplicity, these two patients with grade III are included with grade II for the statistical analysis. The mean percentage ± s.e.m. and median values of total IgG and anti-hTM5 IgG-producing immunocytes for grade 0 were 9 ± 1·6%, median 8% and 32 ± 10·5%, median 33%, respectively. The values (mean percentage ± s.e.m. and median percentage) for the specimens with grade I were 10·5 ± 1·3%, median 9% for total IgG-producing immunocytes and for percentage anti-hTM5 IgG immunocytes the values were 45·8 ± 4·2%, median 46·5%. For grade II (mean percentage ± s.e.m., median percentage) total IgG immunocytes were 15·3 ± 2·2% with median value 13·5%; and the percentages of anti-hTM5 IgG-producing immunocytes (mean ± s.e.m., median percentage) were 38·9 ± 5·4%, and median 37%. There were no significant differences in the total IgG-producing immunocytes and the IgG-producing cells committed to hTM5 in the three histological grades of UC. The age, sex, duration of illness and the treatment did not significantly influence the percentage of total IgG and anti-hTM5 IgG-producing immunocytes. The recto-sigmoid and right colon biopsy specimens from the eight patients with CC showed histological grading of 0 in one patient, grade 1 in one patient, grade II in four patients, and grade III in two patients. Two patients had granulomas. The percentage of IgG-producing cells ranged from 6% to 16% and the IgG-producing B cells committed against hTM5 isoform ranged from 0 to 10% and did not show any correlation with the histological gradings, nor with left side versus right side of the colon.
In seven UC patients, additional biopsy specimens (six to eight specimens) were taken from a single site proximal to the sigmoid colon. Three of these seven patients had proctitis with proximal biopsy specimens histologically graded as ‘0’, while the other four were histologically graded as 1. The recto-sigmoid biopsy specimens of the three proctitis patients were histologically graded as l. The mean percentages ± s.e.m. of total IgG-producing and anti-hTM5 IgG-producing immunocytes in these proximal samples with grade 0 histology were 5·7 ± 2·2% (median 4%), and 8 ± 4% (median 11%), respectively. The mean percentages ± s.e.m. of total IgG-producing immunocytes and anti-hTM5 IgG-producing immunocytes in the recto-sigmoid biopsy specimens of the same three patients were 10·7 ± 2% (median 11%) and 49·3 ± 13·5% (median 44%). Both of the values in the proximal colon were significantly (P < 0·05) lower than the recto-sigmoid area. The percentages (mean ± s.e.m.) of total IgG and anti-hTM5 IgG-producing immunocytes in the four proximal specimens with histological grade 1 were 17·7 ± 4·1% (median 18·5%) and 30·3 ± 7% (median 27%), respectively. The values from the recto-sigmoid area for the same four patients were 16·75 ± 4·4% and 26·3 ± 6·2%, respectively, and they were not significantly different from the values from the proximal colon. We also compared the percentage of IgG-producing immunocytes reactive against hTM5 in the seven proximal UC colon specimens having histological grade ‘0’ (n = 3) and grade 1 (n = 4) against the values of recto-sigmoid biopsy specimens from patients with CC and non-IBD controls. The percentages (mean ± s.e.m.) and median values of anti-hTM5 IgG-producing immunocytes for the seven proximal UC specimens were 20·7 ± 6% and 17%, respectively. These values were significantly higher than the values from recto-sigmoid or proximal colonic biopsy specimens of the eight CC patients (P < 0·02) as well as the 10 non-IBD subjects (P < 0·03) (Table 1).
Discussion
The results from this study show that colonoscopic biopsy specimens provide a sufficient amount of immunocytes to be used for ELISPOT assay to identify autoantigenic protein. IgG-producing cells in the colonic mucosa are significantly increased in both UC and CC compared with non-IBD controls, a finding that has been consistently reported by various investigators [2,3,5,16]. However, the antigenic epitopes recognized by these IgG immunocytes are unknown. Approximately 10–30% of the mucosal immunocytes in IBD belong to the IgG isotype and the remaining cells largely produce IgA (70–90%) [2,15,16]. Although the total number of IgG-producing cells was similar in both UC and CC, the mucosal immunocytes committed to produce antibody against hTM5 was clearly evident in patients with UC. Up to 75% of IgG-producing immunocytes (median value 42%) in UC were committed to the hTM5-related epitope. However, none of the eight patients with CC had an anti-hTM5 immunocyte count above 10%, whereas 22 of 23 patients with UC did. This was statistically highly significant with P < 0·000 01. Similarly, the number of anti-hTM5 IgG immunocytes was also significantly (P < 0·000 01) higher in UC compared with the 10 non-IBD controls. These data suggest that the increase in anti-hTM5 IgG-producing immunocytes is not secondary to inflammation. Twenty-one of 23 UC patients (91%), irrespective of their clinical and histological grades, had IgG-producing mucosal immunocytes in the recto-sigmoid colon biopsy specimens committed to hTM5-related epitope above the mean + 2 s.d. of all the 18 non-UC patients. These data suggest that ELISPOT assay to identify the percentage of anti-hTM5 IgG-producing immunocytes in the recto-sigmoid biopsy specimens may be clinically useful in the diagnosis of UC, particularly in the indeterminate patients with IBD.
In the experimental model of colitis in TCR−/− mice, a significantly higher anti-tropomyosin IgG immunocytes in the colonic mucosa was noted when compared with heterozygous mice without colitis [15]. Furthermore, the degree of anti-tropomyosin IgG-producing B cells correlated with the severity of colitis. In the UC patients, although there were relatively less IgG-producing immunocytes in the colon biopsy specimens with histological grade ‘0’ compared with grades 1 and 2, the proportion of anti-hTM5 IgG-producing immunocytes did not differ significantly. This was probably due to a limited number of grade ‘0’ patients and because of the fact that most of our patients had relatively mild disease and there was probably some overlap intrinsic to the histological grades. However, a heightened anti-hTM5 response was evident in all histological grades (grade 0–3).
TMs have been found to be a major allergen related to various seafood and house dust mite allergies [12,17]. In humans, four TM genes have been identified and various hTM isoforms are synthesized by an alternate RNA-splicing mechanism restricted to three exon regions [9,18–20]. TMs can induce significant autoimmune responses [13]. A biochemical analysis of 109 human autoantigens showed that sequences longer than 27 residues with coiled-coil α helices present in several TM peptides are the most potent autoantigens for various human autoimmune diseases. In the human colon epithelium, the major isoforms of hTM are hTM5 and hTM4 [11]. In this study we used hTM5, which is the most abundant hTM isoform in colon epithelial cells. Because of the limited number of LPMC isolated from the endoscopic biopsy specimens, the immune response against hTM isoforms other than hTM5 was not tested. Furthermore, we have recently demonstrated that hTM5, and not any other hTM isoform, is the isoform which is externalized in colonic epithelial cells [21]. Such an externalization of hTM5 seems to be organ-specific and was not seen in the small intestinal enterocytes, although small intestinal enterocytes contain intracellular hTM5 [11,21]. This is intriguing and may be relevant in UC both for effector immune stimulation as well as for the binding of the autoantibody to colonocytes. Such an organ-specific antigenic display may play an important role for a local trigger of immune response and perpetuation of the disease in the colon and not in the small intestine. The data from this study demonstrate that IgG-producing immunocytes committed to hTM5 are significantly higher in UC patients even in remission and with mild colitis. Such an immune response is lacking in the patients with CC and in non-IBD controls. A less intense anti-hTM5 IgG immunocyte response was noted in the proximal normal colon in patients with distal colitis. These results demonstrate a relationship between the disease process and the increased number of anti-hTM5 IgG-producing immunocytes in the inflamed area.
Using a large number of IBD patients and their first-degree blood relatives, Biancone et al. reported that about two-thirds of Italian patients with UC but not with CD contained circulating IgG autoantibody against hTM5 [22]. They further demonstrated that 21% of the sera from first-degree blood relatives of UC patients had anti-hTM1 antibody. Such a familial autoantibody response was lacking in CD [22]. An anti-TM circulating antibody response in UC, but not in CD, was also reported in Japanese patients [23]. Using the UC serum IgG, this group further identified an hTM-peptide that is involved in antibody-dependent cell-mediated cytotoxicity [23]. Anti-hTM antibodies were not demonstrated in a group of British patients with UC [24]. In this study, two hTM-related proteins from colonic mucosa of 37 kD and 39 kD, which comigrated in SDS–PAGE with porcine tropomyosin, were electroeluted and used in an ELISA as antigen. No reactivity was seen with any of the human sera from UC, CD and non-IBD controls [24]. This complete non-reactivity can be explained due to denaturation of protein(s) by SDS treatment, which has been described with autoantibodies [25]. The non-reactivity may also be due to hTM isoform differences.
A significant hTM5-related immune response may be pathogenetically important because of the surface expression of hTM5 isoform in colon epithelium [21]. Anti-hTM5 isoform autoantibody may bind with colonic epithelium causing activation of complement system. Indeed, in UC, anti-p40 autoantibody was found to be bound with colonic epithelium [7] along with activated complement system [26]. Such an autoantibody response and complement activation was not found in CD [27]. Autoantibodies to intracellular proteins are found in many autoimmune diseases, and, in most cases such autoantibodies are considered non-pathogenic unless the target autoantigen is accessible to the autoantibodies [28]. Several studies have demonstrated that autoantibodies can also be internalized inside the target cells and may cause cell damage [29]. In the experimental mouse model of autoimmune myocarditis, autoantibodies to myosin were found to cause tissue damage and inflammation, presumably by complement fixation [30]. The genetic background of mice was found to also be important in the externalization of myosin and the antibody-mediated myocarditis [30,31]. Molecular mimicry related to a specific peptide in streptococcal M proteins and tropomyosin has been found to be an important pathogenic factor in autoimmune myocarditis [32]. It is unknown if any of the microbial peptide(s) with cross-reactivity to hTM peptide can trigger and perpetuate the chronic inflammatory process seen in UC. While the circulating IgG autoantibody response against tropomyosin in UC has been shown by us [8] and two other independent groups of investigators [22,23], the T cell response to hTMs or hTM-related peptide in UC is unknown. However, TMs biochemically have been found to have the highest number of autoantigenic motifs [13]. Future studies using mucosal B and T cells from colonic biopsy specimens and their responses against hTM5-related peptide(s) may provide important information to identify the immunogenic epitope and to explain the autoimmune mechanisms in UC. Identification of the immunogenic peptide(s) may also have therapeutic applications.
Acknowledgments
This work is supported in part by a research grant, NIADDK, RO1 DK47673, from the National Institutes of Health, Bethesda, MD.
References
- 1.Brandtzaeg P. Autoimmunity and ulcerative colitis: can two enigmas make sense together? Gastroenterology. 1995;109:307–12. doi: 10.1016/0016-5085(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 2.Baklien K, Brandtzaeg P. Comparative mapping of the local distribution of immunoglobulin-forming cells in ulcerative colitis and Crohn's disease of the colon. Clin Exp Immunol. 1975;22:197–209. [PMC free article] [PubMed] [Google Scholar]
- 3.Hibi T, Ohara M, Toda K, et al. In vitro anticolon antibody production by mucosal or peripheral blood lymphocytes from patients with ulcerative colitis. Gut. 1990;31:1371–6. doi: 10.1136/gut.31.12.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targan SR, Landers CJ, Cobb L, et al. Perinuclear anti-neutrophil cytoplasmic antibodies are spontaneously produced by mucosal B cells in ulcerative colitis patients. J Immunol. 1995;155:3262–7. [PubMed] [Google Scholar]
- 5.Scott MG, Nahm MH, Macke K, et al. Spontaneous secretion of IgG subclasses by intestinal mononuclear cells: differences between ulcerative colitis, Crohn's disease and controls. Clin Exp Immunol. 1986;66:209–15. [PMC free article] [PubMed] [Google Scholar]
- 6.Das KM, Dubin R, Nagai T. Isolation and characterization of colonic tissue-bound antibodies from patients with idiopathic ulcerative colitis. Proc Natl Acad Sci USA. 1978;74:4528–32. doi: 10.1073/pnas.75.9.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi F, Das KM. Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound IgG from idiopathic ulcerative colitis. J Clin Invest. 1985;76:311–8. doi: 10.1172/JCI111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das KM, Dasgupta A, Mandal A, et al. Autoimmunity to cytoskeletal protein tropomyosin(s): a new clue to the pathogenetic mechanism for ulcerative colitis. J Immunol. 1993;150:2487–93. [PubMed] [Google Scholar]
- 9.Lin JJ-C, Warren KS, Wamboldt DD. Tropomyosin isoforms in nonmuscle cells. Int Rev Cytol. 1997;170:1–38. doi: 10.1016/s0074-7696(08)61619-8. [DOI] [PubMed] [Google Scholar]
- 10.Novy RE, Lin JL-C, Lin JJ-C. Human fibroblast tropomyosin isoforms: characterization of cDNA clones and analysis of tropomyosin isoform expression in human tissues and in normal and transformed cells. Cell Motil Cytoskeleton. 1993;25:267–81. doi: 10.1002/cm.970250307. [DOI] [PubMed] [Google Scholar]
- 11.Geng X, Biancone L, Dai HH, et al. Tropomyosin isoforms in intestinal mucosa: production of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology. 1998;114:912–22. doi: 10.1016/s0016-5085(98)70310-5. [DOI] [PubMed] [Google Scholar]
- 12.Daul CB, Slattery M, Reese G, et al. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allerg Immunol. 1994;105:49–55. doi: 10.1159/000236802. [DOI] [PubMed] [Google Scholar]
- 13.Dohlman JG, Lupas A, Carson M. Long charge-rich alpha-helices in systemic autoantigens. Biochem Biophys Res Commun. 1993;195:686–96. doi: 10.1006/bbrc.1993.2100. 10.1006/bbrc.1993.2100. [DOI] [PubMed] [Google Scholar]
- 14.Pallone F, Fais S, Squarcia O, et al. Activation of peripheral and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987;28:745–53. doi: 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizoguchi A, Mizoguchi E, Chiba C, et al. Cytokine imbalance and autoantibody production in T cell receptor-α mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–56. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstensen TS, Mollnes TE, Garred P, et al. Epithelial deposition of immunoglobulin G1 and activated complement (C3b and terminal complement complex) in ulcerative colitis. Gastroenterology. 1990;98:1264–71. doi: 10.1016/0016-5085(90)90343-y. [DOI] [PubMed] [Google Scholar]
- 17.Aki T, Kodama T, Fujikawa A, et al. Immunochemical characterization of recombinant and native tropomyosin as a new allergen from the house dust mite, Dermatophagoides farinae. J Allergy Clin Immunol. 1995;96:74–83. doi: 10.1016/s0091-6749(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin LO, Lees-Miller JP, Leonard MA, et al. Four fibroblast tropomyosin isoforms are expressed from the rat alpha-tropomyosin gene via alternative RNA splicing and the use of two promoters. J Biol Chem. 1991;266:8408–10. [PubMed] [Google Scholar]
- 19.Lees-Miller JP, Helfman DM. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991;13:429–37. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- 20.Burgess DR, Broschat KO, Hayden JM. Tropomyosin distinguishes between the two actin-binding sites of willin and affects actin-binding properties of other brush border proteins. J Cell Biol. 1987;104:29–31. doi: 10.1083/jcb.104.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesari KV, Yoshizaki N, Geng X, et al. Externalization of tropomyosin isoform 5 in colon epithelial cells. Clin Exp Immunol. 1999;118:219–27. doi: 10.1046/j.1365-2249.1999.01046.x. 10.1046/j.1365-2249.1999.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biancone L, Monteleone G, Marasco R, et al. Autoimmunity to tropomyosin isoforms in ulcerative colitis (UC) patients and unaffected relatives. Clin Exp Immunol. 1998;113:198–205. doi: 10.1046/j.1365-2249.1998.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamaki S, Takayanagi N, Yoshizaki N, et al. Autoantibodies against the specific epitope on human tropomyosin(s) detected by a peptide-based enzyme immunoassay in sera of patients with ulcerative colitis show antibody dependent cell-mediated cytotoxicity against HLA-DPw9 transfected l-cells. Gut, in press. [DOI] [PMC free article] [PubMed]
- 24.Khoo UY, Bjarnason I, Donaghy A, et al. Antibodies to colon epithelial cells from the serum and colonic mucosal washings in ulcerative colitis. Gut. 1995;37:63–70. doi: 10.1136/gut.37.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bini P, Gabay JE, Teitel A, et al. Antineutrophil cytoplasmic autoantibodies in Wegener's granulomatosis recognize conformational epitope(s) on proteinase 3′. J Immunol. 1992;149:1409–15. [PubMed] [Google Scholar]
- 26.Halstensen TS, Das KM, Brandtzaeg P. Epithelial deposits of immunoglobulin G1 and activated complement co-localize with the 40 kDa colonic autoantigen in ulcerative colitis. Gut. 1993;34:650–7. doi: 10.1136/gut.34.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halstensen TS, Mollnes TE, Garred P, et al. Surface epithelium-related activation of complement differs in Crohn's disease and ulcerative colitis. Gut. 1992;33:902–8. doi: 10.1136/gut.33.7.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naparstek Y. The role of autoantibodies in autoimmune disease. Ann Rev Immunol. 1993;11:79–84. doi: 10.1146/annurev.iy.11.040193.000455. [DOI] [PubMed] [Google Scholar]
- 29.Alarcon-Segovia D, Ruiz-Arguelles A, Liorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996;17:163–4. doi: 10.1016/s0167-5699(96)90258-3. 10.1016/0167-5699(96)80613-x. [DOI] [PubMed] [Google Scholar]
- 30.Lio L, Sindhawani R, Rojkind M, et al. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ specificity. J Exp Med. 1995;181:1123–31. doi: 10.1084/jem.181.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neu N, Rose NR, Beisel KW, et al. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–6. [PubMed] [Google Scholar]
- 32.Fenderson PG, Fischetti VA, Cunningham MW. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989;142:2475–81. [PubMed] [Google Scholar]