Abstract
Introduction of the hu-PBL-SCID mouse model has yielded a potentially useful tool for research in transplantation. The aim of this study was to define the conditions necessary for a reconstituted human immune system to destroy in a consistent manner rat islet xenografts in the alloxan-diabetic hu-PBL-SCID mouse. We examined different time points of hu-PBL reconstitution, different transplantation sites of the islets and several hu-PBL reconstitution protocols. Major differences in graft destruction were observed between the different hu-PBL reconstitution protocols, irrespective of timing of hu-PBL reconstitution or site of transplantation. Although preactivation of hu-PBL did not improve the level of hu-PBL chimerism, histological and immunohistochemical analysis of the grafts revealed a severe human lymphocytic infiltration and β cell destruction only in the grafts of mice receiving preactivated hu-PBL. This β cell injury resulted in impaired glucose tolerance, with in some animals recurrence of hyperglycaemia, and decreased insulin and C-peptide levels after glucose stimulation. Therefore, we conclude that activation of hu-PBL prior to transfer is essential in achieving xenograft infiltration and destruction in hu-PBL-SCID mice. The need for immune manipulation suggests that interactions between hu-PBL and xenografts in this model may be hampered by incompatibilities in cross-species adhesion and/or activation signals.
Keywords: hu‐PBL‐SCID, mouse, pancreatic islets, anti-CD3 activation, destruction, xenograft
INTRODUCTION
Transplantation of tissues and whole organs has changed dramatically the therapeutic arsenal for many diseases, including terminal renal insufficiency and cardiac failure. A major obstacle in making transplantation the treatment of choice for other diseases, such as islet transplantation in type-1 diabetes, is the occurrence of graft rejection and recurrence of the original disease in the graft [1]. Studies in animal models of transplantation, mostly rodent models, have greatly helped in understanding the immune phenomena involved in graft destruction, but major differences between the rodent and human immune systems sometimes limit the value of data obtained in these models [2]. The discovery of the SCID mouse and the observation that this mouse accepts an allo- or xenogeneic immune system and allo- or xenogeneic organ grafts without rejection opened new perspectives in the study of transplant immunology [3,4]. Indeed, a mature human immune system can be introduced into these mice achieving, depending on the induction therapy, variable levels of chimerism in the peripheral mouse blood [5]. However, in order to be useful the human immune cells in these chimeras must recirculate throughout the whole SCID microenvironment and must be able to specifically recognize, invade and finally destroy allo- or xenografts, while leaving the mouse host unaffected. Many papers have appeared on the human peripheral blood lymphocyte reconstituted SCID (hu-PBL-SCID) mouse as a model for the study of transplantation immunology [6–10], but only a few carefully describe the human immune reconstitution protocols and even fewer studies demonstrate convincingly whether immune-specific allo- or xenograft destruction is achieved or whether the destruction of the grafts is a non-specific ‘bystander’ effect, e.g. a graft-versus-host disease (GVHD) reaction provoked by human cells in the SCID mice [6,7,11].
The aim of the present study was to better define the conditions essential for consistent graft destruction in a hu-PBL-SCID-xenoislet model allowing for standardization of this model. To achieve this aim we studied the interactions between hu-PBL and xenogeneic rat islets transferred into SCID mice, using different time points of reconstitution, different transplantation sites of the islet grafts and three different hu-PBL reconstitution protocols.
MATERIALS AND METHODS
Animals
CB17-scid/scid (SCID, H-2d) mice were obtained from the specific pathogen-free animal facility at the Rega-Institute (Leuven, Belgium). The animals were housed in autoclaved microisolator cages and were fed sterilized water and mouse chow. Only male mice, aged 5–8 weeks, were used. Each individual mouse was screened before entering into the study for the presence of mouse T or B lymphocytes (‘leakiness’) [12] in the peripheral blood by flow cytometric analysis (FACSort; Becton Dickinson, Dendermonde, Belgium). The following antibodies were used in a standard two-colour staining: CD90 MoAb (Thy-1.2) (Caltag Labs, Burlingame, CA) and B220 MoAb (PharMingen, Hamburg, Germany). A gate was set to selectively detect mouse lymphocytes and 5000 events were analysed (LYSYS software; Becton Dickinson). SCID mice with > 1·0% mouse T and B lymphocytes/total of lymphocyte gated cells were excluded.
Islet donors were young diabetes-resistant BB/Pfd (RT-1u) rats (8–12 days old), obtained from our own breeding colony in the Proefdiercentrum (Leuven, Belgium).
All experiments were approved and supervised by the Animal Ethics Committee of the Catholic University of Leuven (P98037).
Islet isolation and grafting
Pancreases were removed from young rats under aseptic conditions. The endocrine tissue was digested by fractionated collagenase digestion (Boehringer Ingelheim Bioproducts, Heidelberg, Germany) in Hanks' balanced salt solution (HBSS). Islets were separated from the endocrine cells by dextran gradient centrifugation (Pharmacia Biotech, Roosendaal, The Netherlands) and hand-picked under the stereomicroscope. Islets were suspended in ice-cold RPMI 1640 medium (Gibco BRL, Merelbeke, Belgium) supplemented with 10% fetal calf serum (FCS) and transplanted freshly (within 3 h of preparation).
Recipient SCID mice, rendered diabetic by an alloxan injection intravenously (90 mg/kg; Fluka Chemika, Bornem, Belgium) 24 h prior to islet transplantation, were anaesthetized with avertin (0·02 ml/mg body wt). The target organ (left kidney or spleen) was approached via a lumbotomy. Mice receiving 150 fresh rat islets returned swiftly (48 h) to normoglycaemia, whereas mice that were not transplanted remained hyperglycaemic and died within 1 week.
Hu-PBL reconstitution of SCIDs
Human leucocytes were isolated from buffy coats of healthy volunteers by Ficoll–Hypaque density gradient centrifugation (Nycomed, Oslo, Norway). Recipient SCID mice underwent one natural killer (NK) cell depletion procedure (20 μl antiasialo GM1 (ASGM1)/200 μl PBS per mouse, i.p.) (Wako Chemicals, Neuss, Germany) 1 day prior to the hu-PBL reconstitution. Three different hu-PBL reconstitution protocols were established.
In a first approach, fresh hu-PBL (50 × 106/200 μl PBS per mouse) were injected intraperitoneally into the SCID mice under sterile conditions. In a second protocol, a booster injection of activated hu-PBL was given as described previously [8]. Briefly, initial reconstitution with fresh hu-PBL (30 × 106/200 μl PBS per mouse) was followed 2 days later by a booster injection of 10 × 106 hu-PBL from the same donor, activated in vitro by OKT3 (Ortho Biotech Inc., Raritan, NJ) stimulation. For OKT3 activation, hu-PBL were cultured in RPMI 1640 medium supplemented with antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin) and 2 mm l-glutamine containing 100 ng/ml OKT3 (human anti-CD3 MoAb) for 2 days at 37°C (5% CO2). In the third reconstitution protocol, also a second booster injection of 10 × 106 anti-CD3 antibody-activated hu-PBL was given 2 days after the first booster.
Evaluation of chimerism
The prevalence of human leucocytes in the peripheral blood of the hu-PBL-SCID mice was evaluated by flow cytometry (FACSort) weekly and at the end of the experiments (4 weeks after reconstitution). To avoid the confusion of relative numbers of human cells versus unstable levels of mouse cells (mainly monocytes and NK cells), we chose to determine absolute numbers of human immunocytes. Quantification of chimerism was done using a TriTEST three-colour staining (anti-CD3, anti-CD19, anti-CD45 MoAbs) in combination with TruCOUNT Absolute Count tubes, which contain a lyophilized pellet of a preset number of fluorescent-dyed beads (Becton Dickinson). This test allows simultaneous analysis of an unknown number of human lymphocytes with the Absolute Count beads as an internal reference value [13]. Enumeration of the leucocyte population or subsets was done by dividing the number of cellular events of interest by the number of bead events and test volume (50 μl) and then multiplying by the lot-dependent bead count concentration.
Mixed lymphocyte reaction
To test the functionality of T lymphocytes from the hu-PBL-SCID mice, mixed lymphocyte reactions (MLR) were performed using splenocytes from hu-PBL-SCIDs 4 weeks after reconstitution as responders (R) and irradiated (30 Gy, 60Co source) splenocytes from either unreconstituted SCID mice or from BB rats or fresh hu-PBL from different donors as stimulators (S). Control cultures used splenocytes from unreconstituted SCID mice as responders. Splenocytes were prepared as described previously [14]. After T cell enrichment by a passage through nylon wool, splenocytes (1 × 106/ml, 1 × 105/well) from the hu-PBL-SCID mice were co-cultured for 5 days at a R/S ratio of 1:1. Cells were cultured in quadrinplate in flat-bottomed 96-well microplates (Nunc, Roskilde, Denmark) in complete culture medium (RPMI 1640 supplemented with 10% FCS, 2 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 0·2% 2-mercaptoethanol (2-ME)) at 37°C in 5% CO2 and air. Four days later, 1 μCi of 3H-methylthymidine (Radiochemical Centre Amersham, Aylesbury, UK) was added to each well. After incubation for another 18 h, cells were harvested on glass-filter paper and counted. Proliferation values were expressed as ct/min.
We also tested whether chimera-derived T cells beyond 4 weeks of reconstitution still provide a functional human immune system in the SCID microenvironment. For this purpose, MLR were performed using splenocytes from hu-PBL-SCIDs 5 weeks after reconstitution as responders (R) and irradiated (30 Gy, 60Co source) splenocytes from either unreconstituted SCID mice or BB rats or fresh hu-PBL from different donors as stimulators (S).
Recipient follow up
Mice were weighed once weekly and graft function was evaluated twice weekly by measurement of glycaemia after tail vein puncture (Glucocard; Menarini, Firenze, Italy). The functional capacity of the islet grafts was further assessed by glucose challenge tests as described previously [15]. Briefly, animals, receiving an i.p. injection of 2 g D(+)glucose monohydrate/kg body wt (Merck, Overijse, Belgium) on days 7, 14, 21 and 28 after hu-PBL reconstitution, were bled 30 min after glucose challenge and plasma was collected and stored at −20°C until analysis. Glycaemia was measured by tail vein puncture as described above. Rat insulin was measured by an ultra-sensitive ELISA kit (Crystal Chem Inc., Chicago, IL), while rat C-peptide was measured using a radioimmunoassay (RIA) kit (Nuclilab BV, Ede, The Netherlands). At the end of the study, islet grafts and pancreases were removed for either insulin content determination or histology and immunohistochemistry.
Insulin content was determined as described previously [16]. Briefly, insulin was detected in pancreases by first disrupting the tissues and than extracting the insulin overnight via incubation at 4°C in ethanol with 10% H3PO4 1 m added. Then tissues were sonicated and insulin was determined in the supernatant by RIA, using a rat insulin standard.
For histology and immunohistochemistry, tissues were snap-frozen in 2-methyl-butane 99+% (ACROS Organics, Geel, Belgium) in liquid nitrogen. For general histological analysis, 5-μm cryostat sections were stained with Mayer's haematoxylin and eosin (H–E).
To determine the origin of the infiltrating cells, immunohistochemistry was performed. In brief, 5-μm frozen sections were fixed in acetone and 30% H2O2 for 15 min and after a blocking with 1:20 normal rabbit serum subjected to an overnight incubation with a primary MoAb (biotinylated-conjugated mouse anti-human CD45 MoAb; PharMingen), followed by incubation with a peroxidase-conjugated rabbit anti-mouse IgG (Dako, Merelbeke, Belgium), and visualized using Sigma fast DAB (VEL, Leuven, Belgium). All sections were counter-stained with Mayer's haematoxylin.
Presence of rat insulin-positive cells in the grafts of recipients was checked by insulin staining. In brief, fixation of the 5-μm sections included methanol in combination with 30% H2O2 followed by a blocking with normal goat serum for 15 min. The slides were incubated for 4 h with a polyclonal rabbit anti-insulin antibody (Monosan, Uden, The Netherlands), followed by a secondary incubation with peroxidase-conjugated goat anti-rabbit IgG (Dako) and visualized using Sigma fast DAB. Sections were counter-stained with Mayer's haematoxylin.
Statistical analysis
The following statistical tests were used: the analysis of variance and Fisher's LSD multiple comparison test for chimerism evaluation, in vitro functionality assessment, post-glucose glycaemia, insulin and C-peptide parameters. Significance was defined at the 0·05 level.
RESULTS
Quantitative evaluation of chimerism in hu-PBL-SCID recipients
To evaluate the hu-PBL engraftment of the hu-PBL-SCID mice, we chose to measure absolute numbers of circulating human cells. Human CD45+ leucocytes were detected in the hu-PBL-SCID mouse peripheral blood 4 weeks following injection of 50 × 106 unstimulated hu-PBL (Fig. 1). Almost all human CD45+ cells in the peripheral blood were CD3+ T lymphocytes (> 90%). No or only very small numbers of CD19+ B cells were detected (data not shown).
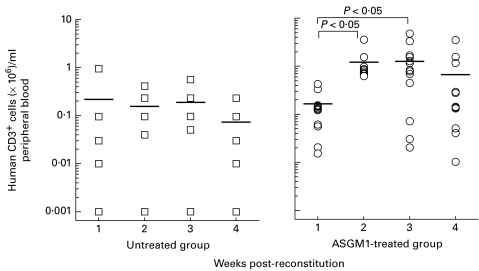
Fig. 1.
Human chimerism in peripheral blood of untreated (n = 5) or antiasialo GM1 (ASGM1)-treated (n = 14) hu-PBL-SCID mice. Chimerism was tested weekly after hu-PBL reconstitution. Natural killer (NK) cell depletion resulted in a clear increase in hu-PBL engraftment levels in the peripheral blood of SCID recipients 2 and 3 weeks after reconstitution compared with untreated hu-PBL-SCID mice (P < 0·05). No rise in chimerism was seen in the absence of NK cell depletion. Data are presented as an absolute count of human CD3+ cells ( × 106)/ml peripheral blood. Means are indicated by a horizontal line.
Depletion of NK cells in recipient mice has been shown to increase the efficacy of a subsequent hu-PBL engraftment [17]. Similarly, we observed that an injection of ASGM1, which depleted NK cells in the mouse peripheral blood (< 1% versus 10% ASGM1+ cells in the controls), increased the human cell engraftment nearly 10-fold (P < 0·05 versus untreated hu-PBL-SCID mice at 2 and 3 weeks post-reconstitution) (Fig. 1). The highest levels of human CD3+ lymphocyte reconstitution were seen 3 weeks after hu-PBL injection (0·17 ± 0·24 × 106 in untreated group versus 1·25 ± 1·30 × 106 human CD3+ cells/ml peripheral blood in ASGM1-treated group; P < 0·05). Thereafter, a gradual decrease of the human lymphocytes in the SCID mice was noticed. The loss of human lymphocyte engraftment was associated with a low degree of GVHD, reflected by weight loss and ruffled fur.
In vitro evaluation of the function of human lymphocytes isolated from hu-PBL-SCID mice
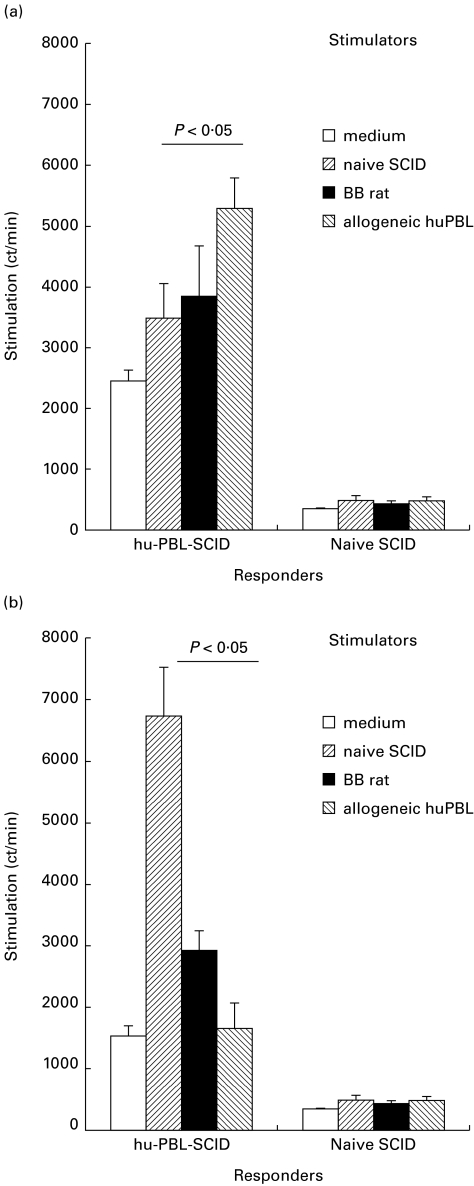
The in vitro immune reactivity of the human immune cells in the hu-PBL-SCID mice at 4 weeks and beyond 4 weeks of reconstitution was tested in MLR. Human T cells were isolated from the hu-PBL-SCID spleen 4 weeks after reconstitution and were found to proliferate to all targets, which indicates their immunocompetence after reconstitution in SCID mice (Fig. 2a). Proliferation of these human T cells was significantly stronger in the presence of allogeneic human cells than in the presence of xenogeneic mouse cells (P < 0·05). On the other hand, no response was detected in unreconstituted SCID mice, as can be expected from their lack in T lymphocytes. At week 5 following reconstitution, the relative reactivity of human lymphocytes from the chimeras against human alloantigens decreased, but there was a ‘squewing’ towards anti-mouse reactivity, as the proliferative capacity of SCID stimulator cells increased (P < 0·05, Fig. 2b). This was probably related to the development of GVHD in these chimeras, which can be expected to affect the validity of this experimental model. In view of these data all further experiments were performed using the ASGM1 depletion protocol and follow up was terminated at 4 weeks after reconstitution.
Fig. 2.
In vitro reactivity of splenocytes from hu-PBL-SCID mice compared with naive SCID mice. (a) Reactivity of splenocytes of hu-PBL-SCID mice harvested 4 weeks after hu-PBL reconstitution. Responder cells were incubated in vitro with irradiated splenocytes from naive SCID mice or from BB rats or with irradiated allogeneic hu-PBL in a 5-day proliferation assay, as described in Materials and methods. (b) Reactivity of splenocytes of the hu-PBL-SCID mice harvested 5 weeks after hu-PBL reconstitution. Responder cells were incubated in vitro with irradiated splenocytes from naive SCID mice or from BB rats or with irradiated allogeneic hu-PBL in a 5-day proliferation assay, as described in Materials and methods. These data represent one of three independent experiments.
Effect of timing of hu-PBL reconstitution on rejection of rat islet xenografts
To study the interactions between a human immune system and islet xenografts in an in vivo situation, a first experiment was designed in which alloxan-diabetic SCID mice (n = 5) received 150 rat islets beneath the kidney capsule and were engrafted 5 days later with unstimulated 50 × 106 hu-PBL from a normal blood donor. As in unreconstituted mice (n = 5), all mice remained normoglycaemic for the duration of the study (Fig. 3a). The pancreatic insulin content at the end of the study clearly demonstrated that the observed normoglycaemia was the result of graft survival and not of recovery of pancreatic β cells of the SCID mice (0·5 ± 0·5 pmol insulin/mg tissue in alloxan-treated mice versus 14·3 ± 6·9 pmol insulin/mg tissue in normal SCID mice; P < 0·001). Immunohistochemical analysis of grafts from both reconstituted and unreconstituted SCID mice showed intact islets with strong insulin staining 4 weeks after hu-PBL reconstitution (Fig. 4a,d). No human immune cells were detected in the grafts, except for a sparse infiltrate of human CD45+ T cells in one of the five reconstituted SCID mice.
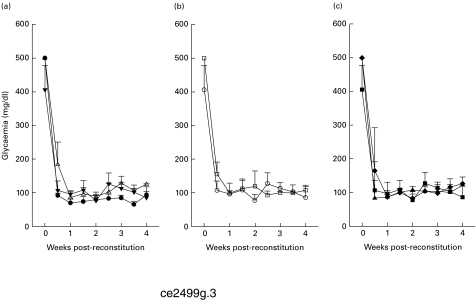
Fig. 3.
Glycaemia of alloxan-diabetic SCID mice transplanted with rat islets. (a) Glycaemia levels of unreconstituted (n = 5, •), hu-PBL reconstituted 5 days before (n = 5, Δ) and 5 days after islet transplantation (n = 5, ▾) SCID mice. Timing of hu-PBL reconstitution had no effect on islet graft survival. (b) Glycaemia levels of kidney-transplanted (n = 5, ○) and spleen-transplanted (n = 5, □) hu-PBL-SCID mice. The site of islet transplantation did not change the incapacity of unstimulated hu-PBL to induce rejection. (c) Glycaemia levels of unboosted (n = 5, ▪), 1 × boosted (n = 6, ▴) and 2 × boosted (n = 5, ♦) hu-PBL-SCID mice. Pre-activation of hu-PBL prior to transfer did not induce recurrence to hyperglycaemia.
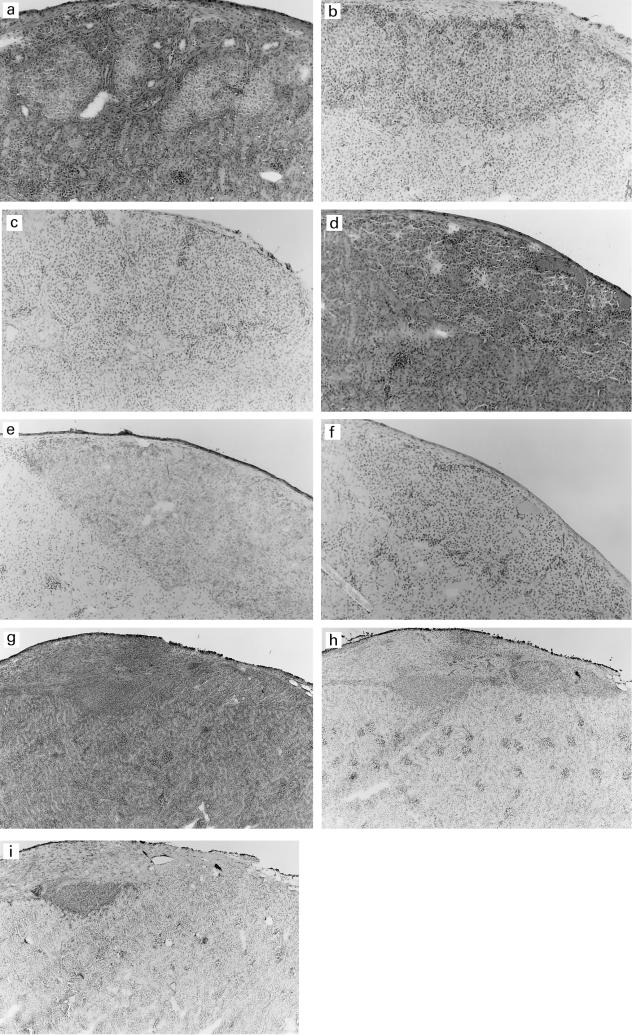
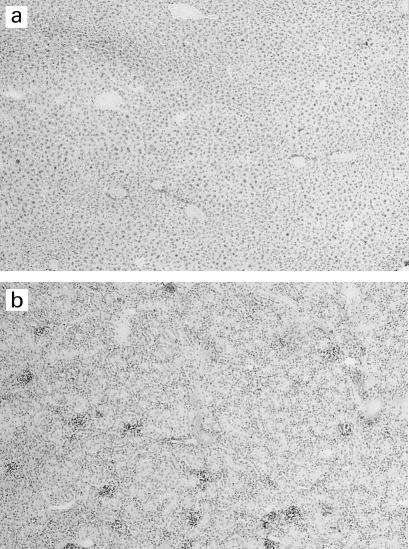
Fig. 4.
Histology and immunohistochemistry of islet grafts from unreconstituted SCID mice (a,b,c), unboosted (d,e,f) and 1 × boosted (g,h,i) hu-PBL-SCID mice 4 weeks after hu-PBL reconstitution. Haematoxylin and eosin (a,d,g), insulin staining (b,e,h) and staining with anti-human CD45 antibody (c,f,i). Mag. × 200 (a–f), × 100 (g–i). Only the grafts from the boosted hu-PBL-SCID mice (g,h,i) show severe human CD45+ T cell infiltrates and clearly reduced insulin-positive cells. No infiltration and high insulin-positive cells are observed in grafts of the unreconstituted SCID mice (a,b,c). No infiltrates were seen in the grafts of unboosted hu-PBL-SCID mice (d,e,f).
Reconstituting the SCID mice with unstimulated 50 × 106 hu-PBL 5 days before or after rat islet transplantation did not change the outcome: all engrafted mice (n = 5) retained their normoglycaemic state for the duration of the experiment (Fig. 3a) and no signs of islet damage or human immune cell infiltration were observed in histological and immunohistochemical sections (data not shown).
Effect of site of islet transplantation on rejection of rat islet xenografts
To determine if the site of transplantation plays a role in, for example, the recruitment of the human leucocytes to the islet xenograft and subsequent rejection of these grafts, rat islets were transplanted into the spleen of alloxan-diabetic SCID mice (n = 5) instead of under the renal capsule. All mice were reconstituted with unstimulated 50 × 106 hu-PBL 5 days after transplantation. All intrasplenically transplanted hu-PBL-SCID mice persisted in their normoglycaemic state until the end of the experiment (Fig. 3b), with intact insulin-positive islets, which lack an infiltration by human CD45+ cells (data not shown).
Pre-activation of human immune cells is essential in mediating tissue infiltration, graft damage and destruction
Since activated lymphocytes preferentially home to sites of inflammation and as in vitro activation of lymphocytes enhances lymphocytic cell adhesion [18,19], we tested whether prior in vitro anti-CD3 activation increased the lymphocytic response towards the xenogeneic rat islet grafts. For this purpose, alloxan-diabetic recipient mice were first transplanted with 150 rat islets under the kidney capsule and 5 days later given 30 × 106 unstimulated hu-PBL followed by one (n = 6) or two (n = 5) boosters with 10 × 106 OKT3-activated hu-PBL. Activation of the human immune cells did not significantly improve the hu-PBL engraftment (1·44 ± 0·77 × 106 in 1 × boosted group and 1·43 ± 1·70 × 106 in 2 × boosted group versus 1·25 ± 1·30 × 106 human CD3+ cells/ml in unboosted group; NS, 3 weeks post-reconstitution).
All mice remained normoglycaemic until the end of the study (Fig. 3c), but histological and immunohistochemical analysis showed a severe CD45+ lymphocytic infiltration in the rat islet xenografts in both boosted groups together with a reduced insulin staining in the grafts at the end of the study (Fig. 4). At the same time point, no human CD45+ cells were detected in other tissues (liver or kidney) from the boosted groups, confirming the absence of GVHD and suggesting specific infiltration into the grafts (Fig. 5).
Fig. 5.
Immunohistochemistry of liver and kidney from 1 × boosted hu-PBL-SCID mice 4 weeks post-reconstitution. Staining with anti-human CD45 antibody (liver (a) and kidney (b)). Mag. × 100 (a,b). No human CD45+ T cell infiltrates were detected in the liver or kidney tissue.
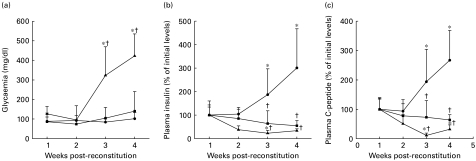
To examine if these histological features, consistent with ongoing xenogeneic islet destruction, affected the metabolic capacity of the transplanted β cells, we performed glucose tolerance tests. Glycaemia, plasma insulin and C-peptide were measured after i.p. glucose challenge in unreconstituted SCID recipients (n = 5), in unboosted (n = 4) and in 1 × boosted (n = 6) hu-PBL-SCID mice once a week. In the unreconstituted SCID mice, normoglycaemia was maintained after glucose challenge throughout the study (Fig. 6a). Moreover, a progressive increase in β cell function was observed, as indicated by an increase in both plasma insulin (301 ± 166% of initial levels, P < 0·05) and C-peptide (267 ± 101% of initial levels, P < 0·05) levels (Fig. 6b,c). Surprisingly, the unboosted hu-PBL-SCID mice did not show this increase in graft function and showed a lack of increase of the levels of insulin (55 ± 22%, NS versus initial levels) and C-peptide (64 ± 18%, NS versus initial levels) (Fig. 6b,c). Nevertheless, these mice maintained normoglycaemia after glucose challenge throughout the study (Fig. 6a). In contrast, a progressive and severe impairment of islet xenograft function was observed in the boosted hu-PBL-SCID mice, resulting in post-glucose hyperglycaemia (422 ± 113 mg/dl) and markedly reduced insulin (34 ± 8%, NS) and C-peptide levels (32 ± 15%, P < 0·05) 4 weeks after hu-PBL reconstitution (Fig. 6a,b,c).
Fig. 6.
Metabolic evaluation of unreconstituted SCID mice (n = 5, •), unboosted (n = 4, ▪) and 1 × boosted (n = 6, ▴) hu-PBL-SCID mice after glucose challenge. Mice received an i.p. glucose (2 g/kg body wt) injection weekly after hu-PBL reconstitution and were bled 30 min after glucose challenge. Post-glucose glycaemia (a), plasma insulin (b) and C-peptide (c) levels were measured as described in Materials and methods. Note that severe metabolic impairment was present in the boosted hu-PBL-SCID mice, as reflected by return to hyperglycaemia, reduced insulin and C-peptide levels. *P < 0·05 versus initial levels; †P < 0·05 versus unreconstituted SCID mice.
DISCUSSION
A number of obstacles restrict the use of the hu-PBL-SCID mouse as a model for studying interactions between a human immune system and an allo- or xenograft. A first problem is the lack of standardized protocols for the reproducible generation and evaluation of hu-PBL-SCID mice. Published methods differ in the source of the immune cells [20,21] or in their number [5,7], in the use of recipient pretreatment, aiming NK cell [17,22] or macrophage depletion [23,24], or in donor cell pretreatment such as activation of the immune cells [8–10,25]. The degree of chimerism has been previously assessed by different methods, i.e. flow cytometry [8,17,20], immunoglobulin measurement [7,8,17], polymerase chain reaction (PCR) detection [9,20] or DNA dot blotting [6], each providing a relative estimate of the hu-PBL reconstitution but none yielding a quantification in absolute terms. In the present study, we adopted a TriTEST three-colour staining with TruCOUNT Absolute Count tubes, to express the level of human chimerism as an absolute number, which should avoid confusions that occur when human cells are related to variable numbers of mouse monocytes and NK cells. We could thus show that human lymphocytes migrate from the peritoneum to the peripheral blood within the first week after their injection and reach maximal circulating numbers after 2–3 weeks post-reconstitution, which confirms previous reports [17].
A second problem is the short period of functionality of the human immune system in these hu-PBL-SCID mice. We tested the proliferative properties of hu-PBL from chimeras during in vitro exposure to allo- and xenoantigens and confirmed that these cells were immune responsive at 4 weeks following hu-PBL reconstitution [26], but that their increasing xenoreactivity to host antigens became responsible for signs of GVHD at week 5, which can be expected to affect the validity of this experimental model [26–28]. In view of these observations, we restricted the use of this model to the 4-week period following reconstitution.
Although the hu-PBL were found to react in vitro to rat antigens, they are not necessarily destructive to the rat islet graft in vivo. Reconstitution of SCID mice with unstimulated human lymphocytes did not result in graft destruction, as shown by the intact insulin-positive islets and an absence of infiltration by human CD45+ cells.
The failure of the human leucocytes to invade and specifically destroy the rat islet grafts was observed irrespective of the timing of reconstitution versus that of the islet implantation. Sawada and co-workers [29] demonstrated in their hu-PBL-skingraft-SCID mouse model that skin xenograft rejection was more frequently observed when reconstitution was performed at the time of transplantation, than when hu-PBL were only injected when the graft was already stably engrafted. They hypothesized that lymphocytes might home more easily to recently transplanted tissues than to a stable graft, since inflamed tissues are expected to express more adhesion molecules on their endothelial cells. However, we did not observe such an influence in our islet graft model.
The rat islet graft was also preserved when implanted in the spleen. An intrasplenic transplantation could bypass the need for species-specific adhesion molecules in lymphocyte migration to the graft, which can be reached in the spleen without passage through endothelial venules [30]. However, mice that were transplanted intrasplenically also maintained normoglycaemia throughout the study, with histologically intact islets.
Our observations thus suggest a state of functional anergy of the hu-PBL in vivo, which might be due to lack of costimulation by species-incompatible SCID mouse antigen-presenting cells [31]. This apparent ‘anergy’ can be avoided by overloading the SCID mouse with high numbers of human lymphocytes, although this condition carries the risk of inducing a non-specific GVHD-type destruction of the allo- and xenograft [32,33]. One potential approach to overcome these barriers may be the use of another human–mouse chimeric model, the Trimera model, in which normal strains of mice are conditioned for hu-PBL reconstitution by a lethal total body irradiation followed by radioprotection with SCID bone marrow [34]. The human T cells in this model have a non-anergized functional status and GVHD is only sporadically reported.
Another way of avoiding this state of anergy in the hu-PBL-SCID mice consists in activating the human immune cells prior to reconstitution. In the case of preactivation, the rat islet grafts in our model became infiltrated by human CD45+ immunocytes, with clear immunohistochemical signs of β cell destruction. This process was reflected in vivo by impaired insulin secretion and disturbed glucose tolerance. However, basal glucose levels remained normal, indicating the presence of an important amount of residual functioning β cells in the graft. In contrast to Shiroki et al. [8,9], we could convincingly demonstrate that preactivation is not fundamental for a better engraftment, since we did not measure higher circulating numbers of human lymphocytes in mice receiving activated lymphocytes, but is essential in mediating host tissue infiltration, probably by bypassing a critical step in the adhesion/activation cascade.
We conclude that the hu-PBL-SCID mouse model can be used as a standardized and reproducible experimental model for the study of graft rejection by human immunocytes, but several restrictions limit the use of this model. The development of GVHD restricts the use of this model to a 4-week post-reconstitution period, and manipulation of the hu-PBL prior to reconstitution is necessary for in vivo reactivity. Although this requirement abrogates the possibility of studying the reactions of a ‘naive’ immune system against an islet graft, the hu-PBL-SCID mouse model may be used for the study of the role of graft composition in graft destruction and for testing immunomodulatory drugs or drug combinations for the prevention of islet allo- or xenograft rejection by human immune cells.
Acknowledgments
This work was supported by the Flemish Research Foundation (FWO grants: Levenslijn 7.0007.96 and 3.0332.98) and a postdoctoral FWO Fellowship for C.M.
REFERENCES
- 1.Casteels K, Waer M, Laureys J, et al. Prevention of autoimmune destruction of syngeneic islet grafts in spontaneously diabetic nonobese diabetic mice by a combination of a vitamin D3 analogue and cyclosporin. Transplantation. 1998;65:1225–32. doi: 10.1097/00007890-199805150-00014. [DOI] [PubMed] [Google Scholar]
- 2.Salmela K. Pancreatic islet transplantation. Ann Chir Gynaecol. 1997;86:149–51. [PubMed] [Google Scholar]
- 3.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–30. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 4.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–9. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 5.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–9. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura T, Niguma T, Fechner JH, et al. Chronic human skin graft rejection in severe combined immunodeficient mice engrafted with human PBL from an HLA-presensitized donor. Transplantation. 1992;53:659–65. doi: 10.1097/00007890-199203000-00032. [DOI] [PubMed] [Google Scholar]
- 7.Murray AG, Petzelbauer P, Hughes CCW, Costa J, Askenase P, Pober JS. Human T-cell-mediated destruction of allogeneic dermal microvessels in a severe combined immunodeficient mouse. Proc Natl Acad Sci USA. 1994;91:9146–50. doi: 10.1073/pnas.91.19.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiroki R, Pointdexter NJ, Woodle ES, Hussain MS, Mohanakumar T, Sharp DW. Human peripheral blood lymphocyte reconstituted severe combined immunodeficient (hu-PBL-SCID) mice. A model for human allograft rejection. Transplantation. 1994;57:1555–62. [PubMed] [Google Scholar]
- 9.Shiroki R, Naziruddin B, Shishido S, Duffy BF, Howard T, Mohanakumar T. Human peripheral blood leukocyte-reconstituted severe combined immunodeficient mouse. Analysis of the human immune response against porcine islet transplantation. Transplantation. 1997;63:818–23. doi: 10.1097/00007890-199703270-00005. [DOI] [PubMed] [Google Scholar]
- 10.MacKenzie DA, Sollinger HW, Hullett DA. Acute graft rejection of human fetal pancreas allografts using donor-specific human peripheral blood lymphocytes in the SCID mouse. Transplantation. 1996;61:1461–4. doi: 10.1097/00007890-199605270-00008. [DOI] [PubMed] [Google Scholar]
- 11.Sultan P, Murray AG, McNiff JM, et al. Pig but not human interferon-γ initiates human cell-mediated rejection of pig tissue in vivo. Proc Natl Acad Sci USA. 1997;94:8767–72. doi: 10.1073/pnas.94.16.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosma GC, Fried M, Custer RP, Caroll A, Gibson DM, Bosma MJ. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988;167:1016–33. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson JK, Stein D, Mui T, Mack R, Hubbard M, Denny T. Evaluation of a method for counting absolute numbers of cells with a flow cytometer. Clin Diagn Lab Immunol. 1997;4:309–13. doi: 10.1128/cdli.4.3.309-313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casteels KM, Mathieu C, Waer M, et al. Prevention of type 1 diabetes in nonobese diabetic mice by late intervention with nonhypercalcemic analogs of 1,25-dihydroxyvitamin D3 in combination with a short induction course of cyclosporin A. Endocrinology. 1998;139:95–102. doi: 10.1210/endo.139.1.5641. [DOI] [PubMed] [Google Scholar]
- 15.Gerling IC, Kotb M, Fraga D, Sabek O, Gaber AO. No correlation between in vitro and in vivo function of human islets. Transplant Proc. 1998;30:587–8. doi: 10.1016/s0041-1345(97)01417-6. [DOI] [PubMed] [Google Scholar]
- 16.Mathieu C, Kuttler B, Waer M, Bouillon R, Hahn H. Spontaneous reestablishment of self-tolerance in BB/Pfd rats. Transplantation. 1994;58:349–54. [PubMed] [Google Scholar]
- 17.Shpitz B, Chambers CA, Singhal AB, et al. High level functional engraftment of severe combined immunodeficient mice with human peripheral blood lymphocytes following pretreatment with radiation and anti-asialo GM1. J Immunol Methods. 1994;169:1–15. doi: 10.1016/0022-1759(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 18.Dustin ML, Springer TA. T cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–24. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 19.Wysocki J, Issekutz TB. Effect of T cell activation on lymphocyte endothelial cell adherence and the role of VLA-4 in the rat. Cell Immunol. 1992;140:420–31. doi: 10.1016/0008-8749(92)90208-7. [DOI] [PubMed] [Google Scholar]
- 20.Alegre M, Peterson LJ, Jeyarajah DR, Weiser M, Bluestone JA, Thistlethwaite JR. Severe combined immunodeficient mice engrafted with human splenocytes have functional human T cells and reject human allografts. J Immunol. 1994;153:2738–49. [PubMed] [Google Scholar]
- 21.Rouleau M, Namikawa R, Antonenko S, Carballido-Perrig N, Roncarolo MG. Antigen-specific cytotoxic T cells mediate human fetal pancreas allograft rejection in SCID-hu mice. J Immunol. 1996;157:5710–20. [PubMed] [Google Scholar]
- 22.Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H, Miyasaka M. Selective long-term elimination of natural killer cells in vivo by an anti-interleukin-2 receptor β chain monoclonal antibody in mice. J Exp Med. 1993;178:1103–7. doi: 10.1084/jem.178.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terpstra W, Leenen PJM, van den Bos C, et al. Facilitated engraftment of human hematopoietic cells in severe combined immunodeficient mice following a single injection of Cl2MDP liposomes. Leukemia. 1997;11:1049–54. doi: 10.1038/sj.leu.2400694. [DOI] [PubMed] [Google Scholar]
- 24.Shibata S, Asano T, Noguchi A, Naito M, Ogura A, Dois K. Peritoneal macrophages play an important role in eliminating human cells from severe combined immunodeficient mice transplanted with human peripheral blood lymphocytes. Immunology. 1998;93:524–32. doi: 10.1046/j.1365-2567.1998.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy WJ, Conlon KC, Sayers TJ, et al. Engraftment and activity of anti-CD3-activated human peripheral blood lymphocytes transferred into mice with severe combined immune deficiency. J Immunol. 1993;150:3634–42. [PubMed] [Google Scholar]
- 26.Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180:1817–27. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray WJ, Bennett M, Anver MR, Baseler M, Longo DL. Human–mouse lymphoid chimeras: host-vs.-graft and graft-vs.-host reactions. Eur J Immunol. 1992;22:1421–7. doi: 10.1002/eji.1830220614. [DOI] [PubMed] [Google Scholar]
- 28.Tary-Lehmann M, Saxon A. Human mature T cells that are anergic in vivo prevail in SCID mice reconstituted with human peripheral blood. J Exp Med. 1992;175:503–16. doi: 10.1084/jem.175.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawada T, DellaPella PA, Seebach JD, Sachs DH, Colvin RB, Iacomini J. Human cell-mediated rejection of porcine xenografts in an immunodeficient mouse model. Transplantation. 1997;63:1331–8. doi: 10.1097/00007890-199705150-00022. [DOI] [PubMed] [Google Scholar]
- 30.Girard JP, Springer TA. High endothelial venules (HEVs): specialised endothelium for lymphocyte migration. Immunol Today. 1995;16:449–57. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 31.Tary-Lehmann M, Saxon A, Lehmann PV. The human immune system in hu-PBL-SCID mice. Immunol Today. 1995;16:529–33. doi: 10.1016/0167-5699(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann-Fezer G, Gall C, Zengerle U, Kranz B, Thierfelder S. Immunohistology and immunocytology of human T-cell chimerism and graft-versus-host disease in SCID mice. Blood. 1993;81:3440–8. [PubMed] [Google Scholar]
- 33.Sandhu JS, Gorczynski R, Shpitz B, Gallinger S, Nguyen HP, Hozumi N. A human model of xenogeneic graft-versus-host disease in SCID mice engrafted with human peripheral blood lymphocytes. Transplantation. 1995;60:179–84. [PubMed] [Google Scholar]
- 34.Reisner Y, Dagan S. The Trimera mouse: generating human monoclonal antibodies and an animal model for human diseases. Tibtech. 1998;16:242–6. doi: 10.1016/s0167-7799(98)01203-7. [DOI] [PubMed] [Google Scholar]