Abstract
Early immunological activation involves an initial phase of cytokine activity and involvement of cell types such as NK cells. Such early immune responses are often decisive in resolution of microbial infection. NK cells reduce parasitaemia and enhance survival in experimental Trypanosoma cruzi infection, although the nature of these protective effects is not well understood. In this study, a detailed analysis of innate cytokine induction in the absence and presence of NK cells during the first 8 days of infection was performed. Following intraperitoneal infection with a high dose of parasites, reverse transcriptase-polymerase chain reaction showed that splenic mRNA for IFN-γ appeared as a peak 24 h after infection and then reappeared 2–3 days later. In NK-depleted animals the first peak of IFN-γ was absent and the second wave was slightly delayed. mRNA for IL-12 and tumour necrosis factor-alpha (TNF-α) as well as IFN-α protein in serum was only recorded 24 h after infection, at the same time as the IFN-γ peak. NK depletion resulted in a small decrease of IL-12 mRNA levels, whereas TNF-α and IFN-α were not affected. NK cytotoxicity remained elevated throughout the 8 days and thus did not parallel the expression of IFN-γ production by NK cells. We conclude that NK cell cytokine production and cytolytic activity play different roles in response to challenge with T. cruzi.
Keywords: NK cell, activation, Trypanosoma cruzi, IFN-γ, IL-12
INTRODUCTION
NK cells enhance host survival in many intracellular infections and NK depletion is known to increase susceptibility to several experimental infections [1–3]. The mechanisms underlying the protective effects are not clear. Activation of NK cells in response to certain pathogens involves increased cytolytic capacity as well as induction of cytokine production of primarily IFN-γ and tumour necrosis factor-alpha (TNF-α). In some cases, direct killing of pathogens or infected cells has been demonstrated in vitro [4–6]. Lately however, more attention has been given to the cytokines, mainly IFN-γ, that NK cells can produce before any T cell-derived cytokines are available.
Murine infection with the intracellular protozoan parasite Trypanosoma cruzi is a suitable model for study of the mechanisms for activation, as well as protective properties of NK cells because the parasite activates both cytotoxicity and IFN-γ production by NK cells. NK cells are also involved in protection against T. cruzi infection [2,7].
Trypanosoma cruzi is the aetiologic agent of Chagas' disease. Upon inoculation into a mammalian host, metacyclic trypomastigotes invade various cell types where they transform into amastigotes and multiply. Within a week, parasites rupture the cell and spread further in the host as bloodstream trypomastigotes. Resolution of the infection is critically dependent on production of IFN-γ which activates microbicidal mechanisms of macrophages (e.g. nitric oxide (NO) from inducible nitric oxide synthase (iNOS)) [7–9]. Ultimately, T cells are required for cure of the disease [10], but the innate immune response plays a crucial role in the very early phase of infection. Thus depletion of NK cells in mice prior to infection results in increased parasitaemia and mortality [2]. The cytotoxic activity of NK cells increases some days after infection [11]. IFN-γ is produced soon after infection with T. cruzi [12] and NK-depleted mice produce reduced amounts of the cytokine at this time [7,13].
The initiation of the immune response to T. cruzi and the way NK cells are activated to produce IFN-γ in vivo are not well understood. Several factors have been described that activate cytotoxicity and/or cytokine production by NK cells. IL-12 and IL-18 are efficient inducers of IFN-γ production by both NK and T cells, and have been demonstrated in vivo during T. cruzi infection [14]. Exogenous IL-12 is protective in an IFN-γ- and TNF-α-dependent manner [15]. IL-12 is produced upon exposure of splenic macrophages to T. cruzi in vitro [16] and anti-IL-12 antibodies mediate susceptibility in vivo [17].
IFN-α/β (type I interferons) are produced 24–48 h after T. cruzi infection [18]. Administration of IFN-α/β or interferon inducers results in reduced parasitaemia and decreased mortality [2,19]. However, type I interferons have many immunoregulatory functions and their exact role in protection is poorly understood.
In the study described here, a kinetic analysis of innate cytokine expression in C57Bl/6 mice during the first 8 days after infection with different doses of T. cruzi was performed. The time period was chosen to extend long enough for adaptive immune mechanisms to become initiated. In order to study the role of NK cells during early infection, both NK-depleted and control mice were infected. The kinetics of cytokine expression was also related to the activation of the cytotoxic activity of NK cells.
MATERIALS AND METHODS
Mice and parasites
C57Bl/6 mice were bred at the animal facilities at the Microbiology and Tumour Biology Centre (MTC). All mice were 4–8 weeks old when inoculated intraperitoneally with trypomastigotes of the Tulahuén strain. Trypanosoma cruzi parasites were obtained from peripheral blood of infected C57Bl/6 mice. Control mice were injected with PBS.
Depletion of NK cells in vivo
Mice were depleted of NK cells by intravenous administration of 25 μl anti-asialo GM1 rabbit antiserum (Wako Pure Chemical Industries, Osaka, Japan) diluted in 200 μl PBS. Control mice received 25 μl of normal rabbit serum (NRS). The antisera were given 24 h prior to infection unless otherwise indicated. The efficiency of the depletion was evaluated in 51Cr-release assays.
Cytotoxicity assay
51Cr-labelled target cells (5 × 103) were added to 96-well V-bottomed microtitre plates containing appropriate numbers of effector cells. The plates were incubated for 4 h at 37°C. After incubation, plates were centrifuged and 100μl of the supernatant were harvested and transferred to precipitin tubes. The amount of released 51Cr was measured and the percentage of specific lysis was calculated from the standard formula [20].
Competitive polymerase chain reaction
Spleen cells [107] were dissolved in 1 ml Ultraspec RNA solution (Biotecx Laboratories, Houston, TX) and total RNA was purified according to the manufacturer's instructions. RNA (2 μg) was denatured at 94°C for 5 min, reverse transcribed at 40°C for 45 min and treated at 94°C for 5 min. The reverse transcription was carried out in a total volume of 40 μl with 7·5 mm DTT (Gibco BRL, Paisley, UK), 0·5 mm nucleotides (dNTP; Pharmacia, Uppsala, Sweden), 1 U/μl RNAsin (Promega, Madison, WI), 5μm random hexanucleotides (pd(N)6; Pharmacia) and 10 U/μl M-MLV reverse transcriptase (Gibco BRL).
Each cDNA sample was amplified in a competitive polymerase chain reaction (PCR) assay [21]. The PCR reaction was performed in 20 μl containing 0·2 mm dNTP, 25 mU/ml taq polymerase (Roche Molecular Systems Inc., Branchburg, NJ), 0·5 μm sense and antisense primers, cDNA and 2 μl of competitor fragments of different lengths, but with the same primer binding sequences as the target DNA. The competitors for each cytokine were diluted in a series of at least five three-fold dilutions for every sample. A negative control containing no template, a competitor control and cDNA controls were included in every assay. Primer sequences, annealing temperatures and competitors for β-actin, IFN-γ, and IL-12 (p40) were the same as previously reported [22,23]. The primer sequences for TNF-α were: sense 5′ TGT CTC AGC CTC TTC TCA TTC 3′, antisense 5′ GAG AAC CTG GGA GTA GAC AAG 3′, annealing temperature 62°C, and for IL-18, sense 5′ CAG TGA ACC CCA GAC CAG AC 3′, antisense 5′ CAA ACC CTC CCC ACC TAA CT 3′, annealing temperature 65°C. PCR products were loaded on a 2% agarose gel, electrophoresed with ethidium bromide and photographed. The original concentration of the competitor was known in all cases. The concentrations of cDNA were calculated from cDNA and competitor bands with equal intensity. The cytokine mRNA concentrations were expressed as percentage of the β-actin mRNA concentration for each sample.
Immunoassays for cytokines in serum
IFN-α and -β in serum were measured separately in dissociation-enhanced lanthanide fluoroimmunoassays (DELFIA) as described [24]. In brief, microtitre plates were coated with sheep anti-IFN-α/β (a gift from Dr Michael Tovey, CNRS, Paris, France), incubated with samples or standards and then with MoAbs to IFN-α or -β labelled with Europium lanthanide chelate. An enhancement solution was added and fluorescence measured in a DELFIA fluorometer.
IFN-γ was measured by a capture ELISA. Rat anti-mouse IFN-γ MoAb R4-GA2 (Immunokontact, Abingdon, UK) was used as the capture antibody (5 μg/ml), and biotinylated anti-mouse IFN-γ MoAb XMG1.2 (4 μg/ml) (Immunokontact) was used for detection. Quantification of IFN-γ in serum was calculated by comparison with a reference linear regression with a known concentration of recombinant IFN-γ (PharMingen, San Diego, CA).
Separation of T cell subsets
T cells positive for CD4 and CD8 were enriched using Dynabeads (Dynal AS, Oslo, Norway) according to the manufacturer's instructions. Briefly, 2·5 × 107 beads were incubated with 108 spleen cells for 30 min at 4°C. The cells were suspended and magnetically separated four times, after which they were dissolved in 1 ml Ultraspec RNA solution.
Cell staining and flow cytometry analysis
For FACS staining, positive cell populations were incubated at 37°C overnight so that the Dynabeads would detach and free beads were magnetically removed from the cell suspensions. Cells (5 × 105) in 50 μl PBS containing 5% fetal calf serum (FCS) were labelled with 4 μg/ml PE-conjugated MoAbs to CD4 (RM4-5; PharMingen) or CD8 (53-6.7; PharMingen). The labelling was performed for 1 h, after which the cells were washed once and fixed for 1–3 days in PBS containing 0·5% bovine serum albumin (BSA) and 1% formaldehyde. After an additional wash, the cells were analysed in a FACScan (Becton Dickinson, Mountain View, CA).
RESULTS
Early cytokine response in C57Bl/6 mice infected with T. cruzi
Innate cytokine responses to T. cruzi were assessed by measuring the amount of cytokine mRNA in the spleen at various time points during the first 8 days of infection. Initially, the mice were inoculated either with a high dose (105 or 5 × 105) or a low dose (50) of trypomastigotes. The high dose is lethal, causing death within 2 weeks. The low dose gives a peak of parasitaemia 3–4 weeks after infection, a time at which a proportion (usually 10–50%) of the animals will die. The surviving mice will be free of detectable blood-form trypomastigotes after about 2 months [25]. In this study, injection of 5 × 105 parasites resulted in low numbers of parasites in the blood at the latest time point, 8 days after infection (data not included). At the other time points and in mice infected with the low dose, no blood trypomastigotes could be detected.
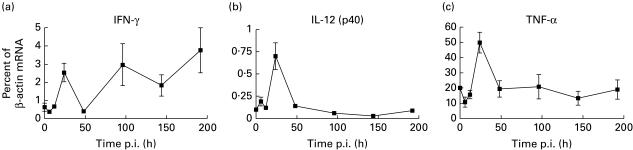
Competitive RT-PCR revealed a substantial increase of IFN-γ mRNA 24 h after infection with the high dose of T. cruzi (Fig. 1a). IFN-γ was also present in serum at this time according to ELISA (data not included). The IFN-γ transcripts had almost completely disappeared 24 h later, but 4 days after infection, a considerable accumulation of IFN-γ mRNA was again observed. The same mice showed a peak of IL-12 (p40) mRNA after 24 h, simultaneous with the first IFN-γ peak (Fig. 1b). IL-12 transcripts never reappeared during the first 8 days, however. Induction of TNF-α mRNA was also apparent after 24 h but not at later time points (Fig. 1c). The levels of splenic mRNA for IL-18 were not above background at any time point (data not included). Contrary to the high dose of parasites, the lower dose did not induce any of the cytokines as measured by PCR in the spleen (Table 1).
Fig. 1.
Accumulation of cytokine mRNA in spleens of C57Bl/6 mice after infection with a high dose of Trypanosoma cruzi trypomastigotes. Results are expressed as moles of cytokine mRNA per mole of β-actin mRNA in percent ± s.e.m. Each point represents the mean of four or five mice and experiments at each time point were repeated at least once with similar results.
Table 1.
Cytokine mRNA levels in spleens 24 h after infection with different doses of Trypanosoma cruzi
| mRNA as percentage of β-actin mRNA* | |||
|---|---|---|---|
| No. of parasites | IFN-γ | IL-12 (p40) | TNF-α |
| 0 | 0·63 ± 0·22 | 0·10 ± 0·02 | 20·0 ± 0·30 |
| 50 | 0·53 ± 0·08 | 0·04 ± 0·02 | 4·73 ± 1·14 |
| 104 | 1·80 ± 0·81 | 0·59 ± 0·01 | 102·7 ± 44·9 |
| 5 × 105 | 2·54 ± 0·51 | 0·70 ± 0·15 | 49·8 ± 6·8 |
Data are expressed as percentage of β-actin mRNA.
The mean and s.e.m. are shown; n = 3–5.
Since the parasites used for inoculation are obtained from peripheral blood of infected mice, it is possible that activated leucocytes in the inoculum migrate to the spleen and contribute to the cytokine production. To test this, mice were infected with an intermediate dose of 104 parasites, thus containing only 2% of the number of leucocytes contained in the highest dose inoculum, and accumulation of cytokine mRNA was assessed after 24 h. These mice exhibited induction of IFN-γ, IL-12 and TNF-α to a similar magnitude as that of mice infected with 5 × 105 parasites (Table 1), demonstrating that the cytokine response observed was indeed an effect of the parasite.
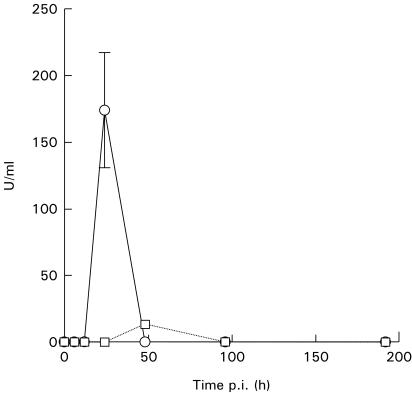
IFN-α and -β were measured in serum by immunoassay. Mice infected with the high dose of parasites demonstrated appreciable concentrations of IFN-α in serum 24 h after infection (Fig. 2). At all other time points IFN-α was undetectable. Mice receiving 50 parasites had low but significant levels after 48 h but were otherwise negative for IFN-α. No IFN-β could be detected in serum from any mouse (data not included).
Fig. 2.
IFN-α in serum of C57Bl/6 mice infected with different doses of Trypanosoma cruz i expressed as U/ml ± s.e.m. The detection limit is 6 U/ml. Each point represents the mean of three mice. □, 50 parasites; ○, 5 × 105 parasites.
Activation of NK cells in response to T. cruzi
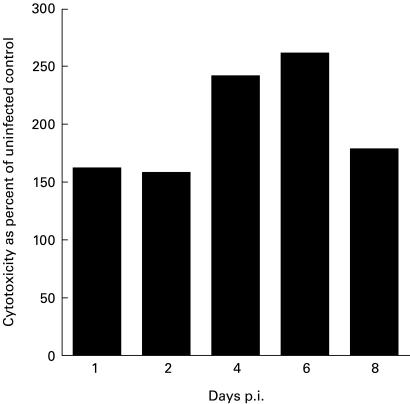
The activation of NK cells in T. cruzi infection was examined both with regard to cytolytic function and IFN-γ production. NK cytotoxicity as measured by lysis of the target cell line Yac-1 was elevated 24 h after infection in accordance with previous reports [11] and remained high throughout the 8-day period (Fig. 3). Infection with 50 parasites did not cause any change in NK cytotoxicity 24 h later (data not included).
Fig. 3.
Lysis of Yac-1 by spleen cells of C57Bl/6 mice at various times after infection with 105 trypomastigotes. 100% = lysis by uninfected control animals at each time point. The E:T ratio is 200:1. The absolute values of cytotoxicity varied between 5% and 20% for uninfected mice and between 20% and 50% for infected mice.
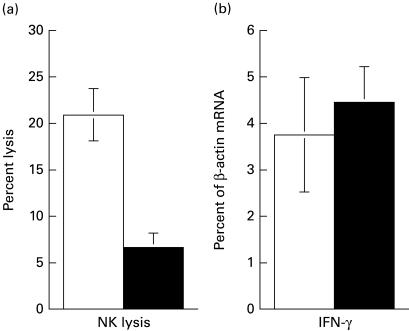
In order to examine in more detail the activities of NK cells during infection with T. cruzi, mice were depleted of NK cells before or during infection. Since only minor alterations in NK activity and cytokine induction were observed after infection with 50 parasites, a high dose (105 parasites) was used in these NK depletion studies. When mice were depleted of NK cells with anti-asialo GM1 antiserum 24 h prior to infection, NK cytolytic activity was almost completely eliminated after 24 h and gradually reappeared during the 8-day test period (Table 2). The IFN-γ mRNA observed in non-depleted control mice 24 h after infection was virtually absent in NK-depleted mice (Table 2), suggesting that NK cells are the dominating source of IFN-γ at this early time point. Six days after infection IFN-γ mRNA levels were detectable at control levels. To investigate if newly recruited NK cells accounted for this IFN-γ production, infected mice were treated with anti-asialo GM1 7 days after infection and IFN-γ transcripts were quantified 24 h later. In spite of a strong reduction in NK cytotoxicity (Fig. 4a), the level of mRNA for IFN-γ was unaffected (Fig. 4b), strongly suggesting that the IFN-γ produced at this time primarily emanates from non-NK cells.
Table 2.
Relative NK cytolytic activity and cytokine mRNA expression in spleen at various times after depletion of NK cells
| Relative cytokine mRNA expression†‡ | ||||
|---|---|---|---|---|
| Time post-infection (h)* | Relative NK cytotoxicity§ | IFN-γ | IL-12 (p40) | TNF-α |
| 24 | 3 ± 3 | 15 ± 1 | 53 ± 15 | 104 ± 27 |
| 96 | 20 ± 4 | 33 ± 13 | B | B |
| 144 | 48 ± 7 | 89 ± 32 | B | B |
| 192 | 78 ± 17 | 125 ± 29 | B | B |
Mice were injected with anti-asialo GM1 intravenously 24 h before infection with 105 trypomastigotes. At the indicated time points postinfection, spleen cells were isolated and tested for cytolytic activity against Yac-1 as well as accumulation of mRNA for the different cytokines.
Only time points at which cytokine expression exceeded the background levels of uninfected ontrol mice are depicted.
Data are expressed as mRNA accumulation in NK-depleted mice/mRNA accumulation in control mice × 100.
Data are expressed as the percentage lysis of NK-depleted mice/%lysis in control mice × 100 at an E:T ratio of 200:1.
B, Background levels.
The mean and s.e.m. are shown. n = 4–5. Experiments at 24 h and 192 h were repeated twice with similar results.
Fig. 4.
NK cytotoxicity (a) and IFN-γ mRNA accumulation (b) in spleens of C57Bl/6 mice 8 days after infection with 105 trypomastigotes. NK depletion was accomplished by i.v. treatment with anti-asialo GM1 antiserum 7 days after infection. Control mice received normal rabbit serum. The E:T ratio is 200:1. Results are averages of four mice per group. Error bars represent s.e.m. □, Control; ▪, NK-depleted.
The levels of IL-12 mRNA 24 h after infection were reduced but not eliminated in NK-depleted mice (Table 2), suggesting that induction of IL-12 may be partly but not completely dependent on NK cells and IFN-γ in this system. In contrast, the number of transcripts for TNF-α was unaffected by NK depletion, indicating that TNF-α is neither mainly produced by, nor dependent on NK cells.
NK depletion did not cause any change in the amount of IFN-α in serum (data not included).
Origin of late IFN-γ
CD4+ and CD8+ T cells were isolated from spleens with magnetic beads 8 days after infection. The procedure resulted in an enrichment of both T cell populations by about six times compared with unseparated spleen cells (Table 3). In the population enriched for CD4+ cells the IFN-γ mRNA levels were about five times higher compared with unseparated spleen cells (Table 3). The IFN-γ mRNA levels in CD8+ enriched cells were the same as in unseparated cells.
Table 3.
Expression of mRNA for IFN-γ in CD4 and CD8 cells enriched by Dynabeads 8 days after infection with Trypanosoma cruzi
| Percent positive cells* | |||
|---|---|---|---|
| Cell population | CD4 | CD8 | IFN-γ/β-actin (%)† |
| Total spleen | 8·5 | 6·7 | 16 ± 2 |
| CD4+ | 51·2 | NT | 78 ± 22 |
| CD8+ | NT | 37·6 | 12 ± 2 |
One spleen was analysed by FACS.
The mean and s.e.m. are shown; n = 6.
NT, Not tested.
The same experiment was performed 12 days after infection, with similar results.
DISCUSSION
We demonstrate in this study that an early peak of IFN-γ transcripts appears in splenocytes between 12 h and 24 h after infection with a high dose of T. cruzi. IFN-γ can also be detected in serum after 24 h. The expression subsided to background levels at 48 h and thereafter increased again. The high dose of parasites was chosen to make cytokine transcription as well as cytotoxicity readily detectable. A dose of 50 parasites, which would more closely mimic natural infection, did not result in any detectable transcription of IFN-γ or IL-12. This dose is infective however, and we believe that innate cytokine production proceeds in a similar fashion, although at a lower rate and possibly with different kinetics. Experimental infection also differs from natural infection in other respects, for example by route of infection and synchronicity of the parasites.
Depletion of NK cells almost completely abrogated IFN-γ transcription 24 h after infection, indicating that NK cells, at least in the spleen, are the dominating source of early IFN-γ. The NK-depleted animals gradually regained full cytolytic activity during the subsequent 8 days, and this was accompanied by increasing numbers of IFN-γ transcripts. Four days after infection NK-depleted mice still had reduced IFN-γ mRNA expression compared with infected control mice. Considering that almost no IFN-γ transcription took place 2 days after infection, this implies a double peak of IFN-γ production by NK cells. A possible explanation for this could be that inoculated blood trypomastigotes cause induction of the first peak and that the second is triggered by release of intracellular parasites 2–3 days later. An alternative explanation is that the IFN-γ mRNA observed at day 4 does not derive from NK cells, but that NK cells trigger IFN-γ production from other cell types and that lack of NK cells delays a second wave of IFN-γ production. From 6 days post-infection NK-depleted and control mice had similar levels of IFN-γ transcripts. Treatment with anti-asialo GM1 at this time did not cause any decrease in the amount of IFN-γ mRNA, suggesting that NK cells are not major IFN-γ producers after the initial induction. Instead, activated T helper 1 cells are the main source. This is in agreement with data from Cardillo et al., who reported that splenocytes from NK-depleted infected mice, upon restimulation in vitro with live trypomastigotes, produced reduced amounts of IFN-γ compared with controls until day 10, after which no difference was observed [7].
NK cell-derived early IFN-γ could exert protective effects in several ways. It could activate macrophage microbicidal mechanisms which could lead to containment of the parasite, as has been suggested in Leishmania major infection [26]. A strongly reduced IFN-γ response during the first 4–6 days, as observed in our study, would certainly give the parasite an advantage. Other studies of L. major infection imply that NK cells, through production of IFN-γ, can direct subsequent T cell development into a Th1 response [27]. However, treatment with exogenous IFN-γ alone has little effect on protection [28], and other cytokines, e.g. IL-12, seem to be necessary for the ultimate formation of a Th1 response. We demonstrate here that the peak of IL-12 mRNA 24 h post-infection is somewhat reduced in mice depleted of NK cells, implying that NK cells could contribute to, but are not necessary for induction of IL-12 in this system. Furthermore, IFN-γ mRNA levels in NK-depleted and control mice are already similar after 6 days, indicating no severe impairment in Th1 development. It thus seems that factors other than NK activation are decisive in finally establishing a Th1 environment during T. cruzi infection.
TNF-α has been implied in defence against T. cruzi [29]. However, in our study the magnitude and kinetics of TNF-α transcription was not affected by depletion of NK cells. TNF-α is therefore not likely to be involved to any significant degree in the protective effects exerted by NK cells.
A distinct peak of IFN-α in serum appeared 24 h after infection and had vanished completely at the later time points. The transient nature of expression is characteristic of type 1 IFN expression in response to many stimuli [30]. It is clear that IFN-α activates the cytolytic function of NK cells [31]. Its role in regulation of cytokine expression is controversial, however. Some authors have reported that IFN-α can stimulate IFN-γ production by NK cells [32]. Others claim that IFN-α suppresses IL-12 activity and thereby IFN-γ production early after viral infection [33]. IFN-α has also been implicated in suppression of IL-4 [34] and induction of iNOS [35].
NK cytolytic activity remained elevated from 24 h until 8 days, although transcription of IFN-γ mRNA in NK cells declined during the period. It thus seems as if the cytotoxic function of NK cells is more persistent than IFN-γ production in response to T. cruzi. The persistence of cytotoxicity appears to be dependent on the dose of parasites, because Hatcher et al. demonstrated that at lower doses the cytotoxicity reverts to control levels after less than a week [11]. The maintenance of elevated cytotoxicity could be explained by more stable cellular alterations associated with cytotoxic function, continuous stimulation of cytotoxicity or down-regulation of IFN-γ production. It should be noted that IFN-α/β or IL-12 are absent 8 days after infection and thus unable to stimulate cytotoxicity directly. Appearance of other cytokines, such as IL-2, may explain the phenomenon. Additionally, mRNA for IL-18 may not be detectable by PCR. Gene expression of IL-18 detectable by in situ hybridization but not PCR has been reported, primarily however at time points later than 8 days post-infection. In the same study IL-12 was detected 3–4 weeks after infection, but not during the first week. Time points before 3 days post-infection were not addressed [14]. Furthermore, it can not be excluded that the early IL-12 has later effects on cytotoxicity. In contrast, IFN-α/β are believed to have only short-term effects on NK cytolytic activity [31]. The discrepancy between NK cytotoxicity and IFN-γ production confirms what has been reported in some other studies. Beige mice lack NK lytic function but possess NK-like cells that produce IFN-γ when stimulated [36], and during cytomegalovirus (CMV) infection IFN-α promotes cytolytic activity while IL-12 induces IFN-γ production by NK cells [37].
In conclusion, we have demonstrated that exposure to T. cruzi in vivo is followed by more or less simultaneous peaks of transcription of IFN-γ, IL-12 and TNF-α in the spleen as well as IFN-α in serum 24 h after infection. IFN-γ at this time is almost completely dependent on the presence of NK cells. The three cytokines are transcribed only transiently, but 3 days later transcription of IFN-γ is resumed, now by CD4+ T cells. We also report that the cytolytic function, and IFN-γ transcription by NK cells, follow different kinetics and are thus probably regulated in different ways.
Acknowledgments
We thank Margareta Hagelin, Maj-Lis Solberg and Mabbe Alter for excellent technical assistance. This study was supported by Sida/SAREC and the Swedish Medical Research Council (MFR).
REFERENCES
- 1.Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52:119–28. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rottenberg M, Cardoni R, Andersson R, et al. Role of T helper/inducer cells as well as natural killer cells in the resistance to Trypanosoma cruzi infection. Scand J Immunol. 1988;28:573. doi: 10.1111/j.1365-3083.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 3.Laskay T, Röllinghoff M, Solbach W. Natural killer cells participate in the early defense against Leishmania major infection in mice. Eur J Immunol. 1993;23:2237–41. doi: 10.1002/eji.1830230928. [DOI] [PubMed] [Google Scholar]
- 4.Santoli D, Trinchieri G, Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. H. Interferon induction and activation of natural killer cells. J Immunol. 1978;121:532–8. [PubMed] [Google Scholar]
- 5.Resnick M, Roguel N, Bercovier H, et al. Lysis of murine macrophages infected with intracellular pathogens by interleukin 2-activated killer (LAK) cells in vitro. Cell Immunol. 1988;113:214–9. doi: 10.1016/0008-8749(88)90019-6. [DOI] [PubMed] [Google Scholar]
- 6.Levitz SM, Dupont MP, Smail EH. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect Immun. 1994;62:194–202. doi: 10.1128/iai.62.1.194-202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardillo F, Voltarelli J, Reed S, et al. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin-10: role of NK cells. Infect Immun. 1996;64:128–34. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed SG. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988;140:4342–7. [PubMed] [Google Scholar]
- 9.Munoz-Fernandez MA, Fernandez MA, Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301–7. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 10.Reed SG. Immunology of Trypanosoma cruzi infections. Chem Immunol. 1998;70:124–43. [PubMed] [Google Scholar]
- 11.Hatcher F, Kuhn R, Cerrone M, et al. Increased natural killer cell activity in experimental American trypanosomiasis. J Immunol. 1981;127:1126–30. [PubMed] [Google Scholar]
- 12.McCabe RE, Meagher SG, Mullins BT. Endogenous interferon-gamma, macrophage activation, and murine host defense against acute infection with Trypanosoma cruzi. J Infect Dis. 1991;163:912–5. doi: 10.1093/infdis/163.4.912. [DOI] [PubMed] [Google Scholar]
- 13.Reed S, Brownell C, Russo D, et al. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J Immunol. 1994;153:3135–40. [PubMed] [Google Scholar]
- 14.Meyer Zum Buschenfelde C, Cramer S, Trumpfheller C, et al. Trypanosoma cruzi induces strong IL-12 and IL-18 gene expression in vivo: correlation with interferon-gamma (IFN-gamma) production. Clin Exp Immunol. 1997;110:378–85. doi: 10.1046/j.1365-2249.1997.4471463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter C, Slifer T, Araujo F. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect Immun. 1996;64:2381–6. doi: 10.1128/iai.64.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frosch S, Kraus S, Fleischer B. Trypanosoma cruzi is a potent inducer of interleukin-12 production in macrophages. Med Microbiol Immunol (Berl) 1996;185:189–93. doi: 10.1007/s004300050030. [DOI] [PubMed] [Google Scholar]
- 17.Albierti J, Cardoso M, Martins G, et al. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–7. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnenfeld G, Kierszenbaum F. Increased serum levels of an interferon-like activity during the acute period of experimental infection with different strains of Trypanosoma cruzi. Am J Trop Med Hyg. 1981;30:1189–91. doi: 10.4269/ajtmh.1981.30.1189. [DOI] [PubMed] [Google Scholar]
- 19.James S, Kipnis T, Sher A, et al. Enhanced resistance to acute infection with Trypanosoma cruzi in mice treated with an interferon inducer. Infect Immun. 1982;35:588–93. doi: 10.1128/iai.35.2.588-593.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullberg M, Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981;153:615–28. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilliland G, Perrin S, Blanchard K, et al. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci USA. 1990;87:2725–6. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laucella S, Salcedo R, Castaños-Velez E, et al. Increased expression and secretion of ICAM-1 during experimental infection with Trypanosoma cruzi. Parasite Immunol. 1996;18:227–39. doi: 10.1046/j.1365-3024.1996.d01-95.x. [DOI] [PubMed] [Google Scholar]
- 23.Guevara-Mendoza O, Une C, Franceschi Carreira P, et al. Experimental infection of Balb/c rnice with Leishmania panamensis and Leishmania mexicana: induction of early IFN-γ but not IL-4 is associated with the development of cutaneous lesions. Scand J Immunol. 1997;46:35–40. doi: 10.1046/j.1365-3083.1997.d01-96.x. [DOI] [PubMed] [Google Scholar]
- 24.Eloranta M-L, Sandberg K, Alm G. The interferon α/β responses of mice to Herpes simplex virus studied at the blood and tissue level in vitro and in vivo. Scand J Immunol. 1996;43:355–60. doi: 10.1046/j.1365-3083.1996.d01-62.x. [DOI] [PubMed] [Google Scholar]
- 25.Rottenberg M, Bakhiet M, Olsson T, et al. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect Immun. 1993;61:5129–33. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laskay T, Diefenbach A, Röllinghoff M, et al. Early parasite containment is decisive for resistance to Leishmania major infection. Eur J Immunol. 1995;25:2220–7. doi: 10.1002/eji.1830250816. [DOI] [PubMed] [Google Scholar]
- 27.Scharton T, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–77. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149. [PubMed] [Google Scholar]
- 29.Castaños-Velez E, Maerlan S, Osorio LM, et al. Trypanosoma cruzi infection in tumor necrosis factor receptor p55-deficient mice. Infect Immun. 1998;66:2960–8. doi: 10.1128/iai.66.6.2960-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Maeyer E, De Maeyer-Guignard J. Interferons and other regulatory cytokines. New York: John Wiley & Sons; 1988. Induction of IFN-α and IFN-β. [Google Scholar]
- 31.Gidlund M, Örn A, Wigzell H, et al. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759–61. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- 32.Hunter C, Gabriel K, Radzanowski T, et al. Type 1 interferons enhance production of IFN-γ by NK cells. Immunol Letters. 1997;59:1–5. doi: 10.1016/s0165-2478(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 33.Cousens L, Orange J, Su H, et al. Interferon-α/β inhibition of interleukin 12 and interferon-γ production in vitro and endogenously during viral infection. PNAS. 1997;94:634–9. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wenner C, Güler M, Macatonia S, et al. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol. 1996;15:1442–7. [PubMed] [Google Scholar]
- 35.Diefenbach A, Schindler H, Donhauser N, et al. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 36.Kawase I, Brooks C, Kuribayashi K, et al. Interleukin 2 induces gamma-interferon production: participation of macrophages and NK-like cells. J Immunol. 1983;131:288–92. [PubMed] [Google Scholar]
- 37.Orange J, Biron C. Characterization of early IL-12, IFN-αβ, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–56. [PubMed] [Google Scholar]