Abstract
To clarify the role of Th1- and Th2-type cytokines in the various outcomes of human alveolar echinococcosis (AE), the cytokine immune response of self-cured patients was studied and compared with those of progressive AE patients and healthy subjects. Self-cured patients were divided into two groups according to the following clinical features: subjects who had positive Echinococcus multilocularis serologies and hepatic calcifications typical of AE were classified as ‘abortive AE’ patients, and those who had positive E. multilocularis serologies but no hepatic lesions or calcifications detectable by ultrasonography were classified as ‘positive serology’ subjects. Secretions of IL-5, IL-10 and interferon-gamma, and expression of IL-5 mRNA were evaluated in peripheral blood mononuclear cells (PBMC) stimulated in vitro with the mitogen phytohaemagglutinin-C or specific E. multilocularis antigenic preparations. The cytokine profile of abortive AE patients was the opposite of that observed in progressive AE patients. An intermediate profile was observed in positive serology subjects. PBMC from abortive AE patients, whether non-stimulated or stimulated with PHA and antigenic preparations, secreted significantly lower levels of IL-10 than those isolated from progressive AE patients. Our observations seem to confirm the regulatory role of IL-10 in the immunopathology of human AE.
Keywords: IL-5, IL-10, interferon-gamma, abortive alveolar echinococcosis, cellular immunity
INTRODUCTION
Human alveolar echinococcosis (AE) is a parasitic infection which occurs after the accidental ingestion of Echinococcus multilocularis eggs. The larval stage of the cestode develops almost exclusively in the liver in humans [1,2]. The immune reaction of infected hosts is characterized by an antibody response with IgE and IgG production [3,4] and a strong cellular immune response indicated by the intense granulomatous infiltration surrounding parasitic lesions [5–7]. Since studies have reported both elevated levels of Th2-type cytokines in patients with evolutive AE [8–10], and prevention of E. multilocularis growth by IL-12 in an experimental murine model [11], the control of the infection seems to require the activation of Th1 cells. Mass screenings have shown that, besides the progressive lesions that characterize AE, there is a small number of patients with ‘abortive forms’ of E. multilocularis infection, characterized by liver calcifications and positive serologies, and a significant number of subjects with permanent specific serologies without liver sequelae [12,13]. To clarify the role of Th1- and Th2-type cytokines in the various outcomes of this infection, we examined the cytokine profile of ‘resistant’ patients with abortive lesions. A previous study reported that IL-5 synthesis was specifically increased in peripheral blood mononuclear cells (PBMC) from AE patients upon stimulation with parasitic antigens [8]. Further reports have indicated that this specific IL-5 production was exclusively due to CD4+ T lymphocyte activation [9,10]. It was also found that basal production of IL-10 by PBMC when permanently stimulated by parasitic antigens from AE lesions could be a cause for the inefficacy of the periparasitic cellular immune response [10]. The aim of the present study was to evaluate the cytokine immune response of 10 patients with positive serologies who managed to reject the parasite (three patients with a calcified liver lesion and seven subjects with no identified lesions in the liver), and to compare it with those of nine chronically infected patients with progressive AE and nine healthy subjects.
We specifically studied IL-10, interferon-gamma (IFN-γ) and IL-5 secretion by PBMC isolated from patients with the different forms of the disease and from healthy control subjects. PBMC were stimulated in vitro with three antigenic preparations of E. multilocularis of increasing specificity, proceeding from a crude extract of the parasite to the purified specific alkaline phosphatase of E. multilocularis. IL-5 secretion was compared with IL-5 mRNA expression in the PBMC.
PATIENTS and METHODS
Blood samples
Blood samples were collected from (i) nine patients with progressive AE (‘progressive AE’ group); none had undergone a curative hepatectomy or a liver transplantation before the study; (ii) three patients with the abortive form of the disease characterized by positive E. multilocularis serologies and hepatic calcifications typical of AE sequelae, as previously described [12,13] (‘abortive AE’ group); (iii) seven healthy subjects who had positive E. multilocularis serologies but no lesions detectable by ultrasonography (‘positive serology’ group); (iv) nine healthy control subjects (‘control’ group). The evaluation of patient and healthy donor specific humoral immune responses against E. multilocularis was carried out using the Emc- and Em2-ELISA as described by Gottstein et al. [14]. These results, expressed as a percentage of the standard serum as described by Gottstein et al. [15], show that specific antibodies against Emc and Em2 antigens ranged, respectively, from 40·4% to 87·9% and from 17·9% to 101·4% in the progressive AE group, from 15·5% to 60·0% and 3·6% to 64·4% in the abortive AE group and from 17·6% to 49·1% and 6·5% to 30·0% in the positive serology group; all nine normal control subjects from the same geographical area had E. multilocularis antibodies under the threshold level.
Antigenic preparations
Crude extract antigen (Emc Ag) was made from a homogenate of E. multilocularis metacestodes as previously described [10]. Vesicular fluid antigen (Emf Ag) free of host protein contamination was prepared according to the method of Hemphill et al. [16]. The alkaline phosphatase antigen (EmAp Ag) was shown to be quite useful for the immunodiagnosis of AE, since both the specificity and sensitivity of EmAp-ELISA reached 100% [17]; however, the usual purification procedure of this antigen by concanavalin A (Con A) affinity chromatography was not suitable for cell cultures and needed to be modified for this study. To avoid contamination by Con A, metacestode alkaline phosphatase (EmAp Ag) extraction was done as follows (all procedures were carried out at 4°C): the metacestodes were washed three times with 0·9% NaCl and homogenized in 100 mm Tris–HCl buffer, pH 7·6, 100 mm NaCl, 1 mm MgCl2, 1 mm CaCl2, 0·02 mm ZnCl2 using a scissors homogenizer (Virtis S23) for 10 min, a grinder (Polytron, Strasbourg, France) for an additional 10 min, and then sonicated for 5 min (15 W). The homogenate was mixed with an equal volume of 1-butanol, cooled to −20°C, and shaken slowly for 3 h at 4°C. The mixture was then centrifuged at 9000 g for 30 min in a refrigerated Sorvall centrifuge (Rotor S34). The aqueous layer was removed. The supernatant fraction and the pellet were again mixed, an equal volume of 1-butanol was added, then shaken overnight at 4°C and then centrifuged at 9000 g for 30 min. The butanol was eliminated, the supernatant fraction was dialysed against 0·9% NaCl for 12 h and then concentrated using a sodium salt of carboxymethylcellulose (Aquacid II; Calbiochem, La Jolla, CA). The sample was stored at −80°C. The total amount of protein in the three antigenic preparations was determined by the BioRad (Ivry‐sur‐Seine, France) protein assay.
Cell cultures
PBMC were isolated from peripheral blood of AE patients and healthy subjects by centrifugation on a Ficoll–Hypaque gradient and resuspended (106 cells in 2 ml) in supplemented RPMI medium as described by Sturm et al. [8]. Cells were cultured with 5 μg of Emc antigen, 2·3 μg of Emf antigen, 3·6 μg of EmAp antigen and 7 μg of non-specific mitogen phytohaemagglutinin (PHA), according to optimal concentrations and time length previously determined [10].
Lymphocyte proliferation assay
The proliferative responses were analysed by 3H-thymidine incorporation. PBMC (105 cells/well) were cultured and stimulated in vitro as described above. After 96 h of stimulation, cells were pulse-labelled with 0·5 μCi of 3H-thymidine and harvested 18 h afterwards. The incorporated radioactivity was counted. Results are expressed as the mean ct/min of triplicate samples ± s.d.
Cytokine assays
PBMC supernatants were collected after 48 h of culture with PHA and after 72 h of culture with antigenic stimulation. Cultures free of the mitogen or antigens were also set up as controls. Cytokine productions were measured using commercial kits for ELISA (Quantikine Immunoassay IL-5 D500; R&D systems, Abingdon, UK; 80-3549-00 Predicta R Human IL-10 ELISA kit, 80-3932-00 Human IFNγ Duoset; Genzyme, Cambridge, UK). Values < 3·0 pg/ml for IL-5, 5·0 pg/ml for IL-10 and 3·0 pg/ml for IFN-γ were below the sensitivity of the assays and recorded as 0.
Detection and measurement of cytokine mRNA by semiquantitative reverse transcriptase-polymerase chain reaction
PBMC were harvested 24 h after culture whatever the stimulation and total mRNA was immediately extracted with Trizol reagent (Gibco, BRL Life Technologies, Cergy‐Pontoise, France) and stored at −70°C for study of the expression of IL-5 mRNA by reverse transcriptase-polymerase chain reaction (RT-PCR). cDNA were obtained by reverse transcription, and specific cDNA sequences were amplified by PCR with a DNA thermal cycler (Minicycler PTC 150; Prolabo, Fontenay‐sous‐Bois, France) in the presence of β-actin and IL-5 sets of sense and antisense primers as previously described by Godot et al. [10]. Any cDNA not giving a suitable β-actin amplification was discarded. Each experiment included a negative control (PCR reagents without cDNA) and a positive control: cDNA from PBMC. PCR amplifications were performed twice and IL-5 gene was considered unexpressed only when both these amplifications gave negative results. Twenty microlitres of the amplified products were electrophoresed in a 2% w/v agarose gel stained with ethidium bromide. IL-5 mRNA was quantified by a densitometric technique using the Molecular Imager system (Gel Doc 1000 UV; BioRad). We calculated a correction factor for each photograph based on the 100 pb molecular weight band density to take the variations of photographic parameters into account. We always normalized the amount of IL-5 mRNA to the level of β-actin mRNA which was not altered after cell stimulation. Results were expressed as a ratio: percentage of IL-5 mRNA expression to 100% of β-actin mRNA expression. Because the mRNA levels were expressed in terms of a ratio with the level of β-actin mRNA, we avoided any possible error in the estimation of both total RNA initial amounts and amplified product amounts dependent on amplification efficiencies.
Statistical analysis
The differences between non-stimulated and stimulated cells in each group were analysed by the non-parametric Wilcoxon signed rank test; P < 0·05 was considered significant. Kruskal–Wallis (KW) test for multiple comparison was used to compare differences between patients infected with E. multilocularis and subjects from the other groups. When the KW value was significant, at least one of the groups was different from at least one of the others. We considered that differences between two groups were significant if the following imbalance was true:
RESULTS
PBMC reactivity from all groups under study was not impaired, since we observed a significantly elevated lymphocyte proliferation in PHA-stimulated PBMC from all groups compared with non-stimulated cells (data not shown). Lymphocyte proliferation, after Emc antigen and both Emf and EmAp purified antigen stimulations, was significantly enhanced in cell cultures from all groups. The proliferative responses to PHA and parasitic antigens were not significantly different in abortive AE patients, positive serology subjects or patients with progressive AE, but were significantly higher than in control subjects (data not shown).
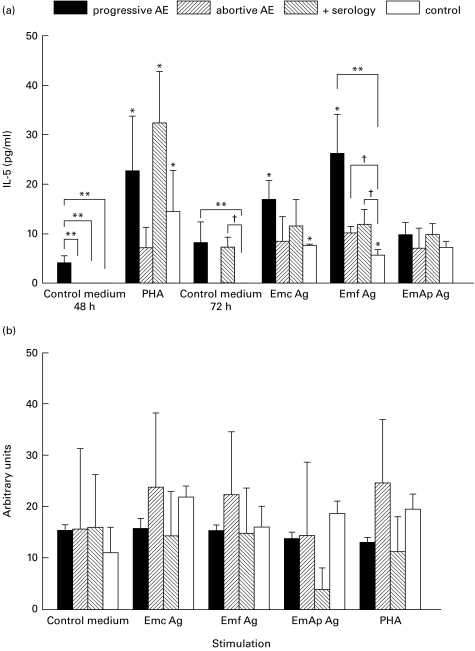
A comparison of cytokine basal secretions showed a significantly higher level of IL-5 (Fig. 1a) in 48 h-cultured PBMC isolated from patients with progressive AE than in those from self-cured subjects and healthy control donors. Concentrations of IL-5 were significantly higher in supernatants from progressive AE patients than in healthy control subjects after PBMC culture with Emf stimulation. However, PHA and all antigenic preparations failed to increase significantly the level of IL-5 in PBMC from the abortive AE group. Analysis of IL-5 mRNA (Fig. 1b) revealed that the expression profile was similar in PBMC from the four groups and that PHA and antigenic preparations did not significantly enhance the expression of IL-5 mRNA in PBMC from any groups.
Fig. 1.
IL-5 production and IL-5 mRNA semiquantification in peripheral blood mononuclear cells (PBMC) after mitogenic or antigenic exposure. (a) The secretion of IL-5 was assessed in PBMC culture supernatants from nine patients infected with Echinococcus multilocularis, three subjects with abortive alveolar echinococcosis (AE), seven subjects from an endemic area with positive E. multilocularis serologies, and nine control subjects with negative E. multilocularis serologies. Values are means ± s.e.m. *P < 0·05 non-stimulated versus stimulated PBMC (Wilcoxon signed rank test); **significant difference between progressive AE group and the other groups; †significant difference between control group and abortive AE group (Kruskall–Wallis test). (b) IL-5 mRNA expression was semiquantified after polymerase chain reaction amplification of cDNA. Results are expressed as a ratio: percentage of IL-5 mRNA expression for 100% β-actin expression. A total of six patients with progressive AE, three subjects with abortive AE, three subjects who had positive E. multilocularis serologies but no parasitic lesions, and six healthy control donors who had negative E. multilocularis serologies were analysed. Control medium, no stimulation; Emc Ag, stimulation with a crude extract antigen of E. multilocularis; Emf Ag, stimulation with the purified vesicular fluid antigen; EmAp Ag, stimulation with the alkaline phosphatase of E. multilocularis; PHA, stimulation with the non-specific mitogen, phytohaemagglutinin.
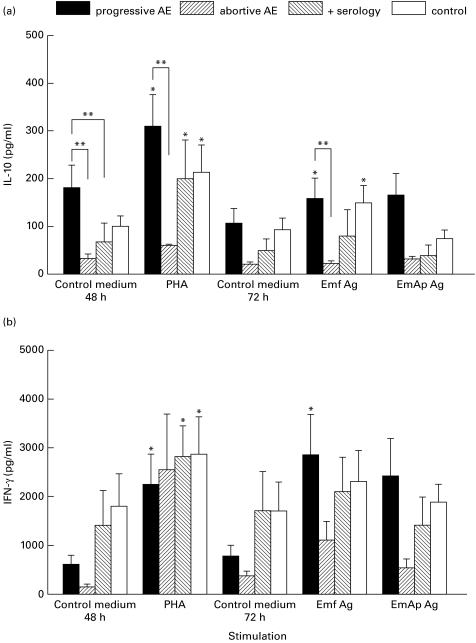
PBMC obtained from progressive AE patients spontaneously produced higher amounts of IL-10 (Fig. 2a), after 48 h of culture, than did those from subjects with abortive AE lesions and subjects with positive serologies. Differences reached statistical significance in both cases. PHA-stimulated PBMC from all subjects except patients with abortive AE were shown to significantly secrete IL-10 compared with non-stimulated cells (Fig. 2a; progressive AE group, P = 0·03; positive serology group, P = 0·04; and control group, P = 0·03); the mean IL-10 level was significantly lower in the abortive AE group than in the progressive AE group. The antigenic preparations did not significantly enhance the amount of IL-10 obtained from either the abortive AE group or the positive serology group. Furthermore, PBMC obtained from patients with abortive AE secreted significantly lower levels of IL-10, upon Emf exposure, than did those of the progressive AE group. The mean concentration of IL-10 was found to be intermediate in the positive serology group compared with that in the abortive AE group and the progressive AE group.
Fig. 2.
Release of IL-10 (a) and IFN-γ (b) by non-stimulated or Emf-, EmAp- or PHA-stimulated peripheral blood mononuclear cells (PBMC). The secretion of the cytokines was assessed in PBMC culture supernatants from nine patients infected with Echinococcus multilocularis, three subjects with abortive alveolar echinococcosis (AE), seven subjects with positive E. multilocularis serologies, and nine control subjects with negative E. multilocularis serologies. Values are means ± s.e.m. *P < 0·05 non-stimulated versus stimulated PBMC (Wilcoxon signed rank test); **significant difference between progressive AE group and the other groups (Kruskall–Wallis test). Control medium, no stimulation; Emf Ag, stimulation with the purified vesicular fluid antigen; EmAp Ag, stimulation with the alkaline phosphatase of E. multilocularis; PHA, stimulation with the non-specific mitogen, phytohaemagglutinin.
PBMC from all subjects except patients with abortive AE increased their IFN-γ secretion significantly after PHA stimulation (Fig. 2b; progressive AE group, P = 0·01; positive serology group, P = 0·02; and control group, P = 0·007); IFN-γ concentrations measured in PHA-stimulated PBMC supernatants did not differ significantly in the four groups of subjects. The mean concentration of IFN-γ was not markedly elevated in PBMC supernatants in any of the groups after antigenic stimulation.
DISCUSSION
The aim of this study was to analyse the immune response of self-cured AE patients. The PBMC proliferative response to E. multilocularis antigens in the patients with abortive lesions was found to be similar to that of patients with progressive AE, whereas cytokine secretion exhibited an opposite cytokine profile; an intermediate profile was observed in patients with positive serologies against E. multilocularis antigens but no parasitic lesions.
AE is one of the most severe helminthic infections in humans because of its cancer-like evolution and its high rate of mortality [2]. The metacestode proliferates in the liver over a long period of time and the clinical symptoms usually appear within a decade after the first contact with the parasite eggs, often revealing an advanced stage of the disease. Mass screening surveys have thus been organized in endemic areas so that diagnosis can be made earlier and patients can be treated more efficiently at the first stages of infection [12,13,18,19]. In Franche-Comté, France, among the 7824 subjects screened, five subjects, who had positive E. multilocularis serologies and hepatic calcifications typical of AE, were classified as cured patients with so-called ‘abortive AE’, as described by Rausch et al. [12]; only three agreed to be included in this study. This limited number reduces the impact of any statistical analysis and can lead to underestimation of significant differences, but the study of the immune response in these few patients was worth doing since it produced some interesting clues on the mechanisms of immune resistance to the cestode for subjects exposed to a similar contamination risk. It also seemed relevant to study the immune responsiveness of screened subjects living in hyper-endemic districts, who had positive E. multilocularis serologies, but without detectable lesions or calcifications of the liver [13]. The strong and efficient immune response in these subjects could mean that oncospheres were eliminated at the intestinal barrier level or at the first stages of larval development in the liver. We found that, upon antigenic stimulation, the lymphoproliferative response of patients with abortive lesions did not differ significantly from that of patients with progressive lesions (data not shown). Gottstein et al. also observed the persistence of a high proliferative response in Alaska patients with lesions containing dead parasites [20]. The authors also reported that patients with severe AE had a depressed proliferation rate. A similar inhibition of specific lymphocyte proliferative responses was also noticed in transplanted patients with recurrence of the disease, whereas transplanted patients considered as cured exhibited a very high proliferative response over a long time period [21]. This was not observed in our study since, in our protocol, we included only patients with medium-sized and stable lesions, i.e. a homogeneous population of patients. As no major differences were observed in the present study, the differences in the cytokine response of both types of patients could not be explained by variations in their mitotic responsiveness to antigenic stimulation.
IL-5 has been shown to be specifically stimulated by E. multilocularis antigens in progressive AE [8,9]. In this study, mRNA semiquantification showed that there was no significant difference in IL-5 mRNA expression between subjects with an efficient immune response against the parasite and patients who developed progressive AE. However, a spontaneous production of IL-5 was almost exclusively observed in non-stimulated cells obtained from progressive AE patients. All E. multilocularis antigenic preparations markedly enhanced the basal level of IL-5 in PBMC isolated from patients with progressive AE; however, they also induced IL-5 secretion in PBMC from all three groups of subjects, including normal controls, as the non-specific mitogen did. Albeit non-significant, stimulation of IL-5 secretion by specific antigens was observed in 2/3 patients with abortive lesions. A similar induction of cytokines by parasitic extracts from E. granulosus was reported by Rigano et al. in healthy controls [22]. The higher increase in IL-5 production observed in patients with progressive AE could be enhanced by a particular genetic background; the increase seems to be the consequence of an expanding IL-5-secreting cell population: we [10] and Jenne et al. [9] demonstrated that this secretion of IL-5 was restricted to CD4+ T lymphocytes in human AE. Echinococcus multilocularis antigenic preparations could regulate the expression of IL-5 gene at the post-transcriptional level, as suggested by the results of IL-5 mRNA expression.
Our study of IL-10 secretion, in AE patients who were different from those previously studied [10], confirmed the permanent secretion of this cytokine, without any stimulation, by PBMC from patients with progressive AE. It also revealed that in non-stimulated PBMC obtained from abortive AE and positive serology subjects, IL-10 concentrations were the lowest among the four groups studied; these concentrations never increased after specific stimulation. PHA induced a moderate secretion of IL-10 in PBMC from these two groups, which remained low in comparison with the IL-10 production by PHA-stimulated cells from progressive AE patients. In human AE [8–10], as in leishmaniasis [23,24], IL-10 seems to promote and/or prolong the disease by blocking both the antigen-presenting cells and effector functions of macrophages, more than by directly inhibiting Th1-type cytokine synthesis. In fact, IFN-γ production was not decreased in PBMC from AE patients. Similar coexistence of Th1- and Th2-type cytokines has been reported in the second phase of rapid development of the larvae in mice experimentally infected with E. multilocularis [25]. This could explain the relative inefficacy of IFN-γ on AE metastases and on the cytokine profile of those patients who have been treated with this interferon [26,27]. In experimental mice however, IL-12 was shown to be fairly efficient in modifying both larval growth and the cytokine profile [11].
All the factors responsible for the susceptibility of AE patients to E. multilocularis proliferation and those responsible for the resistance of patients with abortive lesions or positive serologies have not yet been found. Genetic particularities of the patients infected with E. multilocularis could contribute to the Th2 profile: HLA-B8 DR3 DQ2, a haplotype associated with weak cellular immunity and increased humoral immunity, has been shown to be more frequent in patients with severe AE [28]. We are currently attempting to correlate the immunogenetic characteristics and the cytokine profile of patients with AE.
Acknowledgments
We wish to thank Professors Danièle Lenys and Jean-Philippe Miguet for their contribution to this study, Lois Rose for the preparation of the manuscript and Sabeha Biichlé for care and management of the healthy control subjects. We would like to express our gratitude to the patients, the healthy control subjects and nursing staff without whom this study would not have been possible. This work was supported by the French Ministry of Health and Besançon University Hospital (PHRC 8, UF-1452), and by the European Commission (InterReg II grant).
REFERENCES
- 1.Bresson-Hadni S, Vuitton DA, Lenys D, et al. Cellular immune response in Echinococcus multilocularis infection in humans. 1) Lymphocyte reactivity to Echinococcus antigens in patients with alveolar echinococcosis. Clin Exp Immunol. 1989;78:61–66. [PMC free article] [PubMed] [Google Scholar]
- 2.Miguet JP, Bresson-Hadni S, Vuitton DA. Echinococcosis of the liver. In: McIntyre B, Benhamou JP, Bircher J, Rizetto M, Rodes J, editors. Oxford textbook of clinical hepatology. Oxford: Oxford University Press; 1990. p. 721. [Google Scholar]
- 3.Gottstein B, Felleisen R. Protective immune mechanisms against the metacestode of Echinococcus multilocularis. Parasitol Today. 1995;11:320–5. doi: 10.1016/0169-4758(95)80184-7. [DOI] [PubMed] [Google Scholar]
- 4.Vuitton DA, Bresson-Hadni S, Lenys D, et al. IgE-dependent humoral immune response in Echinococcus multilocularis infection: circulating and basophil-bound specific IgE against Echinococcus antigen in patients with alveolar echinococcosis. Clin Exp Immunol. 1988;71:247–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Bresson-Hadni S, Liance M, Meyer JP, et al. Cellular immunity in experimental Echinococcus multilocularis infection. 2) Sequential and comparative phenotypic study of the periparasitic mononuclear cells in resistant and sensitive mice. Clin Exp Immunol. 1990;82:373–7. doi: 10.1111/j.1365-2249.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liance M, Vuitton DA, Guerret-Stocker S, et al. Experimental alveolar echinococcosis. Suitability of a murine model of intrahepatic infection by Echinococcus multilocularis for immunological studies. Experientia. 1984;40:1436–9. doi: 10.1007/BF01951932. [DOI] [PubMed] [Google Scholar]
- 7.Vuitton DA, Bresson-Hadni S, Laroche L, et al. Cellular immune response in Echinococcus multilocularis infection in humans. 2) Natural killer cell activity and cell subpopulations in the blood and in the periparasitic granuloma of patients with alveolar echinococcosis. Clin Exp Immunol. 1989;78:67–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Sturm D, Menzel J, Gottstein B, et al. Interleukin-5 is the predominant cytokine produced by peripheral blood mononuclear cells in alveolar echinococcosis. Infect Immun. 1995;63:1688–97. doi: 10.1128/iai.63.5.1688-1697.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenne L, Kilwinski J, Scheffold W, et al. IL-5 is expressed by CD4+ lymphocytes from Echinococcus multilocularis-infected patients. Clin Exp Immunol. 1997;109:90–97. doi: 10.1046/j.1365-2249.1997.4031299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godot V, Harraga S, Deschaseaux M, et al. Increased basal production of interleukin-10 by peripheral blood mononuclear cells in human alveolar echinococcosis. Eur Cytokine Netw. 1997;8:401–8. [PubMed] [Google Scholar]
- 11.Emery I, Leclerc C, Sengphommachanh K, et al. In vivo treatment with recombinant IL-12 protects C57BL/6J mice against secondary alveolar echinococcosis. Parasite Immunol. 1998;20:81–91. doi: 10.1046/j.1365-3024.1998.00131.x. [DOI] [PubMed] [Google Scholar]
- 12.Rausch RL, Wilson JF, Schantz PM, et al. Spontaneous death of Echinococcus multilocularis: cases diagnosed serologically (by EM2 ELISA) and clinical significance. Am J Trop Med. 1987;36:576–85. doi: 10.4269/ajtmh.1987.36.576. [DOI] [PubMed] [Google Scholar]
- 13.Bresson-Hadni S, Laplante JJ, Lenys D, et al. Seroepidemiologic screening of Echinococcus multilocularis infection in a European area endemic for alveolar echinococcosis. Am J Trop Med. 1994;51:837–46. doi: 10.4269/ajtmh.1994.51.837. [DOI] [PubMed] [Google Scholar]
- 14.Gottstein B, Eckert J, Fey N. Serological differentiation between Echinococcus granulosus and Echinococcus multilocularis infections in man. Z Parazitenkd. 1983;69:347–56. doi: 10.1007/BF00927876. [DOI] [PubMed] [Google Scholar]
- 15.Gottstein B, Eckert J, Woodtli W. Determination of parasite-specific immunoglobulins using the ELISA in patients with echinococcosis treated with mebendazole. Z Parazitenkd. 1984;70:385–9. doi: 10.1007/BF00927825. [DOI] [PubMed] [Google Scholar]
- 16.Hemphill A, Gottstein B. Immunological and morphological studies on the proliferation of in vivo cultivated Echinococcus multilocularis metacestode. Parasitol Res. 1995;81:605–14. doi: 10.1007/BF00932028. [DOI] [PubMed] [Google Scholar]
- 17.Sarciron ME, Bresson-Hadni S, Mercier M, et al. Antibodies against Echinococcus multilocularis alkaline phosphatase as markers for the specific diagnosis and the serological monitoring of alveolar echinococcosis. Parasite Immnunol. 1997;19:61–68. doi: 10.1046/j.1365-3024.1997.d01-183.x. [DOI] [PubMed] [Google Scholar]
- 18.Gottstein B, Lengeler C, Bachmann P, et al. Sero-epidemiological survey for alveolar echinococcosis (by Em2-ELISA) of blood donors in an endemic area of Switzerland. Trans R Soc Trop Med Hyg. 1987;81:960–4. doi: 10.1016/0035-9203(87)90365-8. [DOI] [PubMed] [Google Scholar]
- 19.Giraudoux P, Vuitton DA, Bresson-Hadni S, et al. Alveolar echinococcosis strategy for eradication of alveolar echinococcosis of the liver. Sapporo: Fuji Shoin; 1996. Mass screening and epidemiology of alveolar echinococcosis in France, western Europe, and Gansu, central China: from epidemiology towards transmission ecology; pp. 243–51. [Google Scholar]
- 20.Gottstein B, Mesarina B, Tanner I, et al. Specific cellular and humoral immune responses in patients with different long-term courses of alveolar echinococcosis (infection with Echinococcus multilocularis) Am J Trop Med. 1991;45:734–42. doi: 10.4269/ajtmh.1991.45.734. [DOI] [PubMed] [Google Scholar]
- 21.Bresson-Hadni S, Beurton I, Bartholomot B, et al. Alveolar echinococcosis. Hepatology. 1998;27:1453–6. doi: 10.1002/hep.510270543. [DOI] [PubMed] [Google Scholar]
- 22.Rigano R, Profumo E, Teggi A, et al. Production of IL-5 and IL-6 by peripheral blood mononuclear cells (PBMC) from patients with Echinococcus granulosus infection. Clin Exp Immunol. 1996;105:456–9. doi: 10.1046/j.1365-2249.1996.d01-796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karp CL. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–8. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melby PC, Andrade-Narvaez F, Darnell BJ, et al. In situ expression of interleukin-10 and interleukin-12 in active human cutaneous leishmaniasis. FEMS Immunol Med Microbiol. 1996;15:101–7. doi: 10.1111/j.1574-695X.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 25.Emery I, Liance M, Deriaud E, et al. Characterization of T-cell immune responses of Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol. 1996;18:1–10. doi: 10.1111/j.1365-3024.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 26.Jenne L, Kilwinski J, Radloff P, et al. Clinical efficacy of and immunologic alterations caused by interferon gamma therapy for alveolar echinococcosis. Clin Infect Dis. 1998;26:492–4. doi: 10.1086/516316. [DOI] [PubMed] [Google Scholar]
- 27.Schmid M, Samonigg H, Stoger H, et al. Use of interferon gamma and mebendazole to stop the progression of alveolar hydatid disease: case report. Clin Infect Dis. 1995;20:1543–6. doi: 10.1093/clinids/20.6.1543. [DOI] [PubMed] [Google Scholar]
- 28.Eiermann T, Bettens F, Tiberghien P, et al. HLA and alveolar echinococcosis. Tissue Antigens. 1998;52:124–9. doi: 10.1111/j.1399-0039.1998.tb02275.x. [DOI] [PubMed] [Google Scholar]