Abstract
The aim of this study was to investigate the immunogenicity of four synthetic peptides, representing linear B cell epitopes of the human La/SSB autoantigen: 145–164 aa (p1), 289–308 aa (p2), 301–318 aa (p3) and 349–364 aa (p4), in rabbits. New Zealand White rabbits were immunized with each of the above peptides attached in four copies on tetrameric sequential oligopeptide carriers (SOC) in duplicate. Control immunizations were also performed (one rabbit each, immunized with Freud's complete adjuvant alone or with the SOC carrier alone). Animals were bled at regular intervals and sera were analysed for anti-La/SSB activity by ELISA assays using as antigen the various synthetic peptides, as well as the whole La/SSB protein. Four months after the last immunization, the animals were killed and peripheral blood mononuclear and spleen cells were co-cultured with either the peptides, the SOC carrier, or 27 peptides, covering the entire length of the human La/SSB molecule (23 amino acids long, overlapping by eight residues to each other). A specific, IgG, anti-peptide antibody response was detected, initially directed against the priming peptide, and subsequently expanded to the other La/SSB synthetic peptides. The antibody titres remained high, even 4 months after the last immunization. Sera from rabbits immunized with either p2 or p3 reacted also with the whole La/SSB protein, as was demonstrated by ELISA and immunoblot assays. No reactivities against either Ro60 or Ro52 autoantigen were found. Rabbit spleen cell reacted not only with the epitope used for the immunization but also with other La/SSB peptides. Immunization of rabbits with the major human La/SSB B cell antigenic determinants, linked to SOC carrier, induces strong and sustained antibody and T cell responses against multiple epitopes of the human La/SSB protein. Thus, La/SSB B cell linear epitopes are probably capable also of functioning as T cell epitopes, in this experimental animal.
Keywords: Sjögren's syndrome, La/SSB, B cell epitopes, T cell epitopes, autoimmunity
INTRODUCTION
La/SSB is an autoantigen which along with two other proteins (Ro52 and Ro60kD), constitutes a complex with hYRNAs and forms ribonucleoprotein particles (hYRNPs). Autoantibodies against this complex are frequently found in the sera of patients with Sjögren's syndrome (SS) and systemic lupus erythematosus (SLE). Autoimmune responses against the hYRNPs are characterized by a remarkable diversity, illustrated by autoantibodies directed towards different proteins of the complex (Ro52, Ro60 and La/SSB), recognizing several conformational or linear epitopes [1–3].
Previous B cell epitope mapping of human La/SSB using the peptide mimotope scanning technique showed that anti-La/SSB autoantibodies from SS and SLE recognize four major linear antigenic epitopes, spanning the amino acid (aa) sequences 145–164 aa (p1), 289–308 aa (p2), 301–318 aa (p3) and 349–364 aa (p4) [4,5]. Using these peptides as antigenic substrates in ELISA it was found that peptides 3 and 4 present high sensitivity and specificity for the detection of anti-La/SSB antibodies. Furthermore, the peptides p1, p2 and p3 also possess features of putative T cell epitopes, as shown by computer prediction analysis [4].
The aim of the present study was to investigate the immunogenicity of these peptides in vivo, as well as to evaluate their ability to induce antibody and T cell responses against the La/SSB autoantigen when injected into experimental animals (New Zealand White female rabbits) in complexes of four copies coupled to a non-immunogenic, sequential oligopeptide carrier (SOC) [6].
MATERIALS AND METHODS
Four La/SSB linear B cell peptides in the form of tetrameric SOC complexes [(peptide)4SOC4] were used as immunogens, as follows.
Peptide synthesis and purification
The four La/SSB linear B cell peptides: 145TLHKAFKGSIFVVFDSIESA164, 289ANNGNLQLRNKEVTWEVLEG308, 301VTWEVLEGEVEKEALKKI318 and 349GSGKGKVQFQGKKTKF364, each attached in four copies to the tetrameric (Gly-Aib-Lys)4 (SOC4) backbone as well as the SOC4 alone, were used for the immunizations. All peptides corresponded to human sequences and were synthesized according to the solid-phase peptide synthesis procedure and purified as previously described [7].
For screening of peptide specificity on T cells, a set of 27 peptides, each consisting of 23 amino acids, overlapping each other by eight residues and covering the entire sequence of the human La/SSB protein, were prepared using the cleavable multipin synthesis system (Chiron Mimotopes, Clayton, Australia) acaccording to the method of Geysen et al. [8]. Synthesis was performed following the Fmoc strategy, based on the principles of solid-phase peptide synthesis [7]. The peptides were cleaved from the pins at neutral conditions that are compatible with biological systems [9–11] (0·05 m HEPES buffer pH 7·6 in sonication bath, for 60 min at room temperature).
Rabbit immunization
Ten New Zealand White female rabbits, 6–8 weeks old, were immunized according to a previously described protocol [12], using in each immunization 0·5 mg immunogen. Successive bleedings were performed before the immunizations (days 26, 53, 99 and 152) and on days 200, 250, 300 and 330. The animals were killed on day 330.
Rabbit no. 1 was immunized with PBS (control animal), rabbit no. 2 with the backbone of SOC4 alone, rabbits nos 3 and 4 were immunized with SOC-p1, rabbits nos 5 and 6 with SOC-p2, rabbits nos 7 and 8 with SOC-p3 and rabbits nos 9 and 10 with SOC-p4.
Elisa
The ELISA procedure was performed as previously described. Briefly, 96-well polystyrene plates (Nunc, Roskilde, Denmark) were coated with 5 μg/ml of each SOC4 peptide or SOC4 alone (50 μl/ml). After blocking, serum samples from preimmunized and post-immunized rabbits at dilutions ranging from 1:50 to 1:3200 were added. Affinity-purified goat antiserum against rabbit IgG (heavy chain-specific) conjugated to alkaline phosphatase (Sigma, St Louis, MO) and nitrophenyl-phosphate disodium, substrate for alkaline phosphatase, were subsequently added. Absorbance was measured at 405 nm. Sera were also tested by a commercially available enzyme-immunoassay for the detection of anti-La (SSB) antibodies (Shield Diagnostics, Diastat, London, UK), using affinity-purified La/SSB protein as antigen and the appropriate conjugate for rabbit antibody detection. The experiments were performed three times on different days.
Immunoblotting
Recognition of the whole La/SSB molecule by antibodies raised in immunized animals was also investigated using (i) LIA strips (Innogenetics, Ghent, Belgium), and (ii) cytoplasmic extracts from rabbit spleen and HeLa cells prepared as previously described [13]. Samples of extracts were electrophorized in 15% SDS–polyacrylamide gels, followed by electrotransfer to nitrocellulose membranes. Subsequently, the nitrocellulose blots were reacted with rabbit sera in 1:30 dilution for 1 h and washed with TBS–Tween-20 0·05% three times for 10 min. Anti-human IgG and anti-rabbit IgG (γ-chain-specific) alkaline phosphatase conjugate was added for 2 h at room temperature. The colour was developed by adding a substrate solution of p-nitrophenyl phosphate on to the strips.
Inhibition assays
In order to evaluate the specificity of the anti-peptide antibody assays and the content of rabbit sera for other anti-peptide antibodies, homologous and cross-inhibition assays were performed. Thus, sera from immunized rabbits in 1:200 dilution were preincubated each with different peptides at concentrations 0·5, 5 and 10 μg/ml and the anti-peptide ELISAs were repeated. The percentage of inhibition of the antibody binding to the peptides immobilized on ELISA plates was calculated as follows: inhibition percentage = 100 × [1 − (inhibited OD405nm/uninhibited OD405nm)]. Sera that recognized the whole La/SSB molecule were preincubated in dilution 1:500 with: (i) the peptide used for the animal immunization, (ii) the remaining three La/SSB peptides, and (iii) the mixture of all four peptides at concentrations 0·5, 5, 10, 50, 100 and 200 μg/ml, overnight at 4°C. Inhibition assays were performed in ELISA plates, precoated with affinity-purified La/SSB antigen (Shield Diagnostics, Diastat).
T cell proliferation assays
Three hundred and twenty-eight days after the first immunization rabbits nos 1, 2, 3, 4, 5, 9 and 10 were killed. Rabbits nos 6, 7 and 8 died between the 53rd and 99th days of the immunization process. Spleen cells and peripheral mononuclear cells were isolated and cultured with 5 μg/ml of different peptides (27 overlapping 23mer peptides of La/SSB, four peptides corresponding to the La/SSB linear B cell peptides and the SOC carrier alone) in triplicate, as follows: 2 × 105 spleen cells/well were cultured in round-bottomed 96-well plates, in RPMI 1640 medium in the presence of 0·2 mm l-glutamine, 100 U/ml penicilln, 100 U/ml streptomycin, and 10% fetal calf serum (FCS) for 5 days. T cell proliferation was assessed by the addition of 1 μCi/well of 3H-thymidine for the last 18 h of culture. Thymidine incorporation was measured using a liquid scintilation beta counter analyser (Packard, Meriden, CT). The stimulation index (SI) was calculated as the ratio of ct/min received in the presence versus absence of the specific antigen. Values of SI > 2 were considered positive. For the peripheral blood mononucelar cell (PBMC) proliferation assay, irradiated spleen cells (30 Gy, 105 cells/well) were used for enrichment of the antigen-presenting cells (APC). Cells added in triplicate in culture medium alone or containing 1 μg/ml phytohaemagglutinin (PHA) (PHA response lasted 3 days) were used as controls, in all the proliferation assays. All experiments were performed in duplicate.
RESULTS
Induction of anti-La/SSB and anti-peptide antibodies
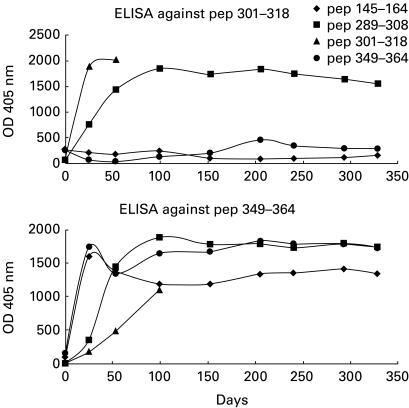
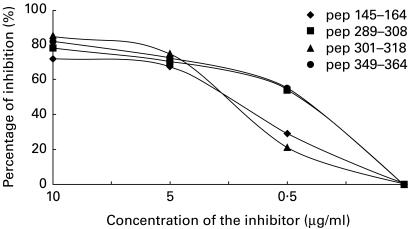
Sera from rabbits immunized with each SOC4–La/SSB peptide conjugate were collected before and after each immunization and were tested for their reactivity against the immunizing as well as the remaining peptides. Sera from rabbits immunized either with Freund's complete adjuvant (FCA) alone (control animal) or the SOC carrier, did not recognize any of the La/SSB synthetic epitope analogues. The antibody activity was gradually increased and after the third boost remained high until the end of the study. The optical density (OD) in serum dilution 1:200 was ranged from 1·400 to 2000 and coefficient variation between the same experiment was < 10%. Sera obtained from the animals immunized with each of the p1, p2 and p3 peptides recognized not only the immunizing but also the other peptides studied. The higher reaction was observed in the ELISA against the immunizing peptide. In contrast, animals immunized with peptide p4 produced antibodies against only the homologous peptide (Fig. 1). Preincubation of sera obtained from rabbits immunized with a particular epitope resulted in significant reduction of the antibody binding to the homologous peptides. In particular, the homologous inhibition of sera from rabbits immunized with the peptides p1 and p2 led to 72% and 78% inhibition, respectively. Also, homologous inhibition using peptides 3 and 4 resulted in 85% and 82% inhibition, respectively (Fig. 2).
Fig. 1.
Sera from rabbits immunized with synthetic peptide analogues of La/SSB are tested against peptide 3 (301VTWEVLEGEVEKEALKKI318) (top) and peptide 4 (349GSGKGKVQFQGKKTKF364) (bottom). Sera from animals immunized with peptide 2 also contained antibodies to peptides 3 and 4. In addition, sera from all animals contained antibodies to peptide 4.
Fig. 2.
Homologous inhibition assays of all anti-peptide ELISAs exhibited a maximum inhibition range of 72–85%.
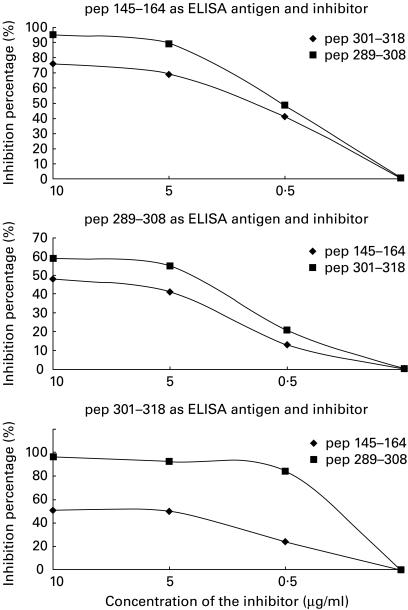
Cross-inhibition assays
All animal sera contained a mixture of antibody specificities recognizing not only the immunizing peptide but also the remaining peptides. Thus, the reactivity of sera from animals immunized with peptide p1 was inhibited by 48%, 51% and 40%, respectively, after serum preincubation with the peptides p2, p3 or p4. Similarly, the reactivity of sera derived from the animal immunized with peptide 2 was inhibited by 95%, 96% and 61%, respectively, after preincubation with the peptides p1, p3 and p4. Sera obtained from rabbits immunized with peptide p3 were inhibited by 76%, 60% and 55%, respectively, after preincubation with peptides p1, p2 and p4 (Fig. 3).
Fig. 3.
Cross-inhibition in the assays of synthetic peptide analogue 1 (145TLHKAFKGSIFVVFDSIESA164) (top) and synthetic peptide analogue 2, to determine the binding of antibodies (bottom) produced by intramolecular spreading in animals immunized with other peptides.
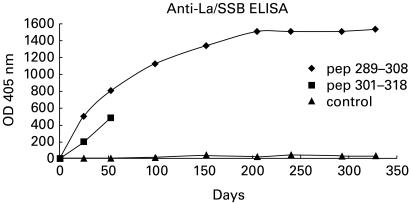
Inhibition of antibody binding to the intact La/SSB antigen
Sera from rabbits immunized with peptides p2 and p3 contained antibodies binding to the intact La/SSB antigen in the ELISA (Fig. 4). Preincubation of these sera in dilution 1:500 with peptides p2 and p3 as well as with a mixture of all peptides resulted in the inhibition of anti-La/SSB reactivity. All sera from the immunized rabbits were tested with LIA test and immunoblotting, using cytoplasmic extract from HeLa cells and rabbit spleen cells. Sera from rabbits immunized with peptides p2, p3 and p4 recognized the recombinant, human and rabbit La/SSB molecule. Binding to Ro/SSA 60 or 52kD autoantigen was not observed either by counterimmunoelectrophoresis or immunoblot.
Fig. 4.
Binding of antibodies, produced after immunization with synthetic peptides 2 and 3 in the intact La/SSB protein. Sera from animals immunized with Freund's complete adjuvant alone have been used as controls.
T cell proliferation assays towards the La/SSB peptides
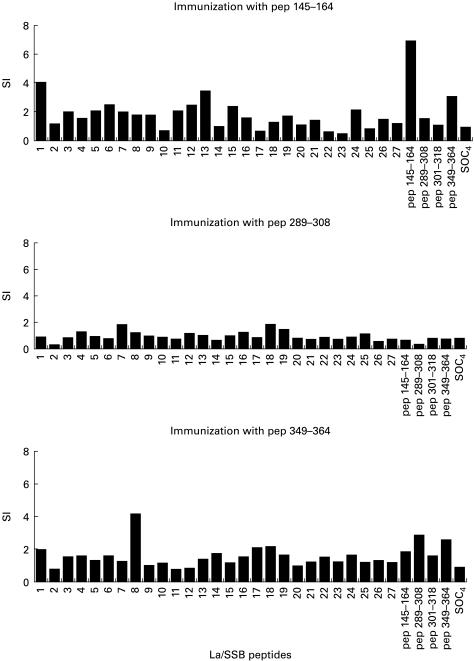
Spleen cells and PBMC isolated from rabbits were co-cultured with 31 La/SSB peptides (27 overlapping peptides and four synthetic epitope analogues). Responses without peptide or SOC4 backbone alone were 12 524 and 9268 ct/min, respectively. Spleen cells obtained from rabbits immunized with p1 responded to the immunizing peptide as well as the peptides spanning the sequences 1–23 aa (no. 1) (28 873 ct/min), 181–203 aa (no. 13) (38 772 ct/min), 376–398 aa (no. 26) (28 806 ct/min), 386–408 aa (no. 27) (28 379 ct/min) and 349–364 aa (p4) (58 894 ct/min). Spleen cells from rabbits immunized with peptide 2 responded also to peptides 286–308 aa (no. 20) (58 894 ct/min) that contain peptide 2 and peptide 4 (28 473 ct/min). Control responses were as follows: without peptide 8999 ct/min and with SOC4 alone 11 088 ct/min. Rabbits immunized with peptide 3 did not reach the end of the experiment. Finally, spleen cells from the rabbits immunized with peptide 4 strongly recognized the peptide of origin and also the sequences 76–98 aa (no. 6) (28 871 ct/min), 106–128 aa (no. 8) (15 944 ct/min), 151–173 aa (no. 11) (15 832 ct/min), 241–263 aa (no. 17) (19 211 ct/min), 346–368 aa (no. 24) (16 078 ct/min) incorporating peptide 4, peptides 1 and 2. (Responses without peptide: 6932 ct/min and with SOC4 alone 5832 ct/min; Fig. 5.) PBMC isolated from the immunized animals proliferated rather poorly in the presence of La/SSB peptides.
Fig. 5.
T cell proliferation assays using 27 overlapping peptides covering the sequence of La/SSB. The four last peptides correspond to linear B cell peptides 1, 2, 3 and 4. The cut-off point for positivity in stimulation index (SI = 2).
Spleen cells and PBMC isolated from control rabbit did not respond to any of La/SSB-related peptides. No response was observed to the SOC backbone alone. Spleen cells and PBMC from all animals were found to respond well to PHA (> 100 000 ct/min), whereas the irradiated spleen cells did not.
DISCUSSION
In humans, reactivity to the native La/SSB protein occurs primarily in patients with SS. This autoantibody response is thought to involve an antigen-driven process, but its precise nature and role remain unclear. Anti-La/SSB reactivities are characterized by polyclonality against multiple peptides of the La/SSB protein as well as by the parallel occurrence of autoantibodies to another member of the heterogeneous hYRNP complex, namely the Ro/SSA protein [1,14]. Despite the fact that there are obvious difficulties in reflecting the human autoimmune response to non-autoimmune experimental animals, a large body of knowledge has been mounted over the last 7 years in regard to antibody response in several animals.
In previous studies the immunization of normal mice with recombinant preparations or short peptides of the La/SSB [15–17] or the Ro/SSA protein [18] has been shown to result in polyclonal antibody responses against peptides which spanned multiple sites on both proteins. Such intramolecular and intermolecular spreading of reactivities has been thought to arise from the activation of antigen-specific T helper clones able to stimulate a diverse array of B cells specific for the constituents of endogenous La/SSB-Ro/SSA protein complexes [15,19,20]. In this study the immunogenicity of the four dominant linear B cell synthetic epitope analogues of the human autoantigen La/SSB was investigated in NZW rabbits. For the immunization, peptides were attached on tetramer sequential oligopeptide carriers (SOC4) as previously described [21,22]. In our hands, immunization with SOC4–peptide conjugates proved an efficient tool. As shown in this study, a high, specific and sustained immune response against the various conjugated peptides was obtained by the immunization of rabbits with the various SOC4–La/SSB peptide conjugates. Furthermore, immunization with La/SSB peptides was found capable of inducing strong B and T cell responses not only against the particular peptide used for the priming of animals, but also against other peptides of the La/SSB antigen. The potent immunogenic property of synthetic peptide polymers, as opposed to monomeric peptide preparations, has been also previously reported [17]. Although healthy animals have been shown to be in a non-tolerant immune state for intracellular proteins which are autoantigenic in humans, the patterns of endogenous autoantigen recognition by humans and experimental animals may differ [23]. In addition, discrete differences in amino acid sequences of peptides applied for immunization may be crucial in the induction and maintenance of responses, as well as for the intramolecular spreading of reactivities [24]. Unfortunately, the current unavailability of the complete sequence of the rabbit La/SSB protein precludes the comparison of amino acid sequence differences between the human-derived La/SSB peptides applied in this study and the reciprocal peptides in the rabbit.
The intramolecular spreading of antibody responses following immunization with peptide polymers appears well documented [12,15–17]. Our findings are in agreement with these reports and further suggest that immunization with synthetic peptides also leads to the intramolecular spreading of T cell reactivities, albeit in a more restricted pattern. These phenomena probably result from the sequential reactions between homotypic and heterotypic antigen-specific B and T cells. B and T cells with specificity for one or more peptides of the La/SSB protein should exist in non-immunized animals, but at quite low frequencies. As in most antibody responses, the induction of anti-La/SSB protein reactions in the immunized rabbits probably involves the activation of helper T cells through the presentation of the priming peptides by rabbit MHC class II-expressing professional APC. Although the peptide binding characteristics of rabbit MHC molecules are yet to be defined, our results suggest that at least three of the four La/SSB peptides used for the immunizations were adequately presented by rabbit APC. The capacity of B cells to capture even minute amounts of circulating antigens and to present protein determinants to peptide-specific T cells is crucial for the T cell-dependent stimulation of B cells and antibody production [19,25]. In the case of immunization with synthetic peptide polymers, as in the present study, B cells specific for the priming peptide should be able to present increased amounts of the same peptide to T cells. Thus, under our experimental conditions, immunization should primarily involve the activation and the expansion of B and T cell populations recognizing the priming peptide, a prediction which is in accordance with our findings. In the process, peptide-specific B cells may capture endogenously derived La/SSB molecules by surface immunoglobulin receptors and present new determinants to T cells. This would presumably result in the activation of one or more T cell clone(s) reacting to secondary epitope(s) of the La/SSB protein, which in turn are able to provide help to diverse B cell clones that recognize multiple peptides of the La/SSB protein.
In this study, despite the notable intramolecular spreading of the anti-La/SSB response, no evidence of intermolecular spreading of reactivities to the Ro/SSA antigen was observed. Intermolecular spreading of antibody responses has been reported to occur following immunization of animals with recombinant La/SSB protein [15,17], as well as with polymeric preparations of peptide sequences residing in the lupus autoantigenic protein Sm [14], mouse La/SSB [16,17] and human Ro (SSA) protein [23]. It is noticeable however, that in those studies certain peptide sequences derived from the above proteins were found incapable of inducing intermolecular diversification of immune responses [16–18,24,26]. Further studies are necessary to clarify the implication of dominance and crypticity features of peptide sequences selected for the priming of animals, in these phenomena [27,28].
Acknowledgments
This work has been supported by a grant (EKBAN-P45) from the Hellenic Secretariat for Research and Technology. Part of the work has been also supported by a grant of the Hellenic Society of Rheumatology and the BMH4-CT96-0595 CA EU concerted action for Sjögren's Syndrome. The authors wish also to thank Ms Pola Papadopoulou for excellent secretarial assistance.
REFERENCES
- 1.Manoussakis MN, Tzioufas AG, Pange PJE, Moutsopoulos HM. Serological profiles in subgroups of patients with Sjögren's syndrome. Scand J Rheumatol. 1986;61:89–92. [PubMed] [Google Scholar]
- 2.Harley JB, Alexander EL, Bias WB, Fox RI, Provost TT, Reichlin M, Yamagata H, Arnett FC. Anti-Ro (SSA) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986;29:196–205. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- 3.Andonopoulos AP, Skopouli FN, Dimou GS, Drosos AA, Moutsopoulos HM. Sjögren's syndrome in systemic lupus erythematosus. J Rheumatol. 1990;17:201–4. [PubMed] [Google Scholar]
- 4.Tzioufas AG, Yiannaki E, Sakarellos-Daitsiotis M, Routsias JG, Sakarellos C, Moutsopoulos HM. Fine specificity of autoantibodies to La/SSB. Epitope mapping molecular mimicry and clinical applications. Clin Exp Immunol. 1997;108:191–8. doi: 10.1046/j.1365-2249.1997.d01-1003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yiannaki EE, Tzioufas AG, Bachmann M, Hantoumi J, Tsikaris V, Sakarellos-Daitsiotis M, Sakarellos C, Moutsopoulos HM. The value of synthetic linear epitope analogues of La/SSB. Specificity, sensitivity and comparison of methods. Clin Exp Immunol. 1998;112:152–8. doi: 10.1046/j.1365-2249.1998.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsikaris V, Sakarellos C, Cung-Thong M, Marraud M, Sakarellos-Daitsiotis M. Concept and design of a new class of sequential oligopeptides carriers (SOC) for covalent attachment of multiple antigenic peptides. Biopolymer. 1996;38:291–3. doi: 10.1002/(SICI)1097-0282(199603)38:3%3C291::AID-BIP1%3E3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Merrifield RB. Solid phase peptide synthesis I. The synthesis of a tetrapeptide. JACS. 1963;85:2149–54. [Google Scholar]
- 8.Geysen HM, Rodda SJ, Mason TJ, Tribbick G, Schoofs PG. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987;202:259–65. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- 9.Maeji NJ, Bray AM, Geysen HM. Multi-pin peptide synthesis strategy for T cell determinant analysis. J Immunol Methods. 1990;134:23–33. doi: 10.1016/0022-1759(90)90108-8. [DOI] [PubMed] [Google Scholar]
- 10.Mutch DA, Rodda SJ, Benstead M, Valerio RM, Geysen HM. Effects of end groups on the stimulatory capacity of minimal length T cell determinant peptides. Peptide RES. 1991;4:132–7. [PubMed] [Google Scholar]
- 11.Reece JR, McGregor DL, Geysen HM, Rodda SJ. Scanning for T helper peptides with human PBMC using pools of short synthetic peptides. J Immunol Methods. 1994;172:41–54. doi: 10.1016/0022-1759(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 12.James JA, Gross T, Scofield H, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B′/B-derived PPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–61. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Venroij WJ, Maini RN. Manual of biological markers of disease. Dordrecht: Kluwer Academic Publishers; 1996. [Google Scholar]
- 14.Scofield RH, Farris AD, Horsfall AC, Harley JB. Fine specificity of the autoimmune response to the Ro/SSA and La/SSB ribonucleoproteins. Arthritis Rheum. 1999;42:99–105. doi: 10.1002/1529-0131(199902)42:2<199::AID-ANR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Topfer F, Gordon T, McCluskey J. Intra- and intermolecular spreading of autoimmunity involving the nuclear self-antigens La (SSB) and Ro (SSA) Proc Natl Acad Sci USA. 1995;92:875–9. doi: 10.1073/pnas.92.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds P, Gordon TP, Purcell AW, Jackson DC, McCluskey J. Hierarchical self-tolerance to T cell determinants within the ubiquitous nuclear self-antigen La (SS-B) permits induction of systemic autoimmunity in normal mice. J Exp Med. 1996;184:1857–70. doi: 10.1084/jem.184.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farris AD, Brown L, Reynolds P, Harley JB, James JA, Scofield RH, McCluskey J, Gordon TP. Induction of autoimmunity by multivalent immunodominant and subdominant T cell determinants of La (SS-B) J Immunol. 1999;162:3079–87. [PubMed] [Google Scholar]
- 18.Scofield RH, Henry WE, Kevrien BT, James JA, Harley JB. Immunization with short peptides from the sequence of the systemic lupus erythematosus-associated 60kda Ro autoantigen results in anti-Ro ribonucleoprotein autoimmunity. J Immunol. 1996;156:4059–66. [PubMed] [Google Scholar]
- 19.Mamula MJ, Janeway Ca., Jr Do B cells drive the diversification of immune responses? Immunol Today. 1993;14:151–2. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- 20.Craft J, Fatenejad S. Self antigens and epitope spreading in systemic autoimmunity. Arthritis Rheum. 1997;10:1374–82. doi: 10.1002/art.1780400803. [DOI] [PubMed] [Google Scholar]
- 21.Tsikaris V, Sakarellos C, Sakarellos-Daitsiotis M, Cung MT, Marraud M, Konidou G, Tzinia A, Soteriadou KP. Use of sequential oloigopeptide carriers (SOCn) in the design of potent Leishmania gp63 immunogenic peptides. Peptide Res. 1996;9:240–7. [PubMed] [Google Scholar]
- 22.Tsikaris V, Sakarellos C, Sakarellos-Daitsiotis M, Orlewski P, Marraud M, Cung MT, Vatzaki E, Tzartos S. Construction and application of a new class of sequential oligopeptide carriers (SOCn) for multiple anchoring of antigenic peptides—application to the acetylocholine receptor (AChR) main immunogenic region. Biol Macromol. 1996;19:195–205. doi: 10.1016/0141-8130(96)01128-2. [DOI] [PubMed] [Google Scholar]
- 23.St Clair EW, Kenan D, Burch Ja, Jr, Keene JD, Pisetsky DS. Anti-La antibody production by MRL-1pr/1pr mice. Analysis of fine specificity. J Immunol. 1991;146:1885–92. [PubMed] [Google Scholar]
- 24.Scofield RH, Kaufman KM, Baber U, James JA, Harley JB, Kurien BT. Immunization of mice with human 60-Kd Ro peptides results in epitope spreading if the peptides are highly homologous between human and mouse. Arthritis Rheum. 1999;2:1017–24. doi: 10.1002/1529-0131(199905)42:5<1017::AID-ANR22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Lanzavecchia A. Receptor mediated antigen uptake and its effect on antigen presentation to class II-restricted T-lymphocytes. Annu Rev Immunol. 1990;8:773–93. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 26.Vlachoyiannopoulos PG, Petrovas C, Tzioufas AG, et al. No evidence of epitope spreading after immunization with the major Sm epitope PPGMRPP anchored to sequential oligopeptide carriers (SOCs) J Autoimmun. 2000;14:53–61. doi: 10.1006/jaut.1999.0344. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–7. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 28.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Mondgil K. Dominance and crypticity of T-cell antigenic determinants. Annu Rev Immunol. 1993;11:729–66. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]