Abstract
Whilst animal studies and a pilot clinical trial suggest that intravitreal triamcinolone acetonide (TA) may be useful in the treatment of age-related macular degeneration (AMD), its mode of action remains to be fully elucidated. The present study has investigated the capacity of TA to modulate the expression of adhesion molecules and permeability using a human epithelial cell line (ECV304) as a model of the outer blood–retinal barrier (BRB). The influence of TA on the expression of ICAM-1 and MHC-I was studied on resting and phorbol myristate acetate (PMA)- or interferon-gamma (IFN-γ)- and/or tumour necrosis factor-alpha (TNF-α)-activated cells using flow cytometry and immunocytochemistry. Additionally, ECV304 cells were grown to confluence in uncoated Transwell chambers; transepithelial resistance (TER) across resting and PMA-activated cells was monitored. TA significantly decreased the paracellular permeability of ECV304 cells and down-regulated ICAM-1 expression, consistent with immunocytochemical observations. PMA-induced permeability changes were dose-dependent and TA decreased permeability of both resting and PMA-activated monolayers. MHC-I expression by ECV304 cells however, was not significantly affected by TA treatment. The modulation of TER and ICAM-1 expression in vitro correlate with clinical observations, suggesting re-establishment of the BRB and down-regulation of inflammatory markers are the principal effects of intravitreal TA in vivo. The results further indicate that TA has the potential to influence cellular permeability, including the barrier function of the retinal pigment epithelium (RPE) in AMD-affected retinae.
Keywords: blood–retinal barrier, macular degeneration, corticosteroids, ICAM-1, epithelium
INTRODUCTION
The aetiology of age-related macular degeneration (AMD) is not established, although a number of studies have reported that the pathogenesis of AMD involves both cell-mediated [1–3] and humoral immunity [4,5]. The efficacy of the anti-inflammatory corticosteroid triamcinolone acetonide (TA) in the treatment of exudative AMD is currently being examined in a prospective randomized clinical trial [6]. Previously we have reported the findings of an open label phase II study, in which intravitreal TA was used to treat subfoveal neovascular membranes, indicating that inhibition of new blood vessel growth and exudation occurred in a significant number of cases [6,7].
Subretinal neovascularization disrupts the outer blood–retinal barrier (BRB) resulting in exudation into the subretinal space and subsequent loss of photoreceptor function. The BRB exists at two principal sites: an inner barrier consisting of retinal vascular endothelial cells and an outer barrier consisting of the retinal pigment epithelium (RPE), in both of which adjacent cells are joined by adherens and occludens junctions. The presence of zonulae occludens (ZO) between adjacent endothelial and RPE cells controls the paracellular passage of metabolites between the neuronal environment and the vasculature, while specific transport systems operate to control the movement of metabolites via the transcellular route [8]. ICAM-1 is constitutively expressed on RPE and choroidal vascular endothelial cell surfaces and is an important component of cell–cell interactions during inflammatory responses, mediating leucocyte adhesion and extravasation [9,10]. It has been suggested that the RPE uses the same principal receptor–ligand pairings to mediate leucocyte traffic as the vascular endothelium of the anterior BRB [11]. Models of the outer BRB have been established to investigate cellular permeability and adhesion molecule expression by the RPE [12], and one study of a model of the inner BRB has examined the effects of hydrocortisone on the formation of barrier properties by cultured vascular endothelial cells [13]. The present study has employed a human cell line with an epithelial phenotype (ECV304) [14] as a model of the outer BRB, to investigate the capacity of TA to modulate cellular permeability and adhesion molecule expression.
MATERIALS AND METHODS
Cell culture
ECV304 cells
A human bladder carcinoma-derived epithelial cell line (ECV304; European Collection of Cell Cultures, Salisbury, UK) was maintained in Dulbecco's modified Eagles' medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Trace Biosciences, Sydney, Australia), 50 mg/ml each of penicillin and streptomycin, and 2 mm glutamine (Trace Biosciences) in a humidified atmosphere of 5% CO2 at 37°C. Cells were subcultured once per week (0·05% trypsin, 0·02% EDTA for 3 min at 37°C) and passages 4–15 were used in the experiments.
Antibodies
For flow cytometry (FCM) the following antibodies were used: mouse anti-human ICAM-1 (CD54; Biodesign International, Saco, ME); mouse anti-human HLA-ABC (Dako Pty Ltd, Sydney, Australia); rabbit anti-human ZO-1 (Zymed, San Francisco, CA, USA) and mouse IgG1 or rabbit IgG (isotype controls) (Dako Pty Ltd). Anti-human CD54 (clone no. 15.2) is directed against the 85–115-kD glycoprotein (ICAM-1). ICAM-1 is a membrane glycoprotein which mediates antigen-independent adhesion of leucocytes via interaction with its ligand LFA-1. ZO-1 is a membrane protein which is a constituent of tight junctions, zonulae occludens, which control the permeability of epithelial and endothelial cell monolayers.
Sheep anti-mouse immunoglobulin F(ab′)2 fraction FITC (Silenus Laboratories Pty Ltd, Sydney, Australia) was used as the secondary antibody for FCM. Biotinylated sheep anti-mouse immunoglobulin and/or donkey anti-rabbit immunoglobulin (Amersham Pty Ltd, Sydney, Australia) were used as secondary antibodies for immunocytochemistry. All antibodies were titrated for FCM or immunolabelling before experimental use and the minimum concentration for saturation labelling was chosen.
Reagents
Phorbol 12-myristate 13-acetate (PMA; Sigma, Sydney, Australia) was dissolved in dimethyl sulphoxide (DMSO; Sigma) as a 6 × 10−2 m stock solution; TA (Sigma) was dissolved in methanol (Biolab Scientific Pty Ltd, Sydney, Australia) as a 10−2 m stock solution. Optimal dose and time responses were established by FCM. For the flow cytometry experiments, a final concentration of 10−6 m and 24 h stimulation with PMA, and 10−6 m and 48 h treatment with TA were used.
Flow cytometry
Cells were grown in 25-cm2 flasks overnight, then 10 ml fresh culture medium were added with the following treatments: control untreated; PMA 10−6 m for 72 h; TA 10−6 m for 48 h; PMA 10−6 m for 24 h, and then TA 10−6 m for 48 h; diluent control (DMSO + methanol). In order to compare the effects of PMA with other proinflammatory cytokines, interferon-gamma (IFN-γ) and/or tumour necrosis factor-alpha (TNF-α), ECV304 cells were treated for 48 h with either IFN-γ (200 U/ml), PMA (10−6 m) and TNF-α (200 U/ml) or with both IFN-γ and TNF-α.
FCM labelling
Following incubation, cultures were washed twice with PBS, then detached from the flasks with 0·05% trypsin/0·02% EDTA for 3 min at 37 °C. This treatment has been reported to not alter the serological detectability of MHC class I [15] and ICAM-1 [16]. Cells (5 × 105) were pelleted by centrifugation at 463 g for 3 min at 4°C, and then resuspended with 100 μl of primary antibody at 4°C. After 1 h incubation, cells were washed through 100 μL FBS by centrifugation (463 g, 3 min, 4°C), and resuspended in 100 μl of FITC-conjugated antibody for 45 min at 4°C. Cells were then centrifuged through FBS as above, and resuspended in 300 μl DMEM/FBS for FCM.
Flow cytometry analysis
Fluorescence between 515 nm and 545 nm was measured using a FACScan (Becton Dickinson, Sydney, Australia) with an argon–ion laser set at an emission of 488 nm for excitation of FITC. Forward and side scatter measurements were within the same range for all populations, and at least 104 events were collected for each sample. Data analysis was performed with the CellQuest program (Becton Dickinson) and results presented either as histograms or bar graphs. Histograms show results from individual experiments, and express number of events versus log10 fluorescence intensity. Bar graphs show average normalized data (n = 3) for peak channel fluorescence which is a quantitative measure of relative cell surface molecule expression.
Transepithelial resistance
ECV304 cells (3·5 × 104) were plated onto uncoated polycarbonate membranes (3 μm pore size) in Transwell inserts (Costar, Cambridge, MA) in a 150-μl volume of culture medium, and 700 μl of culture medium were added to each well. The medium was changed on the following day, and subsequently changed every second day for the duration of the experiment. Electrical resistance was measured from day 3 using a Millipore ERS resistance meter (Millipore, NSW, Australia) as previously described [17].
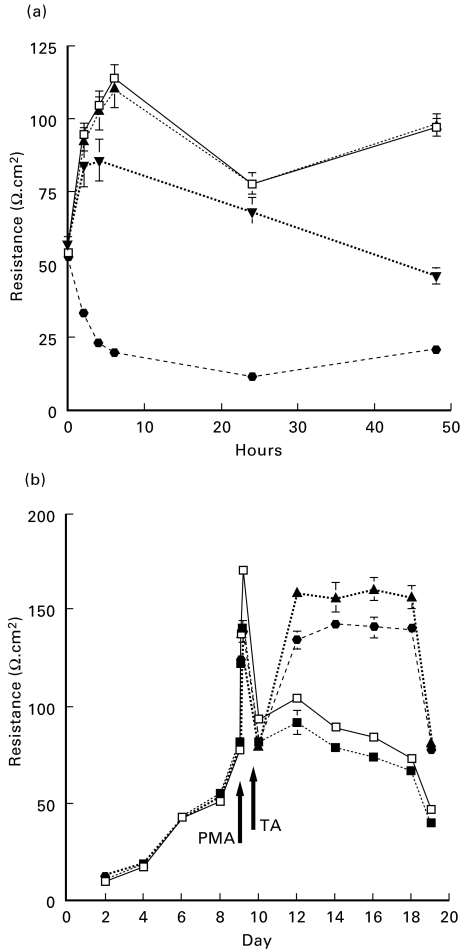
Time–response curves to a range of PMA doses were investigated to establish a threshold effect, using 10−9 m, 5 × 10−9 m and 5 × 10−10 m concentrations.
Treatments of cultured cells were carried out at day 9 after the transepithelial resistance (TER) stabilized. Cells were treated as follows: either with PMA (10−9 m) for 4 h and subsequently DMEM or TA (10−6 m) continuing throughout the experimental period (up to day 19); or TA (10−6 m) alone; to control for the effects of DMSO and methanol, a group of wells was treated with the same concentration of DMSO and methanol alone as the PMA- and TA-treated groups.
The electrical resistances of monolayers were calculated as the average resistance of the different groups, minus the average resistance reading from the background control (membrane only) and then multiplied by the effective growing area (0·33 cm2). Each data point represents the mean of electrical resistance from an individual experiment (n = 5 Transwells) ± s.e.m. (Fig. 1). All experiments were repeated four times.
Fig. 1. (a).
Time–response curves to a range of phorbol myristate acetate (PMA) doses established a threshold effect, using 10−9 m PMA (▾). Four hours post PMA treatment was determined to be optimum to produce a significant differential effect over dimethyl sulphoxide (DMSO)-treated ECV304 monolayers (□) versus PMA @5 × 10−9 m (•) or 5 × 10−10 m (▴) (P = 0·025). (b) Comparison of the resistances of the triamcinolone acetonide (TA)-treated cells (▴) with control cultures (□) showed that the transepithelial resistance (TER) was markedly higher in TA-treated cultures from day 12 to the conclusion of the experiment. Treatment with 10−9 m PMA at day 9 resulted in reduced resistance from 4 h post-treatment (▪). Additionally, treatment with 10−9 m PMA followed by TA (10−6 m) resulted in a significant increase in resistance compared with PMA-treated cultures (•). Each data point represents the mean TER of five Transwells (n = 5) ± s.e.m.
Immunocytochemistry
In parallel with the flow cytometry experiments, 5 × 104 cells (ECV304) were seeded onto coverslips and immunolabelled using ICAM-1 and MHC-I antibodies. Additional coverslips were seeded and grown to confluence for ZO-1 immunolabelling. The coverslips were then fixed in cold acetone for 20 min, washed in PBS, and blocked in 10% serum prior to the primary antibody incubation. The serum used was either sheep or donkey, depending on the secondary antibody. Coverslips were then incubated at 4°C for 1 h with anti-ICAM-1, anti-MHC-I, anti-ZO-1 or negative control (mouse IgG1 or for ZO-1 rabbit IgG), followed by incubation in biotinylated secondary antibody for 30 min. Bound antibody was detected with streptavidin-fluorescein (Zymed) labelling and the specimens examined by fluorescence microscopy.
Electron microscopy
Control Transwell specimens were fixed in 2·5% glutaraldehyde in 0·1 m sodium cacodylate buffer pH 7·4 and postfixed in 2% osmium tetroxide, block stained in uranyl acetate, dehydrated through a series of alcohols and acetone, embedded in Epon-Araldite resin and cured at 60°C. Ultrathin sections were stained with uranyl acetate and lead citrate and examined at 75 kV with an Hitachi 7100FA transmission electron microscope (EM).
Statistical analysis
Results are expressed as mean ± s.e.m., and a two-tailed unpaired Student's t-test was used. Readings with P < 0·05 were considered significant.
RESULTS
Transepithelial resistance
Time–response curves to a range of PMA doses established a threshold effect, using 10−9 m PMA. Four hours post PMA treatment was determined to be optimum to produce a significant differential effect over untreated ECV304 monolayers (P = 0·025) (Fig. 1a). At this concentration of PMA the TER was reduced relative to untreated monolayers, though resistance could still be increased by TA treatment.
The TER of all ECV304 cell cultures reached a peak approximately 9 days after seeding (Fig. 1b). Comparison of the resistances of the TA-treated cells with control cultures showed that the TER was markedly and significantly higher in TA-treated cultures from day 12 to the conclusion of the experiment. Significant increases in TER (ranging from P < 0·0001 to P < 0·01) occurred 3 days after TA treatment (between days 12 and 19). Treatment with 10−9 m PMA at day 9 resulted in reduced resistance from 4 h post-treatment until the conclusion of the experiment. Additionally, treatment with 10−9 m PMA followed by TA (10−6 m) resulted in a significant increase in resistance compared with PMA-treated cultures (ranging from P < 0·0002 to P < 0·01) on day 3 which was preserved until the conclusion of the experiment. Figure 1b illustrates a typical experiment; similar results were obtained in three separate experiments.
Flow cytometry analysis
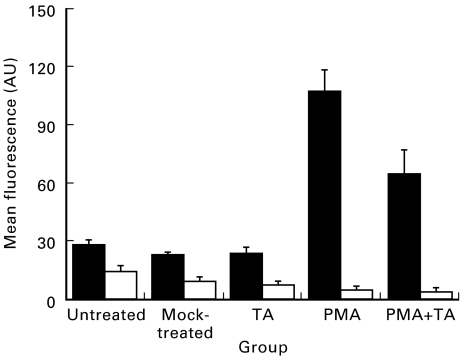
Unstimulated ECV304 cells constitutively expressed moderate levels of ICAM-1 (25 arbitrary units (AU)) and MHC-I (15 AU) compared with the isotype antibody controls (Fig. 2). Similar levels of expression were detectable in cells treated with DMSO/methanol or TA alone (Fig. 2). PMA-activated cells had a significantly (P = 0·0003) up-regulated ICAM-1 expression (110 AU) after 72 h in culture, an approximately four-fold increase over unstimulated levels of expression (Figs 2 and 3a). After 24 h exposure to PMA, cells were additionally exposed to TA (10−6 m) for a further 48 h; treatment of PMA-stimulated cells with TA significantly reduced levels of ICAM-1 expression (70 AU) (PMA versus PMA + TA, P = 0·045) (Fig. 2). MHC-I levels were, conversely, not significantly modulated by either TA (8 AU) or PMA (6 AU) treatment (Figs 2 and 3b). After 24 h initial exposure to PMA, cells were additionally exposed to TA 10−6 m for a further 48 h; treatment of PMA-stimulated cells with TA did not significantly modulate levels of MHC-I expression (5 AU) (Figs 2 and 3b).
Fig. 2.
Unstimulated ECV304 cells constitutively expressed moderate levels of ICAM-1 (▪) and MHC-I (□) compared with the isotype antibody controls. Similar levels of expression were detectable in cells treated with dimethyl sulphoxide (DMSO)/methanol or triamcinolone acetonide (TA) alone. Phorbol myristate acetate (PMA)-activated cells had significantly (P = 0·0003) up-regulated ICAM-1 expression after 72 h in culture, an approximately four-fold increase over unstimulated levels of expression. After 24 h initial exposure to PMA cells were additionally exposed to TA (10−6 m) for a further 48 h; treatment of PMA-stimulated cells with TA significantly reduced detectable levels of ICAM-1 expression (PMA versus PMA + TA, P = 0·045). AU, Arbitrary units. MHC-I levels were not significantly modulated by either TA or PMA treatment. Similarly, after 24 h initial exposure to PMA cells were additionally exposed to TA (10−6 m) for a further 48 h; treatment of PMA-stimulated cells with TA did not significantly modulate levels of MHC-I expression.
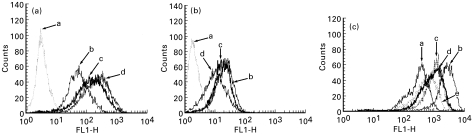
Fig. 3.
Representative FACS histograms showing ECV304 expression of (a) ICAM-1 and (b) MHC-I for: a, IgG control; b, mock-treated; c, phorbol myristate acetate (PMA) + triamcinolone acetonide (TA); and d, PMA. (c) ICAM-1 for: a, mock-treated; b, IFN-γ and tumour necrosis factor-alpha (TNF-α); c, IFN-γ; d, PMA; and e, TNF-α. PMA treatment significantly up-regulated ICAM-1 expression while subsequent treatment with TA significantly reduced ICAM-1 expression. Treatment of PMA-stimulated cells with TA did not significantly modulate levels of MHC-I expression. PMA had a similar effect to other proinflammatory cytokines; IFN-γ produced equivalent levels of ICAM-modulation to PMA, while TNF-α produced less modulation than PMA. IFN-γ and TNF-α in combination produced most pronounced up-modulation of ICAM-1 expression.
The proinflammatory cytokine IFN-γ produced equivalent levels of ICAM-modulation to PMA, while TNF-α produced less modulation than PMA. IFN-γ and TNF-α in combination produced the most pronounced up-modulation of ICAM-1 expression (Fig. 3c).
Morphology
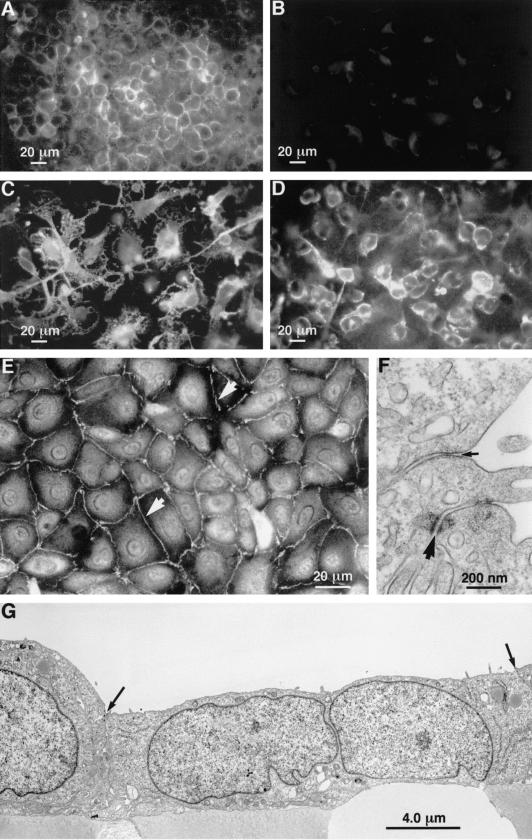
ECV304 cells grown on coverslips showed cell membrane localized ICAM-1 immunostaining (Fig. 4A). Staining with isotype control (non-immune immunoglobulin) indicated insignificant levels of non-specific binding to ECV304 cells (Fig. 4B). PMA stimulation promoted hypertrophy and the formation of long processes in ECV304 cells associated with more intense ICAM-1 expression at the plasma membrane (Fig. 4C). Subsequent treatment with TA resulted in condensation of the ECV304 cell morphology associated with smaller cell size and reductions in processes; the intensity of ICAM-1 expression at the plasma membrane was also visibly diminished (Fig. 4D).
Fig. 4.
(A) On coverslips, resting ECV304 cells showed cell membrane-localized ICAM-1 immunostaining. (B) On coverslips staining with isotype control (non-immune immunoglobulin) revealed insignificant levels of non-specific binding to ECV304 cells. (C) On coverslips, phorbol myristate acetate (PMA) stimulation promoted hypertrophy and the formation of long processes in ECV304 cells was associated with more intense ICAM-1 expression at the plasma membrane. (D) On coverslips, subsequent treatment of PMA-stimulated cells with triamcinolone acetonide (TA) resulted in condensation of the ECV304 cell morphology associated with smaller cell size and reductions in processes. (E) On coverslips, ZO-1 immunofluorescent staining revealed the ECV304 cells to form an integrated monolayer with high levels of immunoreactivity expressed at the cell margins and occasional punctate accumulations of staining (arrowheads). (F) A high power electron micrograph showing details of a zonule occludentes junction (small arrow) and a maculae adherens (large arrow). (G) An electron micrograph illustrating that ECV304 cells form a continuous layer with apposing cells joined by both desmosomes (maculae adherens) and tight junctions (ZO). Both types of junction occurred at the apices of the cells (arrows indicate cell boundaries).
ZO-1 immunostaining of coverslips revealed the ECV cells to form an integrated monolayer (Fig. 4E) with high levels of immunoreactivity expressed at the cell margins with occasional punctate accumulations of staining.
At the ultrastructural level ECV304 cells in Transwells appeared as a continuous layer (Fig. 4G) with apposing cells joined by both desmosomes (maculae adherens) and tight junction-like structures (ZO) (Fig. 4F). Both types of junction occurred at the apices of the cells, with ZO junctions occurring closer to the apices than desmosomes (Fig. 4F,G).
DISCUSSION
Intravitreal administration of corticosteroids, including TA, has been shown to be effective in reducing the incidence of experimentally induced neovascularization in monkeys [18], rabbits [19,20] and pigs [21]. Corticosteroids are commonly used to treat inflammatory eye diseases [22–24] and their therapeutic potential for the treatment of exudative AMD, via intravitreal administration, is currently being examined [6,7,25]. Although this class of drugs is known to display differential capacities to mediate anti-inflammatory and permeability effects, the particular mode of action of TA has not been defined. The present study employed a human cell line with an epithelial phenotype (ECV304)14 to investigate the capacity of TA to modulate cellular permeability and adhesion molecule expression.
The ECV304 cell line was originally suggested to be of endothelial origin and has been employed in many laboratories as a model of human vascular endothelium [26]. Recently, it has been reported that the ECV304 line has identical DNA fingerprint profiles to the T24 epithelial carcinoma cell line [27]. It has been known for some time that ECV304 lacks a number of characteristics of vascular endothelial cells, including VCAM-1, E-selectin [26], factor VIII (von Willebrand's factor) and cadherin-5, whilst expressing epithelial cell proteins including E-cadherin and desmoplakin antigens [14]. Furthermore, ECV304 cells express a number of phenotypic characteristics in common with the human RPE line (D407) including cytokeratin expression, growth characteristics and morphology [28]. ECV304 cells are also reported to express a number of features in common with primary isolates of human RPE cells, including the expression of glucocorticoid receptors [29,30] and ICAM-1 [9,26]. The ECV304 line may now be considered as a potential model of epithelial and RPE cell permeability.
The rationale for the use of anti-inflammatory corticosteroids has been derived from numerous observations of the involvement of both humoral and cell-mediated immunity in the pathogenesis of AMD. Early pathological changes in AMD-affected retinae have been described and related to immunological responses in retinal microglia and vascular elements [3]. Further, the presence of anti-retinal autoantibodies in sera from patients with early AMD has been reported [4,5]. Both the atrophic [31] and neovascular end stages [32] of AMD involve cell-mediated inflammation, and leucocyte sticking and extravasation have been observed ultrastructurally [1]. Chronic inflammatory cells have been reported in surgically excised subfoveal neovascular membranes [2] and it has been recently suggested that IL-1β and TNF-α secreted by macrophages may promote choroidal neovascularization [33]. Glucocorticoids also suppress the activity of monocytes and macrophages, contributing to the efficacy of glucocorticoids in inflammatory diseases [34]. In situ histopathological analyses of fellow eyes showed diminished exudation and microglial/macrophage numbers associated with intravitreal TA administration [7].
The RPE apparently uses the same principal receptor–ligand pairings to mediate leucocyte traffic as the vascular endothelium of the inner BRB [11,35]. Adhesion molecule expression, including ICAM-1, has been described in excised subretinal disciform lesions in association with inflammatory cells [36]. Further, soluble factors from reactive microglia may enhance the expression of ICAM-1 on vascular endothelial cells [37] and we have recently reported microglial activation related to the pathogenesis of AMD [3].
PMA has the potential to mimic the effects of proinflammatory cytokines by inducing expression of ICAM-1 and MHC-I molecules on human endothelial cells [38]. Previously, human vascular endothelial, epithelial and epithelial/endothelial hybrid cell lines have been employed to investigate the modulation of ICAM-1 by dexamethasone [26,30]. The present study has investigated the effects of TA on constitutive and PMA-modulated expression of ICAM-1 and MHC-I antigens on ECV304 cells using flow cytometry. The results demonstrate that PMA-induced expression of ICAM-1 on ECV304 cells is significantly reduced by TA treatment, suggesting that TA has the potential to down-regulate ICAM-1 expression in vivo. Our findings show that IFN-γ results in equivalent levels of ICAM-1 modulation on ECV304 cells compared with PMA treatment. TNF-α produced less modulation than PMA while IFN-γ and TNF-α in combination produced more pronounced up-modulation of ICAM-1 expression. However, MHC-I levels were not significantly modulated by either TA or PMA treatment, consistent with a previous report that MHC antigens may be up-regulated independently of ICAM-1 biosynthesis [39].
The present study indicates that TA has the capacity to decrease the permeability of the ECV304 cell line, both prior to and subsequent to PMA-mediated permeability increases. One earlier study has reported that dexamethasone regulates tight junction permeability of cultured 31EG4 mammary epithelial cells, resulting in increased TER and ZO-1 protein expression [40]. There appear to be no reports of the effect of TA on endothelial or epithelial cell permeability, but the current results suggest that TA has the capacity to modulate both the expression of adhesion molecules and cellular permeability of human epithelium.
Glucocorticoid receptors are widely distributed in mammalian tissues and have been detected in human RPE cells [29] and bovine endothelial cells [41]. RPE cells also express vascular endothelial growth factor (VEGF) antigen [42] and it has been suggested that the capacity of corticosteroids to reduce oedema may be via down-regulation of the VEGF gene [43]. It has been suggested that involution of neovascular membranes in the monkey eye is the result of RPE proliferation, enveloping the newly formed vessels and resorbing subretinal fluid [44,45]. Intra-ocular glucocorticoids, including TA, are reported to induce RPE proliferation [29], and may promote the barrier function of the RPE.
TA may influence the activity of a variety of cell types involved in subretinal fibrovascular lesions including the RPE, vascular endothelial cells and leucocytes. The modulation of epithelial resistance and ICAM-1 expression by TA in vitro is consistent with clinical observations, indicating that reduction of the permeability of the outer BRB and down-regulation of inflammatory stimuli are the principal effects of intravitreal TA in vivo.
Acknowledgments
We thank Professor F.A. Billson for his encouragement and support, the N.S.W. Lions Eye Bank for tissue, Diana van Driel for electron microscopy, digital imaging and reading the manuscript and Lena Caruso for technical advice. Supported by NH&MRC, Sydney Foundation for Medical Research and Sir Zelman Cowen Fund.
REFERENCES
- 1.Penfold PL, Provis JM, Billson FA. Age-related macular degeneration: ultrastructural studies of the relationship of leucocytes to angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1987;225:70–76. doi: 10.1007/BF02155808. [DOI] [PubMed] [Google Scholar]
- 2.Lopez PF, Grossniklaus HE, Lambert HM, et al. Pathologic features of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol. 1991;112:647–56. doi: 10.1016/s0002-9394(14)77270-8. [DOI] [PubMed] [Google Scholar]
- 3.Penfold PL, Liew SC, Madigan MC, Provis JM. Modulation of major histocompatibility complex class II expression in retinas with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997;38:2125–33. [PubMed] [Google Scholar]
- 4.Penfold PL, Provis JM, Furby JH, Gatenby PA, Billson FA. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1990;228:270–4. doi: 10.1007/BF00920033. [DOI] [PubMed] [Google Scholar]
- 5.Gurne DH, Tso MO, Edward DP, Ripps H. Antiretinal antibodies in serum of patients with age-related macular degeneration. Ophthalmology. 1991;98:602–7. doi: 10.1016/s0161-6420(91)32252-8. [DOI] [PubMed] [Google Scholar]
- 6.Challa JK, Gillies MC, Penfold PL, et al. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust NZ J Ophthalmol. 1998;26:277–81. doi: 10.1111/j.1442-9071.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 7.Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone: a pilot study. Aust NZ J Ophthalmol. 1995;23:293–8. doi: 10.1111/j.1442-9071.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 8.Risau W, Engelhardt B, Wekerle H. Immune function of the blood–brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol. 1990;110:1757–66. doi: 10.1083/jcb.110.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elner SG, Pavilack MA, Todd RF, et al. Modulation and function of intracellular adhesion molecule-1 (CD54) on human retinal epithelial cells. Lab Invest. 1992;66:200–11. [PubMed] [Google Scholar]
- 10.Duguid IG, Boyd AW, Mandel TE. Adhesion molecules are expressed in the human retina and choroid. Curr Eye Res. 1992;11:153–9. doi: 10.3109/02713689208999526. [DOI] [PubMed] [Google Scholar]
- 11.Devine I, Lightman SI, Greenwood J. Role of LFA-1, ICAM-1, VLA-4 and VCAM-1 in lymphocyte migration across retinal pigment epithelial monolayers in vitro. Immunology. 1996;88:456–62. doi: 10.1046/j.1365-2567.1996.d01-666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CW, Defoe DM, Caldwell RB. Serum inhibits tight junction formation in cultured pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:1082–93. [PubMed] [Google Scholar]
- 13.Hoheisel D, Nitz T, Franke H, et al. Hydrocortisone reinforces the blood–brain barrier properties in a serum free cell culture system [corrected and republished in Biochem Biophys Res Commun 1998 247:312–5] Biochem Biophys Res Commun. 1998;244:312–6. doi: 10.1006/bbrc.1997.8051. [DOI] [PubMed] [Google Scholar]
- 14.Kiessling F, Kartenbeck J, Haller C. Cell–cell contacts in the human cell line ECV304 exhibit both endothelial and epithelial characteristics. Cell Tissue Res. 1999;297:131–40. doi: 10.1007/s004410051340. [DOI] [PubMed] [Google Scholar]
- 15.Bao S, dos Remedios CG, King NJC. Ontogeny of major histocompatibility complex antigen expression on cultured human embryonic skeletal myoblasts. Transplantation. 1994;58:585–90. doi: 10.1097/00007890-199409150-00010. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Devery J, King NJC. Early induction of interferon-independent virus specific ICAM-1 (CD54) expression by flavivirus in quiescent but not proliferating fibroblasts: implications for virus–host interactions. Virology. 1995;208:437–49. doi: 10.1006/viro.1995.1174. [DOI] [PubMed] [Google Scholar]
- 17.Gillies MC, Su T, Naidoo D. Electrical resistance and macromolecular permeability of retinal capillary endothelial cells in vitro. Curr Eye Res. 1995;14:435–42. doi: 10.3109/02713689509003753. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi T, Koichiro M, Sorgente N, Patterson R, Ryan SJ. Effects of intravitreal administration of steroids on experimental subretinal neovascularization in the subhuman primate. Arch Ophthalmol. 1985;103:708–11. doi: 10.1001/archopht.1985.01050050100026. [DOI] [PubMed] [Google Scholar]
- 19.Chandler DB, Rozakis G, de Juan EJ, Machemer R. The effect of triamcinolone acetonide on a refined experimental model of proliferative vitreoretinopathy. Am J Ophthalmol. 1985;99:686–90. doi: 10.1016/s0002-9394(14)76037-4. [DOI] [PubMed] [Google Scholar]
- 20.Antoszyk AN, Gottlieb JL, Machemer R, Hatchell DL. The effects of intravitreal triamcinolone acetonide on experimental pre-retinal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1993;231:34–40. doi: 10.1007/BF01681698. [DOI] [PubMed] [Google Scholar]
- 21.Danis RP, Bingaman DP, Yang Y, Ladd B. Inhibition of preretinal and optic nerve head neovascularization in pigs by intravitreal triamcinolone acetonide. Ophthalmology. 1996;103:2099–104. doi: 10.1016/s0161-6420(96)30383-7. [DOI] [PubMed] [Google Scholar]
- 22.Jennings T, Rusin MM, Tessler HH, Cunha VJ. Posterior sub-Tenon's injections of corticosteroids in uveitis patients with cystoid macular edema. Jap J Ophthalmol. 1988;32:385–91. [PubMed] [Google Scholar]
- 23.Riordan-Eva P, Lightman S. Orbital floor steroid injections in the treatment of uveitis. Eye. 1994;8:66–69. doi: 10.1038/eye.1994.12. [DOI] [PubMed] [Google Scholar]
- 24.Fang T, Peyman GA, Conway Md. Intravitreal triamcinolone acetonide effectiveness in complex vitroretinal surgery. Invest Ophthalmol Vis Sci. 1999;39:s943. [Google Scholar]
- 25.Danis RP, Ciualla TA, Pratt LM. Short-term visual outcome of exudative age-related macular degeneration treatment with intravitreal triamcinolone acetonide. Invest Ophthalmol Vis Sci. 1999;39:s316. [Google Scholar]
- 26.Dobbie MS, Hurst RD, Klein NJ, Surtees RA. Upregulation of intercellular adhesion molecule-1 expression on human endothelial cells by tumour necrosis factor-alpha in an in vitro model of the blood–brain barrier. Brain Res. 1999;830:330–6. doi: 10.1016/s0006-8993(99)01436-5. [DOI] [PubMed] [Google Scholar]
- 27.Dirks W, Macleod RAF, Drexler HG. ECV304 (endothelial) is really T24 (bladder carcinoma): cell cross-contamination at source. In Vitro 1999; In Vitro Cell Dev Biol Animal. 1995;35:558–9. doi: 10.1007/s11626-999-0091-8. [DOI] [PubMed] [Google Scholar]
- 28.Davis AA, Bernstein PS, Bok D, et al. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci. 1995;36:955–64. [PubMed] [Google Scholar]
- 29.He S, Wang HM, Ye J, et al. Dexamethasone induced proliferation of cultured retinal pigment epithelial cells. Curr Eye Res. 1994;13:257–61. doi: 10.3109/02713689408995786. [DOI] [PubMed] [Google Scholar]
- 30.Wheller S, Perretti M. Dexamethasone inhibits cytokine-induced intercellular adhesion molecule-1 up-regulation on endothelial cell lines. Eur J Pharmacol. 1997;331:65–71. doi: 10.1016/s0014-2999(97)01015-7. [DOI] [PubMed] [Google Scholar]
- 31.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration. The involvement of giant cells in atrophy of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1986;27:364–71. [PubMed] [Google Scholar]
- 32.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 33.Oh H, Takagi H, Takagi C, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1891–8. [PubMed] [Google Scholar]
- 34.Joyce DA, Steer JH, Abraham LJ. Glucocorticoid modulation of human monocyte/macrophage function: control of TNF-alpha secretion. Inflammation Res. 1997;46:447–51. doi: 10.1007/s000110050222. [DOI] [PubMed] [Google Scholar]
- 35.Nagineni CN, Kutty RK, Detrick B, Hooks JJ. Inflammatory cytokines induce intercellular adhesion molecule-1 (ICAM-1) mRNA synthesis and protein secretion by human retinal pigment epithelial cell cultures. Cytokine. 1996;8:622–30. doi: 10.1006/cyto.1996.0083. [DOI] [PubMed] [Google Scholar]
- 36.Heidenkummer HP, Kampik A. Surgical extraction of subretinal pseudotumors in age related macular degeneration. Clinical, morphologic and immunohistochemical results. Ophthalmologe. 1995;92:631–9. [PubMed] [Google Scholar]
- 37.Watanabe T, Tanaka R, Taniguchi Y, et al. The role of microglia and tumor-primed lymphocytes in the interaction between T lymphocytes and brain endothelial cells. J Neuroimmunol. 1998;81:90–97. doi: 10.1016/s0165-5728(97)00163-x. [DOI] [PubMed] [Google Scholar]
- 38.Ritchie AJ, Johnson DR, Ewenstein BM, Pober JS. Tumor necrosis factor induction of endothelial cell surface antigens is independent of protein kinase C activation or inactivation. Studies with phorbol myristate acetate and staurosporine. J Immunol. 1991;146:3056–62. [PubMed] [Google Scholar]
- 39.Li F, Joshua IG, Lian R, Justus DE. Differing regulation of major histocompatibility class II and adhesion molecules on human umbilical vein endothelial cells by serotonin. Int Arch Allergy Immunol. 1997;112:145–51. doi: 10.1159/000237446. [DOI] [PubMed] [Google Scholar]
- 40.Singer KL, Stevenson BR, Woo PL, Firestone GL. Relationship of serine/threonine phosphorylation/dephosphorylation signaling to glucocorticoid regulation of tight junction permeability and ZO-1 distribution in nontransformed mammary epithelial cells. J Biol Chem. 1994;269:16108–15. [PubMed] [Google Scholar]
- 41.Dasarathy Y, Lanzillo JJ, Fanburg BL. Stimulation of bovine pulmonary artery endothelial cell ACE by dexamethasone: involvement of steroid receptors. Am J Physiol. 1992;263:L645–9. doi: 10.1152/ajplung.1992.263.6.L645. [DOI] [PubMed] [Google Scholar]
- 42.Robbins SG, Conaway JR, Ford BL, Roberto KA, Penn JS. Detection of vascular endothelial growth factor (VEGF) protein in vascular and non-vascular cells of the normal and oxygen-injured rat retina. Growth Factors. 1997;14:229–41. doi: 10.3109/08977199709021522. [DOI] [PubMed] [Google Scholar]
- 43.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309–15. doi: 10.1016/s0014-2999(97)01464-7. [DOI] [PubMed] [Google Scholar]
- 44.Miller H, Miller B, Ishibashi T, Ryan SJ. Pathogenesis of laser-induced choroidal subretinal neovascularization. Invest Ophthalmol Vis Sci. 1990;31:899–908. [PubMed] [Google Scholar]
- 45.Miller H, Miller B, Ryan SJ. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Invest Ophthalmol Vis Sci. 1986;27:1644–52. [PubMed] [Google Scholar]