Abstract
Absolute and relative NK cell numbers were determined in peripheral whole blood by flow cytometry in patients with common variable immunodeficiency (CVID) (n = 55) and X-linked agammaglobulinaemia (XLA) (n = 19) on regular immunoglobulin (IVIG) therapy. Absolute CD3−CD16+ NK cell numbers were significantly reduced in CVID patients (median 108/μl, range 23–815), compared with normal subjects (n = 60) (289/μl, range 56–640, P < 0·001). Total lymphocyte concentrations were significantly lower in CVID (median 1587/μl, range 523–7519) compared with normal subjects (median 2019/μl, range 1124–3149, P = 0·004), with the percentage of NK cells also being significantly decreased (median 7·5%, range 3·0–33·0%, compared with 14·2%, range 2·6–30·8%, P < 0·001). In XLA, absolute NK cell numbers (median 140/μl, range 32–551, P < 0·001) but not relative numbers were significantly reduced compared with normal controls. We excluded the possibility that IVIG interferes with in vitro binding of CD16 MoAbs. Further analysis of NK cell subsets showed a deficiency of both CD16+ and CD56+ cells in CVID, most marked in the CD3−CD8dim subpopulation, which may be due to increased homing of these cells to the gut. Serial studies on a small number of patients suggest that IVIG therapy has no short-term effect on NK cells, although we cannot exclude an effect with prolonged use. Although there are no obvious clinical effects of the NK depletion in CVID and XLA, this may be a factor in their predisposition to cancer.
Keywords: natural killer cells, CD16, common variable immunodeficiency, X-linked agammaglobulinaemia, intravenous immunoglobulin therapy, interferon-gamma
INTRODUCTION
Common variable immunodeficiency (CVID) is a complex polygenic primary disorder of immunity characterized by failure of antibody production, associated with defective CD4+ T cell priming to antigen and defective response to mitogens [1–3]. Lymphopenia is common, predominantly affecting the CD4+ T cell subset. T cell cytotoxicity is not suppressed, but lymphokine-activated killer cell (LAK) activity is depressed [4,5]. Affected patients are not prone to the type of viral infections associated with severe T cell defects, but are prone to bacterial infection and unexplained chronic inflammation in various organs, often with granuloma formation [6].
In contrast, X-linked agammaglobulinaemia (XLA) is an inherited single gene disorder due to mutations of an intracellular B cell tyrosine kinase (Btk) [7]. Most patients have no circulating B cells, but are not lymphopenic, have normal T cell function, and are not prone to chronic inflammatory complications. Nevertheless, both CVID and XLA patients suffer from a similar spectrum of microbial infection and are treated with regular immunoglobulin replacement therapy.
Although T cell function and integrity have been extensively investigated in CVID, and to a lesser extent in XLA, there have been few reports regarding NK cells. When analysing routine measurements of circulating lymphocyte subsets in both CVID and XLA patients we noticed a frequent deficiency of cells bearing the NK marker CD16. This led to a more extensive analysis of other NK markers in a large group of patients and to in vitro experiments to try to explain this deficiency.
SUBJECTS AND METHODS
Subjects
Peripheral venous blood was collected in either lithium-heparin or EDTA-containing tubes from 55 patients with CVID (age 44·5 ± 15·4 years (mean ± s.d.); 32 male, 23 female) and 19 male XLA patients (age 33·8 ± 7·4 years) immediately before routine intravenous immunoglobulin (IVIG) replacement therapy. The majority had had IVIG for > 5 years. Six patients were commenced on IVIG during this study. Two patients were on intramuscular immunoglobulin therapy. Single measurements of lymphocyte subsets from 60 normal subjects, from an anonymous donor pool of HIV− adults undergoing routine HIV screening, were used as a healthy control group. All patients gave informed consent, and the study had the approval of the local ethics committee.
For the NK subset assays, 19 normal subjects, 11 CVID and six XLA patients were tested. The in vitro immunoglobulin competition assay was performed on venous blood from four healthy subjects. NK subsets were also studied in four patients with either severe eczema (n = 3) or vasculitis (n = 1) having high dose IVIG (hdIVIG) therapy. Interferon gamma (IFN-γ) studies were performed on six patients with CVID before and after IVIG replacement therapy.
Definition of NK cells
In working with NK cells we used the established definition of CD56+ and/or CD16+ and CD3−‘lymphocytic’ cells within a light-scatter gate for lymphocytes [8]. However, we also investigated CD3+ subpopulations expressing NK cell markers which are sometimes called ‘NK T cells’ or ‘NK-like T cells’[9] in order to see if changes in expression of NK markers were specific for classical NK cells or affected all cells expressing NK markers.
Cell staining
Lymphocyte subsets were determined in whole blood samples using a standard, no-wash technique according to the manufacturer's instructions. Briefly, whole blood (100 μl) was added to mixtures of directly conjugated MoAbs at saturating concentrations, and incubated for 15 min at room temperature. Erythrocytes were lysed by the addition of a lysis buffer (1900 μl) containing 0·8% ammonium chloride, 0·1% potassium carbonate and 0·0037% Na4EDTA for 15 min, followed by immediate acquisition on an Ortho Cytoron (Ortho Diagnostics, Amersham, UK) flow cytometer.
NK cell subsets were measured using a similar staining technique. Whole blood (50 μl) was added to mixtures of MoAbs as detailed below, and incubated at room temperature for 15 min. Erythrocytes were lysed by the addition of Optilyse C (Beckman Coulter, High Wycombe, UK; 500 μl) for 15 min, followed by the addition of PBS 500 μl. For absolute counting purposes, FlowCount beads (Beckman Coulter; 50 μl) of known concentration were added immediately prior to data acquisition by an Epics-XL four-colour flow cytometer (Beckman Coulter). Non-specific binding was determined by using anti-mouse isotype-matched controls.
Monoclonal antibodies
For lymphocyte subsets, the following mixtures of MoAbs were used for staining cells: (i) isotype controls directly conjugated to FITC, PE and PE-Cy5; (ii) CD16/FITC (clone 3G8), CD19/PE, CD3/PE-Cy5 (all from Ortho Diagnostics). For NK subset staining, various antibody combinations were used from the following: CD3/PE-Cy5 (Immunotech, Bournbrook, UK), CD8/ECD (Coulter), CD16/FITC (clone NKP15, Leu-11a; Becton Dickinson, Cowley, UK), CD16/PE-Cy5 (clone 3G8; Immunotech), CD56/PE (Immunotech), CD57/FITC (Immunotech). Isotype controls used were also directly conjugated to FITC (Becton Dickinson), PE (Becton Dickinson) and PE-Cy5 (Immunotech).
Data acquisition and analysis
For lymphocyte subsets, an acquisition period of 60 s was used. The flow cytometer was compensated for spectral overlap using standard compensation controls, and calibrated for absolute counts using the Ortho-count calibration kit (Ortho Diagnostics) and Dako fluorescence QC beads (Dako, Cambridge, UK). In addition, the laboratory participates in a national quality assessment programme for absolute lymphocyte subset counts. List mode data from the lymphocyte subset experiment were analysed using standard Immunocount II (Ortho Diagnostics) software.
For NK subset experiments, a minimum of 10 000 events within a lymphocyte light-scatter gate was acquired. Colour compensation for spectral overlap was applied electronically offline using WinList 4 (Verity, Topsham, ME) following calibration with appropriately stained control cells. Listmode data were analysed using Winlist 4.0 (Verity). Cells bearing NK markers within a tight lymphocyte light-scatter gate were determined by counting quadrant statistics from CD16/57 and CD56/57 dot plots, gated on seven different populations of cells (i.e. total lymphocytes, CD3+8−, CD3+8dim, CD3+8bright, CD3−8−, CD3−8dim and CD3−8bright cells). Absolute subset concentrations were derived from the numbers of FlowCount beads in the sample using the method of Schlenke et al. [10].
In vitro competition assay for anti-CD16 MoAbs with IgG
Peripheral blood mononuclear cells (PBMC) from peripheral blood were isolated by density gradient centrifugation (Ficoll–Paque; Pharmacia Biotech AB, Uppsala, Sweden) and resuspended at 6 × 106/ml in PBS. Cell aliquots (300 μl) were added to known concentrations (0, 2·5, 5, 10, 20 and 30 mg/ml) of IVIG preparations (Octagam; Octapharma, Solihull, UK) made up in PBS (600 μl) to give a final cell concentration of 2 × 106/ml and incubated at room temperature for 15 min. Aliquots (50 μl) of these cell/immunoglobulin suspensions were then added to MoAbs at saturating concentrations, and incubated for 15 min. For each immunoglobulin concentration cells were stained in triplicate using a combination of MoAbs against CD3, CD8, CD56, CD14 and one of three anti-CD16 MoAbs (Ortho Diagnostics, Becton Dickinson, or Immunotech). The stained cells were washed in PBS and resuspended in 0·5% paraformaldehyde/PBS prior to flow cytometric analysis.
Effects of in vivo IVIG replacement therapy and high-dose IVIG on NK subsets
To determine the effects of IVIG therapy on NK subsets, four patients receiving hdIVIG for systemic vasculitis (n = 1) or atopic eczema (n = 3) had NK subsets measured immediately before and immediately following a complete (usually 3 days) treatment with hdIVIG at 2 g/kg body weight. In addition, routine lymphocyte subsets in six patients on IVIG replacement therapy and six patients on hdIVIG determined before the start of IVIG and after various numbers of treatments were analysed.
Intracellular NK IFN-γ assay
Intracellular IFN-γ was measured in NK cells before and after IVIG therapy (200–400 mg/kg every 3 weeks) in six CVID patients. An adaptation of the intracellular cytokine method for whole blood samples was used [11]. Briefly, 250 μl whole blood was cultured in 500 μl medium (RPMI 1640; Life Technologies, Paisley, UK) with monensin, phorbol myristate acetate and ionomycin for 2 h. Cells were stained with CD3, CD56 and CD16 reagents prior to cell fixation and permeabilization. Intracellular IFN-γ staining was then performed using directly FITC-conjugated anti-IFN-γ antibodies (Serotec, Oxford, UK). Cells were analysed using four-colour flow cytometry as previously described. Cytokine-positive cells were defined using gates set by non-stimulated cells cultured with monensin only.
Statistical analysis
Absolute concentrations and percentages of lymphocyte subsets and NK subsets were not normally distributed when tested by histograms and normal probability plots, and no simple transformation produced a normal distribution. Therefore, they were compared using the Mann–Whitney U-test (SPSS Inc., Chicago, IL). Pearson's correlation coefficient (r) was used, where correlation between parameters is given. Significance was set at the P < 0·05 level.
RESULTS
In vitro competition assay for anti-CD16 MoAbs with IgG
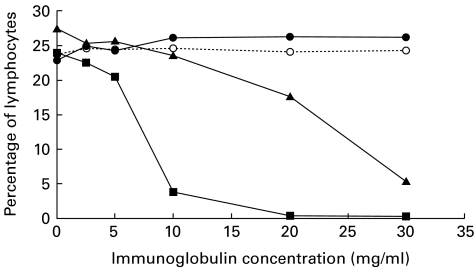
To investigate the competitive effects of IgG on binding of anti-CD16 MoAbs used for staining, cells from one normal subject were stained in triplicate with CD16 monoclonals from three manufacturers in the presence of various concentrations of a commercial IVIG preparation. The effect of increasing concentrations of IVIG products on binding of anti-CD16 to its receptor (Fcγ RIII) varies depending on the anti-CD16 MoAb used for staining (Fig. 1). The Becton Dickinson anti-CD16 (clone NKP15) was subject to interference from IVIG, with a strong inhibition of binding at physiologic immunoglobulin concentrations and complete inhibition at 20 mg/ml. The Immunotech anti-CD16 (clone 3G8) was only inhibited at high concentrations (equivalent to 20 mg/ml or more). The Ortho Diagnostics clone 3G8 was not affected by IVIG. All these antibodies were similar in sensitivity in the absence of immunoglobulins. As an internal control, increasing concentrations of the IVIG preparation did not influence numbers of CD56+ cells. Estimates of the different CD16+ lymphoid populations (e.g. CD3−/+, CD56−/+) and also CD16+ monocytes were equally affected (data not shown). These results were confirmed by experiments from three other healthy donors using a single tube for each immunoglobulin concentration and anti-CD16, respectively (data not shown).
Fig. 1.
Effect of increasing concentrations of intravenous immunoglobulin (IVIG) on relative NK cell numbers from a single donor staining with anti-CD16 MoAbs (Ortho Diagnostics anti-CD16 clone 3G8 (•), Becton Dickinson anti-CD16 clone NKP15 (▪), Immunotech anti-CD16 clone 3G8 (▴)). For comparison, the percentage of lymphocytes staining with anti-CD56 (○) is shown. Increasing IgG concentrations do not reduce staining with the Ortho MoAb, but significantly interfere with the Becton Dickinson MoAb, and to a lesser extent with the Immunotech clone.
NK cells in routine lymphocyte subset determination
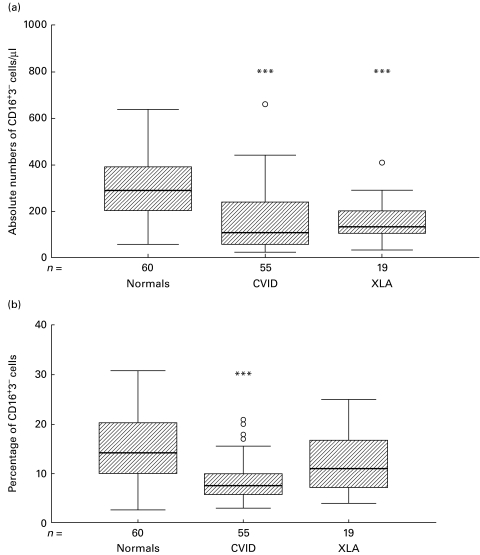
In routine testing, NK cells are defined as CD16+CD3− cells within a light-scatter gate for lymphocytes when the Ortho Diagnostics MoAb (clone 3G8) was used. Absolute CD3−CD16+ NK cell numbers were significantly reduced in CVID patients (n = 55, median 108·0/μl, range 23·0–815·0), compared with normal subjects (n = 60, 288·5/μl, range 56·0–640·0, P < 0·001) (Fig. 2a). Total lymphocyte counts were significantly lower in CVID (median 1587·0/μl, range 522·5–7519·0) compared with normal subjects (median 2019·0/μl, range 1124·0–3149·0, P = 0·004), with the percentage of NK cells also being significantly decreased (median 7·5%, range 3·0–33·0%, compared with 14·2%, range 2·6–30·8%, P < 0·001) (Fig. 2b) and with 60% of CVID patients having NK cell concentrations below the normal range (9–16%). Within the CVID patients, there was no significant effect of age or sex on NK cell numbers. Absolute numbers of NK cells showed a significant positive correlation with the total lymphocyte count in CVID (r = 0·82, P < 0·001) and normal subjects (r = 0·36, P = 0·005).
Fig. 2.
Boxplots of absolute (a) and relative (b) numbers of NK cells in lymphocyte subset determinations in common variable immunodeficiency (CVID) (n = 55), X-linked agammaglobulinaemia (XLA) (n = 19) and healthy controls (n = 60). The hatched boxes show the median and the range between the 25th and the 75th percentile, tails show whiskers (defined as values within 1·5 of the interquartile range). ○, Outliers. *P < 0·05; **P < 0·01; ***P < 0·001 by Mann–Whitney U-test when compared with normals.
In XLA, absolute numbers of NK cells (n = 19, median 139·5/μl, range 32·2–550·5) were significantly reduced compared with normal controls (P < 0·001). The NK cell percentage was not significantly decreased compared with normals, but showed a downward trend (median 11·0%, range 4·0–25·0%) (Fig. 2a,b). Total lymphocyte counts were also lower than in normal controls (median 1445·0/μl, range 786·0–2652·5, P < 0·001).
NK subset assays
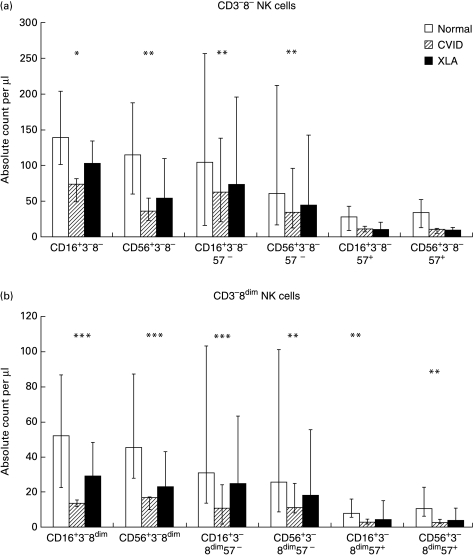
As we found significant numbers of CD16+ cells within a monocytic light-scatter gate, we analysed them for CD56/CD14 expression. Since they were almost exclusively CD56− (data not shown), only cells within a lymphocytic light-scatter gate were considered in this study. Cells within a lymphocytic gate were further subdivided according to CD3 (+/–) and CD8 (bright/dim/negative) expression; thus six subpopulations were obtained. NK subsets were defined within two major subpopulations: CD3−8− and CD3−8dim (see Fig. 3a,b, respectively). However, cells expressing NK cell markers were also found within the CD3+ populations. Within each subpopulation further subdivision was made depending on expression of CD16, CD56 and CD57. The results a summarized in Table 1.
Fig. 3.
(a) CD16+3−8− and CD56+3−8− NK cell subsets and co-expression of CD57 in normal subjects, common variable immunodeficiency (CVID) and X-linked agammaglobulinaemia (XLA) patients. (b) CD16+3−8dim and CD56+3−8dim NK cell subsets and co-expression of CD57 in normal subjects (n = 19), CVID (n = 11) and XLA patients (n = 6). Bars represent the median, tails represent the 25th to 75th percentiles. *P < 0·05; **P < 0·01; ***P < 0·001, by Mann–Whitney U-test when compared with normal subjects.
Table 1.
Lymphocyte subsets and NK cell subsets (based on the established NK markers CD16 or CD56) for normal subjects (n = 19), common variable immunodeficiency (CVID) (n = 11) and X-linked agammaglobulinaemia (XLA) (n = 6) patients.
| Population (subpopulation) | Normals, cells/μl | CVID cells/μl | XLA cells/μl | P (CVID versus controls) | P (XLA versus controls) |
|---|---|---|---|---|---|
| CD56+ | 218 | 94 | 129 | 0·004 | NS |
| CD56+57− | 149 | 70 | 109 | 0·02 | NS |
| CD56+57+ | 57 | 27 | 26 | NS | NS |
| CD56+3− | 183 | 46 | 70 | 0·002 | NS |
| CD56+3−8− | 115 | 36 | 54 | 0·006 | NS |
| CD56+3−8dim | 45 | 17 | 23 | 0·0003 | NS |
| CD56+3+ | 51 | 33 | 56 | NS | NS |
| CD56+3+8− | 6 | 8 | 40 | NS | 0·04 |
| CD56+3+8dim | 11 | 6 | 8 | NS | NS |
| CD56+3+8bright | 15 | 16 | 4 | NS | 0·02 |
| CD16+ | 247 | 102 | 144 | 0·006 | NS |
| CD16+57− | 166 | 80 | 107 | 0·0009 | 0·01 |
| CD16+57+ | 42 | 19 | 14 | NS | NS |
| CD16+3− | 199 | 88 | 135 | 0·01 | NS |
| CD16+3−8− | 139 | 74 | 103 | 0·01 | NS |
| CD16+3−8dim | 52 | 14 | 29 | 0·0002 | NS |
| CD16+3+ | 9 | 10 | 13 | NS | NS |
| CD16+3+8− | 2 | 1 | 1 | 0·04 | NS |
| CD16+3+8dim | 1 | 1 | 2 | NS | NS |
| CD16+3+8bright | 8 | 6 | 9 | NS | NS |
| Lymphocytes | 1898 | 1347 | 1499 | NS | NS |
| CD3−8− | 511 | 515 | 265 | NS | 0·003 |
| CD3−8dim | 59 | 19 | 27 | 0·0001 | 0·04 |
| CD3+ | 1240 | 822 | 1183 | NS | NS |
| CD3+8− | 764 | 523 | 834 | NS | NS |
| CD3+8dim | 52 | 32 | 35 | NS | NS |
| CD3+8bright | 362 | 235 | 364 | NS | NS |
The numbers represent the median of cells per microlitre.
P, P value based on Mann–Whitney U-test and compared with normal subjects. NS (not significant), P > 0·05.
CVID patients showed a reduced number of CD56+ cells (irrespective of CD3 and CD8 expression) (n = 11, median 94·2/μl, range 45·4–341·2) compared with normals (n = 17, median 217·6/μl, range 92·1–556·2, P = 0·004), a reduced percentage of CD56+ cells (median 7·2%, range 2·6–23·3%, compared with 11·3%, range 7·0–29·3%, P = 0·008), a reduced number of CD16+ cells (median 102·0/μl, range 44·1–423·1, compared with 247·3/μl, range 50·0–407·2, P = 0·006) and a reduced percentage of CD16+ cells (median 7·2%, range 2·4–22·9%, compared with 11·4%, range 0·6–12·9%, P = 0·018). Interestingly, the reduction in CVID was mainly due to a decrease in CD57− cells. CD57 expression in some subpopulations (e.g. CD3+8bright) actually showed a trend towards higher numbers compared with normal controls.
There were significantly fewer CD3−8dim cells in CVID patients compared with normal controls (median 18·6/μl, range 6·1–26·8, versus 59·5/μl, range 12·5–208·8, P < 0·001) (Table 1). This population accounts for about 30% of circulating NK cells. CVID patients had significantly fewer CD16+CD3−8dim (median 13·5/μl, range 5·4–22·6) and CD56+CD3−8dim cells (median 16·7/µ l, range 3·7–28·6) compared with normal controls (median 52·0/μl, range 13·6–106·3, P < 0·001 for CD16+CD3−8dim, median 45·4/μl, range 18·0–184·7, P < 0·001 for CD56+CD3−8dim). Analysis for CD57 revealed that both CD3−8dimCD57+ and CD3−8dimCD57− NK cells were significantly decreased in CVID compared with normal controls.
Within the CD3−8− population (which contains about 60% of NK cells and all B cells), NK cells were also significantly depleted (Fig. 3a). CVID patients showed a median of 74·0/μl (range 32·2–372·9) CD16+CD3−8− cells and 35·6/μl (range 17·7–202·7) CD56+CD3−8− cells compared with 139·1/μl (range 34·7–254·2) CD16+CD3−8− cells (P = 0·01) and 114·7/μl (range 40·2–310·9) CD56+CD3−8− cells (P = 0·006) in normal subjects, but NK cell subsets co-expressing CD57 were similar to normal levels.
As expected, XLA patients had a decreased number of CD3−8− cells due to the absence of B cells. However, XLA patients also had decreased absolute and relative numbers of CD3−8dim cells. In the analysis of NK cell subsets the absolute numbers of CD16+3− and CD56+3− cells were lower than normals, but this did not reach significance, probably because only six patients were studied, whereas there were 19 patients in the routine lymphocyte subset testing. Unlike CVID, XLA patients showed a decreased number and proportion of CD57+ cells in several subpopulations (CD3−8−, CD3−8dim) (data not shown).
In non-classical NK subpopulations, such as CD16+3+8+ cells, there were no significant differences between CVID and normal controls. Although normally present in very low numbers, CD56+ cells were significantly lower in XLA than in normal controls within the CD3+8bright subpopulation, and higher within the CD3+8− (predominantly CD4+ T cells) subpopulations. These changes were not seen in CVID.
Effects of IVIG on NK cell subsets
There was no correlation between serum IgG concentration and NK cell concentration in CVID and XLA patients on standard IVIG therapy, as the range of IgG concentrations was small, being maintained at about 9 mg/ml. In addition, NK cell concentrations measured repeatedly in 39 patients on average 1 year apart were not significantly different, despite interim IVIG replacement therapy, and there was a lack of correlation between NK cell numbers and duration of IVIG (r = 0·184 and r = 0·0031 for relative and absolute numbers, respectively, P > 0·05 for both). In six patients, in whom lymphocyte subsets were determined before starting continuous IVIG replacement therapy (five CVID, one hypogammaglobulinaemia associated with thymoma), and in six individuals who received hdIVIG (five eczema, one vasculitis), NK cell concentrations and percentages in routine lymphocyte subset determinations did not change significantly (data not shown).
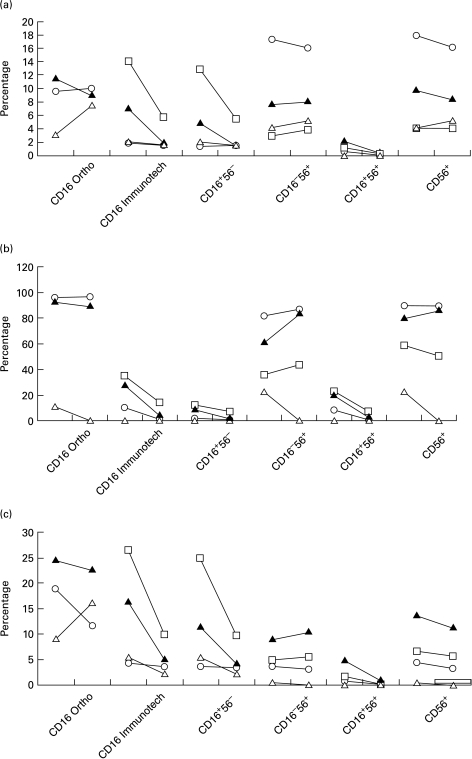
In four subjects receiving hdIVIG, NK subsets were measured (on two occasions in one of the subjects) immediately prior to, and immediately following hdIVIG (2 g/kg body weight) (Fig. 4). Using the Immunotech anti-CD16 MoAbs, there were reductions in the proportions of CD16+ cells within the total lymphocytes, and in the CD3−8− and CD3−8dim subpopulations; these changes were not seen in the CD56+ cells, suggesting that the high IgG concentrations obtained following IVIG may compete for monoclonal anti-CD16 binding. This explanation was supported by the finding that within the CD3−8dim subpopulation, the CD16+56+ cells became CD16−56+. Furthermore, there was no fall in CD16+ cells, when Ortho MoAb was used.
Fig. 4.
NK cells before and after high dose intravenous immunoglobulin (hdIVIG) in patients with eczema (n = 3, ○, Δ, and □) and vasculitis (n = 1, ▴) within the total lymphocytes (a), the CD3−CD8dim subpopulation (b) and the CD3−CD8− subpopulation (c). The beginning of the lines represent the values before hdIVIG, the end of the lines the values immediately after hdIVIG. Double-positive cells for CD16 and CD56 become negative for CD16, especially in the CD3−CD8dim subpopulation. Unless otherwise stated the Immunotech MoAb for CD16 is used. CD56 does not change. A discrepancy between Ortho Diagnostic and Immunotech antibodies is demonstrated.
Effects of replacement-dose IVIG on IFN-γ production of NK cells
There is a wide range of IFN-γ expression in NK cells, with 5–65% of NK cells from CVID patients being positive after stimulation. Cambronero et al. (personal communication) previously found that NK cells in CVID patients expressed similar levels of IFN-γ compared with normal subjects. Replacement-dose IVIG (400 mg/kg body weight every 4 weeks) did not change IFN-γ expression when assessed immediately post-infusion (data not shown).
DISCUSSION
NK cells are an important component of the innate host defence system, targeting and killing tumour cells and viral infected cells. NK cells express a surface receptor for IgG (CD16, Fcγ RIII) which binds the Fc portion of IgG and mediates antibody-dependent cell-mediated cytotoxicity [12,13]. Most NK cells express neural cell adhesion molecule (NCAM; CD56) on their surface, which is capable of binding to other surface-bound NCAM by homotypic adhesion [14]. The significance of this property is well established for the development of the nervous system, but not understood for NK cells [15]. A minority of NK cells weakly express CD8, theoretically facilitating binding to MHC class I molecules. It was this subset which was most strikingly depleted in the CVID patients, although there was also a marked depletion of the CD3−8− NK subset.
Both CVID and XLA patients had lower total lymphocyte counts compared with normal controls; this is well recognized in CVID [16,17], but not previously appreciated in XLA [18]. The moderate reduction in CD16+ cells in XLA probably reflects a general decrease in all lymphocyte populations.
There have been few previous reports of decreased NK cell numbers and activity in CVID, and in most only a few patients were studied, and CD57 was used as the NK cell marker [4,19,20]. However, CD57 is probably an activation rather than a lineage marker for NK cells [21,22]. Moreover, a substantial number of these CD57+ cells could be T cells [8]. Baumert et al. [23] investigated 20 CVID patients on regular IVIG replacement therapy and found decreased relative numbers of CD16+CD3− and CD16+CD8+ cells (also classified as CD8dim), but normal CD16+CD3+ cells. They showed that CVID patients with a decreased CD4/CD8 ratio had an increased percentage of CD8+ cells co-expressing CD57. These results agree with our finding of increased CD57+ cells in CVID patients and are consistent with the concept of a more inflammatory pattern of pathology [24]. Although circulating NK cell numbers are clearly depleted in CVID patients, this appears to have no short-term clinical relevance, since the patients are not prone to herpes viruses, as has been reported in rare patients with isolated NK cell deficiencies [25], nor to most other viral infections. Furthermore, previous studies on in vitro cytotoxicity of PBMC from CVID patients to the K562 cell line (presumed NK activity) have been normal, suggesting a compensatory mechanism for the low numbers [4]. Our demonstration of normal IFN-γ expression by CVID NK cells supports their functional integrity. Nevertheless, CVID patients and to a lesser extent those with XLA are prone to cancer [26,27], particularly lymphoma, and a deficiency of NK cells could compromise tumour surveillance and control of oncogenic viruses.
Since several studies have demonstrated altered expression of NK cell markers after IVIG [28,29], it is reasonable to speculate that the NK depletion is related to this therapy. There are also reports showing effects of immunoglobulins on NK activity in vitro[30,31]. Although our pre- and post-IVIG NK cell subsets were measured in a distinct patient group receiving hdIVIG, this did not appear to alter NK cell numbers. Furthermore, reanalysis of NK cell numbers from routine testing of patients receiving IVIG replacement therapy (n = 6) or hdIVIG (n = 6) did not show a specific effect on NK cell numbers or percentages (data not shown). In addition, there was no change in IFN-γ expression after replacement IVIG in the small group of patients tested, suggesting that the perceived ‘anti-inflammatory’ effect of IVIG is not dependent on major alterations in the function of NK cells.
This contrasts with other studies showing marked down-regulation of CD56+ cells by hdIVIG in women with recurrent abortions [29]. However, another study showed an increase in CD16+ cells 24 h after infusion of hdIVIG for Kawasaki disease [28]. Thus, changes in NK cells after IVIG may be disease-specific.
Our in vitro experiments showed that IVIG did compete with two of the commercially available MoAbs to CD16 (Becton Dickinson, and to some degree Immunotech), but not with the Ortho antibodies. Although the Ortho clone shares the same name (3G8) as the Immunotech clone which is susceptible to competition, they were generated from spleen cells of different mouse strains and different myeloma cell lines, so there are probably subtle differences in epitope specificity. Our finding with the Ortho MoAb contrasts with one study showing complete inhibition of MoAb binding (also demonstrated for clone 3G8; Medarex, Annandale, NJ) by physiological immunoglobulin concentrations of 10 mg/ml [32]. We conclude that the marked overall depletion of NK cells in CVID patients is real, particularly since we also found low numbers of CD56+ cells, and that it is unlikely to be due to a short-term effect of IVIG replacement therapy.
In CVID, it is likely that the same underlying defect(s) affect T cells and NK cells. In XLA, Btk may play a subtle role in T lymphocyte and NK cell survival in adults. Sequential testing of a larger group of CVID and XLA patients before and during IVIG therapy is needed to test whether the lymphopenia and NK cell depletion are associated with prolonged use of IVIG, but the available evidence does not support this possibility.
The marked reduction in the CD3−8dim NK population may be specific for CVID. A distinct function for this subset is not known. It is known that CD16+3−8dim NK cells have a higher binding affinity to target cells than their CD8− counterparts [33], but this was measured on K562 cells which do not express MHC class I, suggesting that a binding pair distinct from CD8/MHC 1 is responsible for the higher affinity. NK cells also have a higher density of perforin granules compared with cytotoxic T cells when measured with intracellular staining and flow cytometry [34], but it is not known if there is a difference in density between the CD8+ and CD8− NK cells. However, the cytolytic activity of the CD3+ NK cell subset is intermediate between those of Tc cells and CD3− NK cells [35].
CD3−8dim NK cells express α/α-homodimeric CD8 rather than the α/β-heterodimer [8]. There is evidence that CD8 T cells expressing this homodimer preferentially home to the gut epithelium [36], and this homing may be increased in patients with inflammatory bowel disease [37]. Therefore, it is feasible that the low numbers of circulating CD3−8dim NK cells in CVID are due to the common occurrence of bowel inflammation in these patients [2,38]. Further studies to characterize the intraepithelial lymphocytes in CVID are now needed to support this hypothesis.
Acknowledgments
We would like to thank Professor George Janossy, Dr Margarita Bofill, and the staff of the immunopathology laboratory for the routine measurement of lymphocyte subsets. We also thank Jean M. Fletcher, Margaret E. North, and Luisa Baptista for assistance. We thank Octapharma for helping to support this research and Serotec for providing the monoclonal antibodies used for the intracellular cytokine assay.
REFERENCES
- 1.Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Quart J Medicine. 1993;86:31–42. [PubMed] [Google Scholar]
- 2.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 3.Kondratenko I, Amlot PL, Webster AD, Farrant J. Lack of specific antibody response in common variable immunodeficiency (CVID) associated with failure in production of antigen-specific memory T cells. Clin Exp Immunol. 1997;108:9–13. doi: 10.1046/j.1365-2249.1997.d01-993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckert K, Schmitt M, Garbin F, Wahn U, Monier JC. Thymosin alpha 1 effects, in vitro, on lymphokine-activated killer cells from patients with primary immunodeficiencies: preliminary results. Int J Immunopharmacol. 1994;16:1019–25. doi: 10.1016/0192-0561(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe JS, Strober W, Sneller MC. Functional abnormalities of CD8+ T cells define a unique subset of patients with common variable immunodeficiency. Blood. 1993;82:192–200. [PubMed] [Google Scholar]
- 6.Spickett GP, Farrant J, North ME, Zhang JG, Morgan L, Webster AD. Common variable immunodeficiency: how many diseases? Immunol Today. 1997;18:325–8. doi: 10.1016/s0167-5699(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 7.Vetrie D, Vorechovsky I, Sideras P, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 8.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–38. [PubMed] [Google Scholar]
- 9.Leite-De-Moraes MC, Dy M. Natural killer T cells: a potent cytokine-producing cell population. Eur Cytokine Network. 1997;8:229–37. [PubMed] [Google Scholar]
- 10.Schlenke P, Frohn C, Klueter H, et al. Evaluation of a flow cytometric method for simultaneous leukocyte phenotyping and quantification by fluorescent microspheres. Cytometry. 1998;33:310–7. doi: 10.1002/(sici)1097-0320(19981101)33:3<310::aid-cyto4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Sewell WA, North ME, Webster AD, Farrant J. Determination of intracellular cytokines by flow-cytometry following whole-blood culture. J Immunol Methods. 1997;209:67–74. doi: 10.1016/s0022-1759(97)00150-6. [DOI] [PubMed] [Google Scholar]
- 12.Ritz J, Schmidt RE, Michon J, Hercend T, Schlossman SF. Characterization of functional surface structures on human natural killer cells. Adv Immunol. 1988;42:181–211. doi: 10.1016/s0065-2776(08)60845-7. [DOI] [PubMed] [Google Scholar]
- 13.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carson WE, Fehniger TA, Caligiuri MA. CD56bright natural killer cell subsets: characterization of distinct functional responses to interleukin-2 and the c-kit ligand. Eur J Immunol. 1997;27:354–60. doi: 10.1002/eji.1830270203. [DOI] [PubMed] [Google Scholar]
- 15.Ronn LC, Hartz BP, Bock E. The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol. 1998;33:853–64. doi: 10.1016/s0531-5565(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 16.Wright JJ, Wagner DK, Blaese M, Hagengruber C, Waldmann TA, Fleisher TA. Characterization of common variable immunodeficiency: identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood. 1990;76:2046–51. [PubMed] [Google Scholar]
- 17.Farrant J, Spickett G, Matamoros N, et al. Study of B and T cell phenotypes in blood from patients with common variable immunodeficiency (CVID) Immunodeficiency. 1994;5:159–69. [PubMed] [Google Scholar]
- 18.Crockard AD, Boyd NA, McNeill TA, McCluskey DR. CD4 lymphocyte subset abnormalities associated with impaired delayed cutaneous hypersensitivity reactions in patients with X-linked agammaglobulinaemia. Clin Exp Immunol. 1992;88:29–34. doi: 10.1111/j.1365-2249.1992.tb03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S. Deficiency of monoclonal antibody-defined NK cells (HNK-1+) in primary immunodeficiency disorders. Immunol Letters. 1982;5:327–9. doi: 10.1016/0165-2478(82)90122-5. [DOI] [PubMed] [Google Scholar]
- 20.Ohno T, Kanoh T, Suzuki T, et al. Comparative analysis of lymphocyte phenotypes between carriers of human immunodeficiency virus (HIV) and adult patients with primary immunodeficiency using two-color immunofluorescence flow cytometry. Tohoku J Exp Med. 1988;154:157–72. doi: 10.1620/tjem.154.157. [DOI] [PubMed] [Google Scholar]
- 21.Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983;131:1789–96. [PubMed] [Google Scholar]
- 22.Phillips JH, Hori T, Nagler A, Bhat N, Spits H, Lanier LL. Ontogeny of human natural killer (NK) cells: fetal NK cells mediate cytolytic function and express cytoplasmic CD3 epsilon, delta proteins. J Exp Med. 1992;175:1055–66. doi: 10.1084/jem.175.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumert E, Wolff-Vorbeck G, Schlesier M, Peter HH. Immunophenotypical alterations in a subset of patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1992;90:25–30. doi: 10.1111/j.1365-2249.1992.tb05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spickett GP, Zhang JG, Green T, Shrimankar J. Granulomatous disease in common variable immunodeficiency: effect of immunoglobulin replacement therapy and response to steroids and splenectomy. J Clin Pathol. 1996;49:431–4. doi: 10.1136/jcp.49.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries E, Koene HR, Vossen JM, et al. Identification of an unusual Fc gamma receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood. 1996;88:3022–7. [PubMed] [Google Scholar]
- 26.Kinlen LJ, Webster AD, Bird AG, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985;1:263–6. doi: 10.1016/s0140-6736(85)91037-2. [DOI] [PubMed] [Google Scholar]
- 27.Filipovich AH, Shapiro RS. Tumors in patients with common variable immunodeficiency. J Immunol Immunopharmacol. 1991;11:43–46. [Google Scholar]
- 28.Finberg RW, Newburger JW, Mikati MA, Heller AH, Burns JC. Effect of high doses of intravenously administered immune globulin on natural killer cell activity in peripheral blood. J Pediatr. 1992;120:376–80. doi: 10.1016/s0022-3476(05)80900-x. [DOI] [PubMed] [Google Scholar]
- 29.Rigal D, Vermot-Desroches C, Heitz S, Bernaud J, Alfonsi F, Monier JC. Effects of intravenous immunoglobulins (IVIG) on peripheral blood B, NK and T cell subpopulations in women with recurrent spontaneous abortions: specific effects on LFA-1 and CD56 molecules. Clin Immunol Immunopathol. 1994;71:309–14. doi: 10.1006/clin.1994.1091. [DOI] [PubMed] [Google Scholar]
- 30.Engelhard D, Waner JL, Kapoor N, Good RA. Effect of intravenous immune globulin on natural killer cell activity: possible association with autoimmune neutropenia and idiopathic thrombocytopenia. J Pediatr. 1986;108:77–81. doi: 10.1016/s0022-3476(86)80772-7. [DOI] [PubMed] [Google Scholar]
- 31.Sulica A, Gherman M, Galatiuc C, Manciulea M, Herbermann R. Inhibition of human natural killer cell activity by cytophilic immunoglobulin G. J Immunol. 1982;128:1031–6. [PubMed] [Google Scholar]
- 32.Edberg JC, Kimberly RP. Cell type-specific glycoforms of Fc gamma RIIIa (CD16): differential ligand binding. J Immunol. 1997;159:3849–57. [PubMed] [Google Scholar]
- 33.Vitale M, Zamai L, Neri LM, Manzoli L, Facchini A, Papa S. Natural killer function in flow cytometry: identification of human lymphoid subsets able to bind to the NK sensitive target K562. Cytometry. 1991;12:717–22. doi: 10.1002/cyto.990120805. [DOI] [PubMed] [Google Scholar]
- 34.Rutella S, Rumi C, Lucia MB, Etuk B, Cauda R, Leone G. Flow cytometric detection of perforin in normal human lymphocyte subpopulations defined by expression of activation/differentiation antigens. Immunol Letters. 1998;60:51–55. doi: 10.1016/s0165-2478(97)00132-6. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Wolf IG, Blume KG, Johnston V, Huhn D, Negrin RS. Propagation of large numbers of T cells with natural killer cell markers. Br J Haematol. 1994;87:453–8. doi: 10.1111/j.1365-2141.1994.tb08297.x. [DOI] [PubMed] [Google Scholar]
- 36.Latthe M, Terry L, MacDonald TT. High frequency of CD8 alpha alpha homodimer-bearing T cells in human fetal intestine. Eur J Immunol. 1994;24:1703–5. doi: 10.1002/eji.1830240737. [DOI] [PubMed] [Google Scholar]
- 37.Rust C, Orsini D, Kooy Y, Koning F. Reactivity of human gamma delta T cells to staphylococcal enterotoxins: a restricted reaction pattern mediated by two distinct recognition pathways. Scand J Immunol. 1993;38:89–94. doi: 10.1111/j.1365-3083.1993.tb01698.x. [DOI] [PubMed] [Google Scholar]
- 38.Teahon K, Webster AD, Price AB, Weston J, Bjarnason I. Studies on the enteropathy associated with primary hypogammaglobulinaemia. Gut. 1994;35:1244–9. doi: 10.1136/gut.35.9.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]