Abstract
Captopril is an orally active inhibitor of angiotensin-converting enzyme (ACE) which is widely used as an anti-hypertensive agent. In addition to its ability to reduce blood pressure, captopril has a number of other biological activities. Recently the drug was shown to inhibit Fas-induced apoptosis in human activated peripheral T cells and human lung epithelial cells. In this study, we investigated whether captopril blocks activation-induced apoptosis in murine T cell hybridomas, and found that captopril inhibited IL-2 synthesis and apoptotic cell death upon activation with anti-CD3 antibody. In addition, captopril inhibited an inducible caspase-3-like activity during activation-induced apoptosis. On the other hand, captopril did not interfere with Fas signalling, since anti-Fas antibody-induced apoptosis in Fas+ Jurkat cells was unaffected by the drug. Furthermore, we examined whether captopril blocks activation-induced apoptosis by interfering with expression of Fas, Fas ligand (FasL), or both on T cell hybridomas. FasL expression on activated T cells was significantly inhibited by captopril, whereas up-expression of Fas was partially inhibited, as assessed by cell surface staining. Taking all data together, we conclude that captopril prevents activation-induced apoptosis in T cell hybridomas by interfering with T cell activation signals. Captopril has been reported to induce systemic lupus erythematosus syndrome, and our findings may be useful for elucidating the mechanism of captopril-induced autoimmunity.
Keywords: angiotensin-converting enzyme, captopril, T cell activation, apoptosis
INTRODUCTION
Angiotensin-converting enzyme (ACE) inhibitors are widely utilized in the treatment of systemic hypertension and congestive heart failure. Captopril (D-3-mercapto-2-methyl-propionyl-L-proline) [1,2], among them, is the most extensively used compound. Like all other ACE inhibitors, captopril blocks conversion of angiotensin I to the potent vasoconstrictor angiotensin II and inactivates simultaneously the vasodilator peptide bradykinin.
Besides its blood pressure-lowering properties, captopril has various immunomodulatory functions. The drug exhibits beneficial effects on rheumatoid arthritis [3,4] and prevention of complications in insulin-dependent diabetes mellitus [5]. Additionally, captopril successfully inhibits inflammation in schistosomiasis [6], experimental lupus diseases [7], or experimental autoimmune encephalomyelitis (EAE) [8]. Some ACE inhibitors including captopril are capable of suppressing the production of monocytes/macrophage-derived proinflammatory cytokines such as tumour necrosis factor (TNF), IL-1, IL-6, and IL-12 [9–11]. These immunomodulatory actions of captopril have been explained by several mechanisms, including anti-proliferation [12–15], anti-oxidant activity [16–18], inhibition of metalloproteases [19,20], and elevation of prostaglandin [21–23]. Some of these properties may be related to the presence of thiol groups in its structure and are independent of its effect on the renin angiotensin system [24,25].
As captopril has recently been found to inhibit Fas-induced apoptosis in human activated T cells [26] and lung epithelial cells [27], we investigated whether captopril also blocks activation-induced apoptosis in T cells. We examined this in murine T cell hybridomas, since these cells have been widely used for the study of the mechanisms of activation-induced apoptosis [28–30]. T cell hybridomas undergo apoptosis upon activation with a high density of immobilized anti-TCR/CD3 antibodies. We found that captopril prevented IL-2 synthesis and apoptotic cell death upon activation with anti-CD3 antibody. Activation of murine T cell hybridomas through TCR/CD3 cross-linking leads to expression of Fas (CD95) and its ligand (FasL) that subsequently interact, resulting in apoptotic cell death [31–33]. Therefore, we further examined whether captopril prevents cell death by interfering with expression of Fas, FasL, or both on T cell hybridomas, as assessed by cell surface staining. We observed that the induction of FasL expression by activation with anti-CD3 antibody is significantly inhibited in the presence of captopril. Cell surface Fas levels were consistently lower on T cells activated with anti-CD3 antibody in the presence of captopril than on activated T cells. In contrast to previous studies [26,27], captopril did not influence Fas-induced apoptosis in Fas+ Jurkat cells. These results suggest that captopril inhibits activation-induced expression of Fas and FasL on T cell hybridomas and therefore may block activation-induced apoptotic cell death by inhibiting Fas–FasL interaction. Taking these facts together, we conclude that captopril inhibits activation-induced apoptosis in T cell hybridomas by interfering with T cell activation signals.
MATERIALS AND METHODS
Reagents and antibodies
Captopril, N-succinyl-L-proline and lisinopril were purchased from Sigma Chemical Co. (St Louis, MO). These reagents were dissolved in PBS, with pH adjusted to 7·4 and used for the experiments. Anti-mouse Fas antibody (Jo-2, hamster IgG) was purchased from PharMingen (San Diego, CA). Anti-mouse FasL MoAb, MFL1, was kindly donated by Dr H. Yagita (Juntendo University, Tokyo, Japan). Anti-human Fas antibody (CH-11) was obtained from Medical Biological Laboratories (Nagoya, Japan). Hamster anti-mouse CD3ε MoAb (145-2C11) was purified from ascites fluid by protein A-affinity chromatography. Twenty-four-well plates or 96-well plates were coated with purified anti-CD3 antibody as descried previously [30].
Cells
Murine T cell hybridoma N3-6-71 was described previously [30]. Jurkat T cells or N3-6-71 cells were maintained in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS; Hyclone Labs, Logan, UT), 5 × 10−5 m 2-mercaptoethanol (2-ME), 1 mm sodium pyruvate, 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. In the experiments for evaluation of the effects of ACE inhibitors on T cells, the concentration of FCS was reduced to 1% since FCS contains large amounts of ACE and may interfere with these inhibitor activities.
Determination of cell proliferation
N3-6-71 cells (2 × 104) were incubated in control uncoated 96-well plates or in anti-CD3ε (5 μg/well) antibody-coated 96-well plates in the presence or absence of captopril for 24 h, followed by a 2-h pulse with 1 μCi of methyl-3H-thymidine (ICN, Costa Mesa, CA). The cells were then harvested on glass-filter paper, and uptake of 3H-thymidine was quantified by liquid scintillation counting.
Trypan blue dye exclusion assay
N3-6-71 cells were seeded into control uncoated 24-well plates or into anti-CD3 (5 μg/well)-coated 24-well plates at a density of 5 × 105 cells/ml. Captopril, N-succinyl-L-proline, or lisinopril was added immediately in appropriate concentrations to the assay plates. After 50 μl of culture supernatant were removed for the measurement of IL-2 at 24 h of culture, the cells were harvested by vigorous pipetting and cell viability was determined by trypan blue dye exclusion.
Quantification of DNA fragmentation
Quantification of DNA fragmentation was determined as described by Chow et al. and Matzinger [34,35]. N3-6-71 cells (5 × 105 cells/ml) were labelled with 2 μCi/ml of methyl-3H-thymidine at 37°C for 6 h. After being washed three times, they were cultured in uncoated 24-well plates or in anti-CD3-coated 24-well plates (5 × 104 cells/well), in the presence or absence of indicated concentrations of captopril. After 24 h of culture at 37°C, the cells were lysed by addition of lysis buffer (5 mm Tris–HCl, 20 mm EDTA, 0·5% Triton X-100, pH 8·0) and harvested onto fibreglass filters (PHD cell harvester; Cambridge Technology, Cambridge, MA). Intact chromatin adheres to the filters, but DNA fragments pass through. The radioactivity was measured by liquid scintillation counter and percentage DNA fragmentation was calculated as described previously [36].
IL-2 assay
IL-2 in culture supernatant was measured using a commercially available ELISA (BioSource Int., Camarillo, CA) according to the manufacturer's instructions. Recombinant murine IL-2 was used as a standard.
Assay for caspase-3-like activity
Cells (2 × 106) were lysed in 100 μl of extract buffer (150 mm NaCl, 50 mm Tris–HCl, pH 7·2), 1% NP-40 containing the protease inhibitors (PMSF, leupeptin, antipain, and pepstatin A) and centrifuged at 15 000 rev/min for 15 min, and the supernatant was kept at −70°C. Fifty micrograms of cytosolic extracts were diluted with 200 μl of dilution buffer (10 mm HEPES pH 7·0, 40 mmβ-glycerophoshate, 50 mm NaCl, 2 mm MgCl2, 5 mm EGTA, 1 mm DTT, supplemented with 0·1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and 100 μg/ml bovine serum albumin (BSA)) and incubated at 30°C for 30 min with 10 μm fluorescent substrate Ac-DEVD-MAC (Peptide Institute, Osaka, Japan). Release of 7-amino-4-methycoumarin (AMC) was measured in a fluorospectrophotometer with a filter setting of 380 nm (excitation) and 460 nm (emission). Increase in fluorescence was standardized using free AMC (Peptide Institute). Values are given as release of AMC in pmole/min per mg protein.
Assessment of anti-Fas antibody-induced apoptosis in Jurkat cells
Jurkat cells (5 × 105) were seeded into 24-well plates with or without CH-11. Captopril was added immediately in appropriate concentrations. Twenty-four hours later, the cells were harvested and assessed for their cell viability by trypan blue dye exclusion.
Cell surface staining of Fas and FasL
N3-6-71 cells (5 × 105) were cultured in uncoated 24-well plates or in anti-CD3-coated 24-well plates, in the presence or absence of captopril. After 5 h of incubation, the cells were harvested and stained with anti-mouse Fas antibody, Jo2. After being washed twice, the cells were stained with a secondary FITC-conjugated goat anti-hamster IgG and analysed using a FACScan (Becton Dickinson, Mountain View, CA). For staining of cell surface FasL, the cells were incubated either with anti-mouse FasL antibody followed by a FITC-conjugated goat anti-hamster secondary antibody or with only a FITC-conjugated goat anti-hamster secondary antibody. The cells were analysed with Lysis II software using a FACScan.
RESULTS
Captopril blocks activation-induced apoptosis in T cell hybridomas
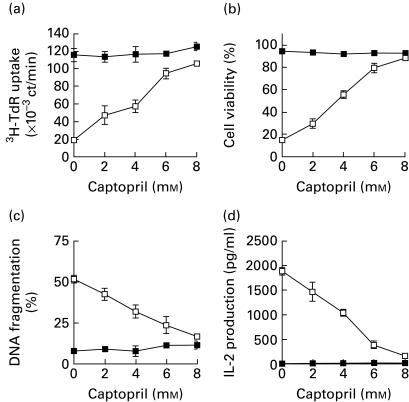
Activation of T cell hybridomas through TCR/CD3 complex results in IL-2 production and cell growth arrest, followed by apoptosis [28–30]. We first examined whether captopril could inhibit IL-2 production and activation-induced apoptosis in murine T cell hybridoma N-3-6-71. As shown in Fig. 1a, after N-3-6-71 cells were stimulated with immobilized anti-CD3 antibody for 24 h, T cell proliferation was significantly inhibited. When cell viability was assessed by trypan blue dye exclusion, extensive cell death was observed in 24-h activation with anti-CD3 antibody (Fig. 1b). In addition, activation with anti-CD3 antibody induced approximately 50% DNA fragmentation in N3-6-71 cells (Fig. 1c). As shown in Fig. 1a,b,c, activation-induced apoptosis was blocked by captopril in a dose-dependent manner, almost complete inhibition being achieved at 8 mm. Captopril was found to exert its effect even when added after 3 h of cultivation (data not shown). Captopril by itself had no effect on T cell proliferation and cell viability (Fig. 1a,b). When N3-6-71 cells were activated with immobilized anti-CD3 antibody for 24 h, a large amount of IL-2 was detected in the culture supernatant of the T cells (Fig. 1d). IL-2 production in N3-6-71 cells activated with immobilized anti-CD3 antibody was suppressed in the presence of captopril in a dose-dependent manner. Thus, captopril prevents IL-2 production and apoptosis in T cell hybridomas following activation with anti-CD3 antibody. Similar results were obtained in the experiments using another T cell hybridoma, 2-45-12 [30] (data not shown).
Fig. 1.
Captopril inhibits activation-induced cell death and IL-2 production in T cell hybridoma N3-6-71. (a) Cell proliferation. N3-6-71 cells were cultured in control uncoated 96-well plates or in anti-CD3 antibody-coated 96-well plates (2 × 104 cells/well), in the presence or absence of the indicated concentrations of captopril. After 24 h, the cells were pulsed with 3H-thymidine, and harvested after an additional 2 h. Incorporation of 3H-thymidine into cellular DNA was measured in a scintillation counter. (b) Cell viability. N3-6-71 cells were cultured in control 24-well plates or in anti-CD3 antibody-coated 24-well plates (5 × 105 cells/well), in the presence or absence of the indicated concentrations of captopril. After 50 μl of supernatant were removed from each well for the measurement of IL-2 at 24 h of cultivation, the cells were harvested and cell viability was determined by trypan blue dye exclusion. (c) DNA fragmentation. 3H-thymidine-labelled N3-6-71 cells were cultured in control 96-well plates or in anti-CD3 antibody-coated 96-well plates (2 × 104 cells/well), in the presence or absence of the indicated concentrations of captopril. Twenty-four hours later, the cells were harvested and the percentage of DNA fragmentation was determined as described in Materials and methods. (d) IL-2 production. N3-6-71 cells were cultured as described above for 24 h. Concentrations of IL-2 in the culture supernatant were measured by ELISA. Mean values of triplicates ± s.d. of representative experiments are shown (a, b, c, d). Error bars are less than symbol width when they are not apparent (a, b, c, d). ▪, Control; □, anti-CD3.
Captopril inhibits an enhanced caspase-3-like activity during activation-induced apoptosis
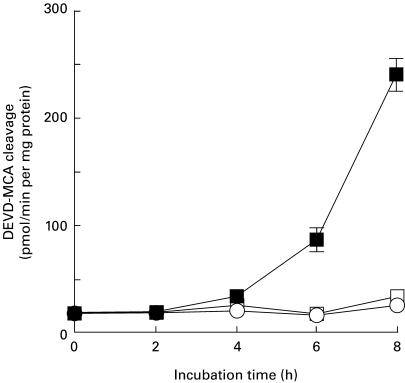
In the last few years, a family of cysteine proteases termed ‘caspases’ has been implicated in the effector process of apoptosis in several systems [37]. Although at least 14 members of the caspase family have been identified to date, caspase-3 (CPP32/Apopain/YAMA) is thought to be a key apoptotic enzyme in mammalian cells for the nuclear changes associated with apoptosis [38]. Since it has been demonstrated that caspase-3 is activated during activation-induced apoptosis in T cells [39], we next prepared cytosolic extracts from T cell hybridoma N3-6-71 cells stimulated with anti-CD3 antibody, and thereafter measured caspase-3-like activity in the cytosolic extracts using a fluorescent substrate Ac-DEVD-MAC. Originally, the cleavage of the amino acid sequence DEVD was attributed to caspase-3 [40], but can probably be catalysed by several caspases [41]. Therefore, DEVD cleaving activity is here referred to as caspase-3-like activity. We previously indicated that DNA cleavage in N3-6-71 cells was detected after 6–8 h of activation with anti-CD3 antibody [30]. As shown in Fig. 2, caspase-3-like activity was detected 6 h after activation with anti-CD3 antibody and increased up to 8 h. The kinetics of caspase-3-like activity seemed to correlate well with that of DNA fragmentation in N3-6-71 cells. Captopril (8 mm) that was able to prevent entirely activation-induced cell death inhibited the inducible caspase-3-like activity in T cell hybridoma N3-6-71.
Fig. 2.
Captopril inhibits caspase-3-like activity in activation-induced apoptosis of T cell hybridomas. N3-6-71 cells were cultured in control 24-well plates or in anti-CD3 antibody-coated 24-well plates (5 × 105 cells/well), in the presence or absence of 8 mm captopril. At indicated times, the cells were harvested and the cytosolic extracts were prepared as described in Materials and methods. Caspase-3-like activity was determined by excitation fluorometery of released 7-amino-4-methycoumarin (AMC) from the cleaved substrate Ac-DEVD-MAC. Results are expressed as the mean ± s.d. of triplicate cultures. ▪, Anti-CD3; ○, captopril; □, anti-CD3 + captopril.
Captopril does not affect anti-Fas antibody-induced apoptotic cell death in Jurkat cells
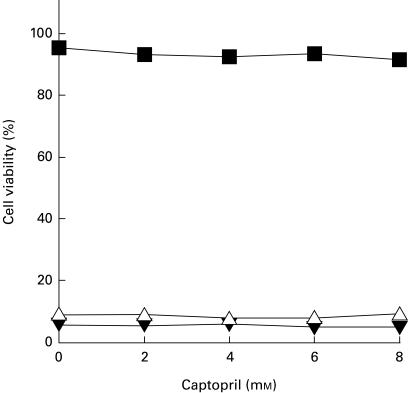
Jurkat cells constitutively express high levels of Fas molecules on their surface and readily die via apoptosis upon cross-linking with anti-Fas antibody [42]. Therefore, we used Jurkat cells to examine the effect of captopril on Fas-induced apoptosis. When Jurkat cells were treated with anti-Fas antibody, a high percentage of cell death was observed as assessed by trypan blue dye exclusion (Fig. 3). Captopril showed no or little toxicity when added alone at the concentrations tested. The addition of captopril that was able to block activation-induced apoptotic cell death in T cell hybridoma N3-6-71 cells did not exhibit any significant effects on anti-Fas antibody-induced apoptosis of Jurkat cells. In our studies, captopril failed to inhibit anti-Fas antibody-induced cell death, suggesting that the drug does not affect activation-induced apoptosis in T cell hybridomas by blocking Fas signalling leading to apoptosis.
Fig. 3.
Captopril does not inhibit Fas-mediated apoptosis in Jurkat cells. Jurkat cells were cultured in 24-well plates (5 × 105 cells/well), with or without captopril. Apoptosis was induced by the addition of anti-human Fas antibody, CH-11. Twenty-four hours later, cell viability was determined by trypan blue dye exclusion. Results are expressed as the mean ± s.d. of triplicate cultures. Error bars are less than symbol width when they are not apparent. ▪, Control; Δ, CH-11 (50 ng/ml); ▾, CH-11 (100 ng/ml).
Captopril prevents activation-induced Fas and FasL expression on T cell hybridomas
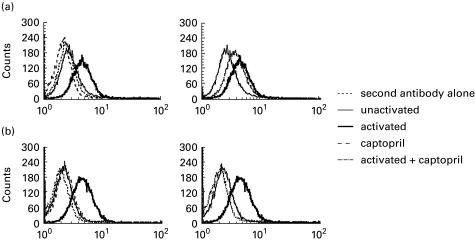
It has been demonstrated that activation-induced up-expression of Fas and FasL on T cell hybridomas leads to apoptotic cell death by Fas–FasL interaction [31–33]. We further examined whether captopril inhibits activation-induced cell death in N-3-6-71 cells by interfering with the expression of Fas, FasL, or both on their surface. The levels of Fas and FasL expression on inactivated N3-6-71 cells were similar to that on background staining with secondary reagents only, indicating that inactivated N3-6-71 cells did not express Fas and FasL molecules on their surface (Fig. 4a,b). Treatment of N-3-6-71 cells with captopril alone had no effect on basal levels of Fas and FasL expression. As expected, an increase in Fas expression on N-3-6-71 cells was observed after 5 h post-activation with anti-CD3 antibody (Fig. 4a). Cell surface Fas levels were lower on the cells activated with anti-CD3 antibody in the presence of 8 mm captopril than on the activated cells. This partial inhibition was consistently observed in a number of experiments. Thus, captopril, at the concentration that completely blocked activation-induced cell death, moderately blocked Fas expression on the cell surface of N3-6-71.
Fig. 4.
Effects of captopril on activation-induced cell surface (a) Fas and (b) its ligand (FasL) expression on T cell hybridomas. N3-6-71 cells were cultured in control 24-well plates or in anti-CD3-coated 24-well plates (5 × 105 cells/well), in the presence or absence of 8 mm captopril. After 5 h, the cells were stained with anti-Fas antibody (a) or anti-FasL antibody (b), and then stained with a secondary FITC-conjugated goat anti-hamster antibody. Flow cytometric analysis was performed using a FACScan. These results are representative of a number of independent experiments.
FasL was apparently absent on unactivated cells, and rapidly expressed after 5 h of activation with anti-CD3 antibody (Fig. 4b). The levels of FasL expressed on unactivated N3-6-71 cells and those that were activated with anti-CD3 antibody in the presence of 8 mm captopril were approximately equal. Thus, activation-induced FasL expression on N3-6-71 cells was significantly inhibited by captopril. These results suggest that the inhibitory effect of captopril on activation-induced apoptosis in T cell hybridomas is due to blocking Fas and FasL expression on their surface.
Effects of the other ACE inhibitors on activation-induced apoptosis in T cell hybridomas
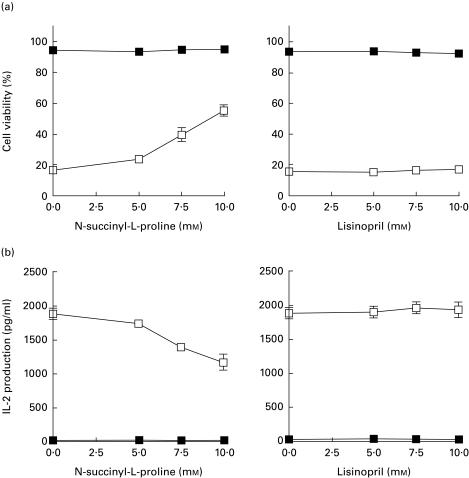
Since the action of captopril in activation-induced apoptosis may be related to the presence of thiol groups in its structure, it was interesting to examine whether non-thiol ACE inhibitors also prevent activation-induced apoptosis in T cell hybridoma N3-6-71 cells. N-succinyl-L-proline is known to be a specific inhibitor of ACE, although its activity is weaker than captopril [43]. Activation-induced cell death in T cell hybridomas was partially inhibited by the addition of N-succinyl-L-proline. Additionally, N-succinyl-L-proline partially inhibited IL-2 production from N3-6-71 cells after 24 h of activation with anti-CD3 antibody (Fig. 5a). On the other hand, lisinopril (1-carboxyl-3-phenylpropyl-L-lysyl-L-proline) [44] at concentrations ranging from 5 to 10 mm did not affect activation-induced apoptosis and IL-2 synthesis in N3-6-71 cells (Fig. 5b). These results indicate that T cell activation signals were interfered with partially by N-succinyl-L-proline, but not by lisinopril.
Fig. 5.
Influence of non-thiol angiotensin-converting enzyme (ACE) inhibitors on activation-induced cell death and IL-2 production in T cell hybridomas. (a) Cell viability. N3-6-71 cells were cultured in control 24-well plates or in anti-CD3 antibody-coated 24-well plates (5 × 105 cells/well), in the presence or absence of the indicated concentrations of N-succinyl-L-proline or lisinopril. After 50 μl of culture supernatant were removed from each well for the measurement of IL-2 at 24 h of cultivation, the cells were harvested and then cell viability was assessed by trypan blue dye exclusion. (b) IL-2 production. N3-6-71 cells were cultured in described above and concentrations of IL-2 in the culture supernatant were measured by ELISA. These results are expressed as the mean ± s.d. of triplicate cultures (a,b). ▪, Control; □, anti-CD3.
DISCUSSION
It has been reported that captopril inhibits proliferation of canine renal epithelial cells [12], human neuroblastoma cells [13], lung fibroblasts [14] and mammary ductal carcinoma cells [15]. Among patients on clinical trials, captopril has been shown to slightly lower the incidence of death due to cancer [45]. In addition, captopril is shown to slow the growth of experimental tumours [46,47]. In contrast to these reports, in our experiments, captopril did not affect the proliferation or cell viability of murine T cell hybridomas in 24-h cultivation. The drug also showed no or little toxicity in Jurkat cells when added alone at the concentrations tested.
In this study, we showed that captopril inhibited not only IL-2 production upon activation with anti-CD3 antibody, but also activation-induced apoptosis in murine T cell hybridoma N3-6-71 cells. In addition, captopril inhibited an enhancement of caspase-3-like activity during activation-induced apoptosis. It has been recently demonstrated that Fas-induced apoptosis in activated human peripheral T cells is inhibited by captopril or other thiol compounds but not by non-thiol anti-oxidants [26]. In addition, captopril is shown to inhibit Fas-induced apoptosis in human lung epithelial cell line [27]. Deas et al. [26] concluded that the inhibition of T cell apoptosis by captopril is the result of sulfhydryl redox regulation of critical molecules involved in the apoptosis signalling cascade. In contrast to these reports, we showed that captopril did not interfere with the signal transduction events leading from Fas ligation to the apoptotic process itself, because anti-Fas antibody-induced apoptosis in Fas+ Jurkat cells was unaffected by treatment with the drug, at the concentrations that completely blocked activation-induced apoptosis in T cell hybridomas. Therefore, the results of our experiments suggest that captopril does not affect activation-induced apoptosis in T cell hybridomas by blocking Fas signalling leading to apoptosis.
Fas and FasL were virtually absent on unactivated N-3-6-71 cells, but were rapidly induced after activation with anti-CD3 antibody. Our results strongly indicate that captopril blocked anti-CD3 antibody-induced FasL expression on T cells. Fas expression on their surface was partially affected by captopril, when analysed by flow cytometry. Since activation-induced apoptosis in T cell hybridomas is the result of interaction of de novo synthesized Fas and FasL, we conclude that captopril inhibits activation-induced cell death in murine T cell hybridomas predominantly by blocking Fas and FasL expression and thus preventing their interaction, leading to activation of the apoptosis program. The actions of captopril in T cell activation appear to be similar to those of the popular immunosuppressants cyclosporin A (CsA) or FK506. These drugs are well known to have inhibitory effects on IL-2 production as well as apoptotic cell death upon activation with TCR/CD3 cross-linking in T cell hybridomas [28,48]. The induction of FasL expression by activation with anti-CD3 antibody is completely inhibited in the presence of these drugs. However, activation-induced cell surface Fas expression on T cell hybridomas is partially inhibited by these immunosuppressive drugs. In contrast, anti-CD3 antibody-induced Fas mRNA expression is unaffected by CsA or FK506. Brunner et al. suggested the possibility that CsA or FK506 may interfere with the transport of synthesized Fas to the cell surface [48]. Although it seems that the signal transduction pathway leading to Fas expression is different from that of FasL, the mechanism by which Fas or FasL expression is regulated has not yet been clarified. Further studies are needed to elucidate the differential modulation of Fas and FasL expression by captopril.
Calcineurin plays a pivotal role in TCR/CD3-mediated signal transduction leading to the transcriptional activation of cytokines such as IL-2. CsA binds to cyclophilin and FK506 binds to FKBP upon entering T cells [49]. Thereafter, cyclophilin–CsA and FKBP–FK506 complexes independently associate with calcineurin and inhibit its protein phosphatase activity [50]. Calcineurin modulates the activity of several transcription factors that bind to the IL-2 promoter, including NF-AT, NF-κ B, and AP-1. The nuclear NF-AT has been shown to bind to the promoter/enhancer region of the FasL gene and increases its transcription [51]. It is likely that captopril also interferes with the common target of CsA and FK506, resulting in prevention of IL-2 production and apoptotic cell death upon T cell activation.
ACE has been identified as a membrane-bound enzyme in several types of cells, including lymphocytes and macrophages [52]. T lymphocytes contain high levels of ACE, approximately 28 times more per cell than monocytes. ACE is not expressed in B lymphocytes, or at a much lower level than in T lymphocytes. The biological functions of ACE in T lymphocytes remain largely unknown. ACE was highly expressed even in T cell hybridoma N-3-6-71, as examined by Western blot analysis (data not shown). ACE has two homologous active NH2- and COOH-terminal domains and displays activity toward a broad range of substrates. Captopril is a potent inhibitor of the NH2-terminal domain, whereas the reverse is observed for lisinopril [53]. Among non-thiol ACE inhibitors, lisinopril hardly affected activation-induced apoptosis and IL-2 production in T cell hybridomas. In contrast, N-succinyl-L-proline inhibited T cell activation signalling to some extent. Although the binding property of N-succinyl-L-proline against the two domains of ACE is unknown, N-succinyl-L-proline is likely to be a potent inhibitor of the NH2-terminal domain of ACE, because the thiol group of captopril was replaced by a carboxyl group in N-succinyl-L-proline and therefore the structure of N-succinyl-L-proline is similar to that of captopril. If so, the activity of ACE, especially the NH2-terminal active domain, may be to participate in T cell activation signals through TCR/CD3 complex, because captopril or N-succinyl-L-proline inhibited T cell activation signalling, and this hypothesis is now being tested. Although the inhibitory activity of captopril against ACE is thought to be stronger than that of N-succinyl-L-proline [43], the former exhibited strong inhibitory effects on T cell activation signalling compared with the latter in this study. The thiol group present on captopril is not only essential to its ability to inhibit Zn2+-dependent ACE, but also enables the drug to block other enzymes with transition metals at their active sites [19,20,54,55]. Therefore, we cannot exclude the possibility that inhibition of metal-dependent enzymes other than ACE might be responsible for the effects of captopril on T cell activation.
In conclusion, we showed that captopril blocks activation-induced apoptosis in T cell hybridomas by interfering with T cell activation signals, but the mechanism(s) underlying this action is presently unclear. It has been reported that captopril induces systemic lupus erythematosus syndrome [56–61]. T cell hybridomas have been used as a valuable model for negative selection in the thymus and for extrathymic deletion of T cells in the periphery. Captopril may interfere with clonal deletion and acquisition of self tolerance in vivo and cause autoimmunity by interfering with this process.
Acknowledgments
We are grateful to Dr H. Yagita for providing us with the antibody. We thank Ms Y. Nakano (Department of Immunology, National Institute of Infectious Diseases) for her technical assistance in FACS analysis.
REFERENCES
- 1.Cushman DW, Cheung HS, Sabo E, et al. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry. 1977;16:5484–91. doi: 10.1021/bi00644a014. [DOI] [PubMed] [Google Scholar]
- 2.Migdalof BH, Antonaccio MJ, McKinstry DN, et al. Captopril: pharmacology, metabolism and disposition. Drug Metab Rev. 1984;15:841–69. doi: 10.3109/03602538409041080. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe I. Angiotensin converting inhibitors in rheumatoid arthritis. Arthritis Rheum. 1984;27:840. doi: 10.1002/art.1780270724. [DOI] [PubMed] [Google Scholar]
- 4.Martin MF, Surrall KE, McKenna F, et al. Captopril: a new treatment for rheumatoid arthritis? Lancet. 1984;1:1325–8. doi: 10.1016/s0140-6736(84)91821-x. [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 6.Weinstock JV, Ehrinpreis MN, Boros DL, et al. Effect of SQ 14225, an inhibitor of angiotensin I-converting enzyme, on the granulomatous response to Schistosoma mansoni eggs in mice. J Clin Invest. 1981;67:931–6. doi: 10.1172/JCI110142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herlitz H, Tarkowski A, Svalander C, et al. Beneficial effect of captopril on systemic lupus erythematosus-like disease in MRL lpr/lpr mice. Int Arch Allergy Appl Immunol. 1988;85:272–7. doi: 10.1159/000234517. [DOI] [PubMed] [Google Scholar]
- 8.Constantinescu CS, Ventura E, Hilliard B, et al. Effects of the angiotensin converting enzyme inhibitor captopril on experimental autoimmune encephalomyelitis. Immunopharmacol Immunotoxicol. 1995;17:471–91. doi: 10.3109/08923979509016382. [DOI] [PubMed] [Google Scholar]
- 9.Schindler R, Dinarello CA, Koch KM. Angiotensin-converting-enzyme inhibitors suppress synthesis of tumour necrosis factor and interleukin 1 by human peripheral blood mononuclear cells. Cytokine. 1995;7:526–33. doi: 10.1006/cyto.1995.0071. [DOI] [PubMed] [Google Scholar]
- 10.Fukuzawa M, Satoh J, Sagara M, et al. Angiotensin converting enzyme inhibitors suppress production of tumor necrosis factor-alpha in vitro and in vivo. Immunopharmacology. 1997;36:49–55. doi: 10.1016/s0162-3109(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 11.Constantinescu CS, Goodman DB, Ventura ES. Captopril and lisinopril suppress production of interleukin-12 by human peripheral blood mononuclear cells. Immunol Letters. 1998;62:25–31. doi: 10.1016/s0165-2478(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 12.Bauer H, Lametschwandtner A, Steiner M, et al. Influence of angiotensin converting enzyme inhibitor (captopril) on kidney epithelial cells in vitro: studies on potassium (86Rb) influx and cellular proliferation. Clin Chim Acta. 1990;187:47–53. doi: 10.1016/0009-8981(90)90260-y. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Re RN, Prakash O, et al. Angiotensin-converting enzyme inhibition reduces neuroblastoma cell growth rate. Proc Soc Exp Biol Med. 1991;196:280–3. doi: 10.3181/00379727-196-43189. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen L, Ward WF, Ts'ao CH, et al. Captopril inhibits proliferation of human lung fibroblasts in culture: a potential antifibrotic mechanism. Proc Soc Exp Biol Med. 1994;205:80–84. doi: 10.3181/00379727-205-43681. [DOI] [PubMed] [Google Scholar]
- 15.Small W, Jr, Molteni A, Kim YT, et al. Captopril modulates hormone receptor concentration and inhibits proliferation of human mammary ductal carcinoma cells in culture. Breast Cancer Res Treat. 1997;44:217–24. doi: 10.1023/a:1005827119296. [DOI] [PubMed] [Google Scholar]
- 16.Roberts NA, Robinson PA. Copper chelates of antirheumatic and anti- inflammatory agents: their superoxide dismutase-like activity and stability. Br J Rheumatol. 1985;24:128–36. doi: 10.1093/rheumatology/24.2.128. [DOI] [PubMed] [Google Scholar]
- 17.Jay D, Cuella A, Jay E, et al. Superoxide dismutase activity of the captopril-iron complex. Mol Cell Biochem. 1995;146:45–47. doi: 10.1007/BF00926880. [DOI] [PubMed] [Google Scholar]
- 18.Jay D. Captopril and glutathione inhibit the superoxide dismutase activity of Hg (II) Arch Inst Cardiol Mex. 1998;68:457–61. [PubMed] [Google Scholar]
- 19.Sorbi D, Fadly M, Hicks R, et al. Captopril inhibits the 72 kDa and 92 kDa matrix metalloproteinases. Kidney Int. 1993;44:1266–72. doi: 10.1038/ki.1993.378. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa T, Kubota T, Kabuto M, et al. Captopril inhibits glioma cell invasion in vitro: involvement of matrix metalloproteinases. Anticancer Res. 1995;15:1985–9. [PubMed] [Google Scholar]
- 21.Johnsen SA, Aurell M. Immunosuppressive action of captopril blocked by prostaglandin synthetase inhibitor. Lancet. 1981;1:1005. doi: 10.1016/s0140-6736(81)91777-3. [DOI] [PubMed] [Google Scholar]
- 22.Linz W, Wiemer G, Gohlke P, et al. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- 23.Johnsen SA, Persson IB, Aurell M, et al. PGE2 production after angiotensin-converting enzyme inhibition. Scand J Urol Nephrol. 1997;31:81–88. doi: 10.3109/00365599709070307. [DOI] [PubMed] [Google Scholar]
- 24.Ward WF, Kim YT, Molteni A, et al. Radiation-induced pulmonary endothelial dysfunction in rats: modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. 1988;15:135–40. doi: 10.1016/0360-3016(88)90357-4. [DOI] [PubMed] [Google Scholar]
- 25.Reddy MK, Baskaran K, Molteni A, et al. Inhibitors of angiotensin-converting enzyme modulate mitosis and gene expression in pancreatic cancer cells. Proc Soc Exp Biol Med. 1995;210:221–6. doi: 10.3181/00379727-210-43942. [DOI] [PubMed] [Google Scholar]
- 26.Deas O, Dumont C, Mollereau B, et al. Thiol-mediated inhibition of FAS and CD2 apoptotic signaling in activated human peripheral T cells. Int Immunol. 1997;9:117–25. doi: 10.1093/intimm/9.1.117. [DOI] [PubMed] [Google Scholar]
- 27.Uhal BD, Gidea C, Bargout R, et al. Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am J Physiol. 1998;275:L1013–7. doi: 10.1152/ajplung.1998.275.5.L1013. [DOI] [PubMed] [Google Scholar]
- 28.Shi YF, Sahai BM, Green DR. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989;339:625–6. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- 29.Ucker DS, Ashwell JD, Nickas G. Activation-driven T cell death. I. Requirements for de novo transcription and translation and association with genome fragmentation. J Immunol. 1989;143:3461–9. [PubMed] [Google Scholar]
- 30.Odaka C, Kizaki H, Tadakuma T. T cell receptor-mediated DNA fragmentation and cell death in T cell hybridomas. J Immunol. 1990;144:2096–101. [PubMed] [Google Scholar]
- 31.Brunner T, Mogil RJ, LaFace D, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–4. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 32.Dhein J, Walczak H, Baumler C, et al. Autocrine T-cell suicide mediated by APO-1/ (Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 33.Ju ST, Panka DJ, Cui H, et al. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 34.Chow SC, McConkey DJ, Orrenius S, et al. Quantitation of DNA fragmentation using fiberglass filters. Anal Biochem. 1989;183:42–45. doi: 10.1016/0003-2697(89)90168-1. [DOI] [PubMed] [Google Scholar]
- 35.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Vacchio MS, Ashwell JD. 9-cis-retinoic acid inhibits activation-driven T-cell apoptosis: implications for retinoid X receptor involvement in thymocyte development. Proc Natl Acad Sci USA. 1993;90:6170–4. doi: 10.1073/pnas.90.13.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alnemri ES, Livingston DJ, Nicholson DW, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 38.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 39.Alam A, Braun MY, Hartgers F, et al. Specific activation of the cysteine protease CPP32 during the negative selection of T cells in the thymus. J Exp Med. 1997;186:1503–12. doi: 10.1084/jem.186.9.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 41.Thornberry NA, Rano TA, Peterson EP, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–11. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb RA, Nordberg J, Skowronski E, et al. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc Natl Acad Sci USA. 1996;93:654–8. doi: 10.1073/pnas.93.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ondetti MA, Rubin B, Cushman DW. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977;196:441–4. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 44.Patchett AA, Harris E, Tristram EW, et al. A new class of angiotensin-converting enzyme inhibitors. Nature. 1980;288:280–3. doi: 10.1038/288280a0. [DOI] [PubMed] [Google Scholar]
- 45.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. Engl J Med. 1992;327:669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 46.Volpert OV, Ward WF, Lingen MW, et al. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J Clin Invest. 1996;98:671–9. doi: 10.1172/JCI118838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hii SI, Nicol DL, Gotley DC, et al. Captopril inhibits tumour growth in a xenograft model of human renal cell carcinoma. Br J Cancer. 1998;77:880–3. doi: 10.1038/bjc.1998.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunner T, Yoo NJ, LaFace D, et al. Activation-induced cell death in murine T cell hybridomas. Differential regulation of Fas (CD95) versus Fas ligand expression by cyclosporin A and FK506. Int Immunol. 1996;8:1017–26. doi: 10.1093/intimm/8.7.1017. [DOI] [PubMed] [Google Scholar]
- 49.Friedman J, Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell. 1991;66:799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Farmer J, Jr, Lane WS, et al. Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP–FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 51.Holtz-Heppelmann CJ, Algeciras A, Badley A, et al. Transcriptional regulation of the human FasL promoter-enhancer region. J Biol Chem. 1998;273:4416–23. doi: 10.1074/jbc.273.8.4416. [DOI] [PubMed] [Google Scholar]
- 52.Costerousse O, Allegrini J, Lopez MA, et al. Angiotensin I-converting enzyme in human circulating mononuclear cells: genetic polymorphism of expression in T-lymphocytes. Biochem J. 1993;290:33–40. doi: 10.1042/bj2900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei L, Clauser E, Alhenc-Gelas F, et al. The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J Biol Chem. 1992;267:13398–405. [PubMed] [Google Scholar]
- 54.Palatini P, Dabbeni-Sala F, Finotti P. Inhibition of dopamine beta-hydroxylase by captopril. Biochem Pharmacol. 1989;38:1011–3. doi: 10.1016/0006-2952(89)90293-1. [DOI] [PubMed] [Google Scholar]
- 55.Orning L, Krivi G, Bild G, et al. Inhibition of leukotriene A4 hydrolase/aminopeptidase by captopril. J Biol Chem. 1991;266:16507–11. [PubMed] [Google Scholar]
- 56.Kale SA. Drug-induced systemic lupus erythematosus. Differentiating it from the real thing. Postgrad Med. 1985;77:231–5. doi: 10.1080/00325481.1985.11698908. 238–9, 242. [DOI] [PubMed] [Google Scholar]
- 57.Hill GS. Drug-associated glomerulopathies. Toxicol Pathol. 1986;14:37–44. doi: 10.1177/019262338601400105. [DOI] [PubMed] [Google Scholar]
- 58.Patri P, Nigro A, Rebora A. Lupus erythematosus-like eruption from captopril. Acta Derm Venereol. 1985;65:447–8. [PubMed] [Google Scholar]
- 59.Sieber C, Grimm E, Follath F. Captopril and systemic lupus erythematosus syndrome. BMJ. 1990;301:669. doi: 10.1136/bmj.301.6753.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pelayo M, Vargas V, Gonzales A, et al. Drug-induced lupus-like reaction and captopril. Ann Pharmacother. 1993;27:1541–2. doi: 10.1177/106002809302701226. [DOI] [PubMed] [Google Scholar]
- 61.Bertin P, Kamdem J, Bonnet C, et al. Captopril-induced lupus. Clin Exp Rheumatol. 1993;11:695. [PubMed] [Google Scholar]