Abstract
Increased serum cytokine levels have been reported in patients with autoimmune thyroid disease, but less is known about their levels in patients with Graves' ophthalmopathy (GO). It is not known whether GO is a cell-mediated or humoral autoimmune disease. We investigated whether serum cytokines are elevated in GO patients and whether the cytokines were Th1- or Th2-derived. In addition, elevated cytokines might reflect the activity of GO, and thus we investigated whether cytokine levels could predict the clinical response to orbital radiotherapy. We studied 62 consecutive patients with moderately severe untreated GO and 62 healthy controls, matched for sex, age and smoking habits. Serum concentrations of IL-1RA, sIL-2R, IL-6, sIL-6R, tumour necrosis factor-alpha (TNF-α) RI and II and sCD30 were measured using highly sensitive ELISAs, in the patients before and 3 and 6 months after radiotherapy. All patients were euthyroid, with anti-thyroid drugs, before and during the entire study period. All baseline cytokine and cytokine receptor levels were significantly elevated in GO patients compared with healthy controls, except for IL-1RA. The levels did not correlate with parameters of the thyroid disease, nor with the duration, activity or severity of GO. However, backward logistic regression analysis showed that IL-6, sCD30 and TNFαRI were able to predict a beneficial response to orbital radiotherapy. We therefore conclude that both Th1- and Th2-derived cytokines are elevated in GO patients compared with its controls. IL-6, sCD30 and TNFαRI had some value for predicting therapeutic outcome to orbital irradiation, and may thus reflect active eye disease.
Keywords: Graves' ophthalmopathy, IL-1RA, sIL-2R, IL-6, IL-6R, TNFαR, sCD30, smoking, thyroid disease
Introduction
Graves' ophthalmopathy (GO) is an orbital disease of presumed autoimmune aetiology, closely connected with Graves' hyperthyroidism. Histological examination of orbital tissue in the active stages of GO shows a lymphocytic infiltrate consisting mainly of T-helper and T-suppressor cells, together with some B cells, macrophages and a few plasma cells and mast cells [1,2]. The immune process in the orbit leads to proliferation of fibroblasts and stimulation of glycosaminoglycan (GAG) production, which in turn leads to swelling of orbital tissue [1,3–5]. These features cause the clinical manifestations of proptosis, diplopia, periorbital swelling and inflammation [5,6]. Once the active inflammation has subsided fibrotic tissue is left.
Lymphocytes produce different cytokines which can have an effect on retrobulbar fibroblasts [7,8]. IL-1 and IL-4 stimulate the proliferation, and GAG production by fibroblasts is stimulated by interferon-gamma (IFN-γ), tumour necrosis factor-beta (TNF-β) and IL-1 and inhibited by IL-1RA [8–10]. Some cytokines can induce the expression of modulatory molecules like HLA-DR, intercellular adhesion molecule-1 (ICAM-1), and heat shock protein (hsp72) on retrobulbar fibroblasts [11–14]. Whether these in vitro data are relevant for the in vivo situation is unclear, because only some of the above mentioned cytokines, e.g. IL-4, IL-1 and IFN-γ, have been found in orbital tissues using polymerase chain reaction (PCR) or immunohistochemistry [15,16].There are only scanty data about their serum levels in GO. In patients with Graves' hyperthyroidism increased levels of soluble IL-2 receptor (sIL-2R), IL-6, IL-6 receptor (IL-6R), soluble CD30 (sCD30) have been found [17–21]. In GO we only know that sIL-2R and IL-6R levels are elevated, and that IL-1 and IL-1RA increase in response to radiotherapy in GO [20,22,23].
To understand better the pathogenesis of GO it would be important to know which cytokines are present in the lymphocytic infiltrate. Such knowledge might help to establish whether GO is mainly a humoral (Th1), or a cellular (Th2) immune process, or both. To answer this question, analysis of orbital tissues would be necessary. Since it is difficult to obtain orbital tissue from a large number of patients, measuring serum levels seems to be a helpful first step. Another reason why serum cytokine levels are potentially important is the distinction between active and inactive eye disease, as the former but not the latter may be susceptible to immunosuppressive therapy. Pathogenically, the difference between active and inactive GO is the presence of a lymphocytic infiltrate during the active stage. This is clinically of little relevance however, and other methods are necessary to separate these stages, since the stage of the disease indicates which treatment should be given. Since cytokines seem to be involved as intermediary effectors in GO, we wondered whether serum measurements might help to differentiate between active and inactive disease.
The aim of our study is (i) to determine whether there is a difference in cytokine levels between GO patients and healthy controls, and (ii) to establish whether cytokine levels correlate with the activity and/or severity of GO. Because Graves' hyperthyroidism itself is associated with elevation of serum cytokines, we only studied GO patients, who were euthyroid for > 2 months. Since GO patients smoke more frequently than the general population, and smoking might have an influence on cytokine levels, we matched controls for age, sex and smoking habits. We chose to measure a panel of stimulatory (IL-6, TNFαRI and II) [24,25], inhibitory (IL-1RA), Th1-derived (sIL-2R, TNFαRI and II), and Th2-derived (IL-6, IL-6R) cytokines and sCD30. sCD30 is a member of the TNF receptor superfamily, and is released by T cells that secrete Th2-type cytokines [26–28].
PATIENTS and METHODS
Patients and controls
We studied 62 consecutive patients with moderately severe untreated GO, who qualified for retrobulbar irradiation. The patients had severe soft tissue involvement, and/or proptosis of ≥ 25 mm, and/or impaired eye muscle motility (mostly with diplopia). None had optic nerve involvement. All patients were euthyroid, mostly on anti-thyroid drugs, for at least 2 months before start of treatment (defined as: free T4 (fT4) 10–23 pmol/l, total T3 1·3–2·7 nmol/l, thyroid-stimulating hormone (TSH) < 4·0 mU/l). Blood for cytokine measurements was withdrawn from the patients before, and 3 and 6 months after treatment. Controls were recruited from the general population by advertisement, and participated in a study to determine reference values. They were matched for age (within + 5 or − 5 years), sex, and smoking habits. Smokers were defined as individuals who currently smoked cigarettes and non-smokers did not.
Retrobulbar radiotherapy consisted of 10 fractions of 2 Gy, in 2 weeks (20 Gy in total). Six months later the response was assessed according to changes in major and minor criteria. Major criteria were changes in grade of diplopia (0 = no diplopia, 1 = intermittent, 2 = inconstant, 3 = constant diplopia) [29], and changes in eye muscle motility (≥ 8° elevation). Minor criteria were (i) a change in appearance on colour slides (pre- and post-treatment) [30], (ii) a change in lid aperture of ≥ 2 mm, or (iii) a change in proptosis of ≥ 2 mm. Amelioration in more than one major criterion or in two minor criteria was considered a response. No response was defined as no change at all, or changes in 1 minor criterion only. If a patient deteriorated this was considered as no response. Orbital radiotherapy resulted in a response in 34/62 (55%) patients. No change or deterioration was observed in 28/62 (45%) patients.
Clinical characteristics
The activity of the ophthalmopathy was scored using the classic inflammation parameters: rubor, dolor, tumour and functio laesa [31]. This Clinical Activity Score consists of 10 items, for each item present 1 point is given, thus the maximal score is 10. We also noted duration of both the eye disease and the thyroid disease in months since the first signs or symptoms, and the Total Eye Score, calculated as the sum of multiplying each NO SPECS class present by the grade in that class (for that purpose we substituted 1, 2, and 3, respectively, for grades a, b, and c) [32], as an overall measurement of disease severity.
Methods
fT4 was determined with either a coated tube 125I radioimmunoassay (SPAC; Byk-Sangtec Diagnostica, Dietzenbach, Germany), or a solid-phase time-resolved fluoroimmunoassay (Delfia; Wallac Oy, Turku, Finland). TBII was measured by TRAK assay (BRAHMS Diagnostica, Berlin, Germany). Anti-thyroid peroxidase (TPO) antibodies were measured by immunofluorescence.
Serum samples were kept stored at −20°C until use. All samples were measured in duplicate. sCD30 (detection limit 0·5 U/ml) was measured by an ELISA (Bender MedSystems, Vienna, Austria). Highly sensitive, commercially available ELISA kits (Quantikine; R&D Systems, Minneapolis, MN) were used to measure serum concentrations of IL-1RA (detection limit 14 pg/ml), sIL-2R (detection limit 24 pg/ml), IL-6 (detection limit 0·094 pg/ml), sIL-6R (detection limit 140 pg/ml), TNFαRI (detection limit 30 pg/ml) and II (detection limit 10 pg/ml) and expressed in pg/ml.
Statistical analysis
To analyse differences between GO patients and healthy controls we used either t-tests or in case of an abnormal distribution, the Mann–Whitney U-test. To assess whether one or more cytokine or cytokine receptors could predict for the response to orbital radiotherapy, stepwise, backward, logistic regression analysis was performed in which response was the dependent variable. In short, this means that all cytokine values are added to the regression analysis and then they are stepwise eliminated from the formula if they have no statistically significant influence on the dependent variable, e.g. response to treatment. At the end, these variables with a P < 0·10 are left in the regression equation. On the basis of this analysis, a receiver-operator-characteristics (ROC) curve was drawn, and the area under the curve (AUC) was calculated. Correlations were calculated with two-tailed Pearson correlation coefficients. Changes in serum cytokine concentrations were evaluated with repeated measurement F-tests.
Results
Serum cytokine levels in patients and controls
Patients and controls were well matched and had similar fT4 levels. Patients had significantly higher levels of all Th1- and Th2-derived cytokines with the exception of IL-1RA (Table 1). This could not be explained by differences in smoking behaviour in view of the matching procedure, although differences in some cytokine concentrations were noted between smokers and non-smokers (Table 2).
Table 1.
Serum cytokine concentrations in patients with untreated moderately severe Graves' ophthalmopathy and healthy matched controls
| Patients | Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 62 | 62 | |||||||
| Age (years) | 53·1 ± 10·3 | 53·2 ± 9·7 | |||||||
| F/M | 43/19 | 43/19 | |||||||
| Smoking, yes/no | 32/30 | 32/30 | |||||||
| fT4 (pmol/l) | 15·5 ± 4·7 | 15·9 ± 3·5 | P | ||||||
| sIL-2R | 1339 ± 421 | 949 ± 227 | < 0·0001 | ||||||
| IL-1RA | 394 ± 249 | 384 ± 162 | NS | ||||||
| IL-6 | 3·16 ± 2·7 | 1·98 ± 2·0 | 0·009 | ||||||
| IL-6R | 35 623 ± 7860 | 30 434 ± 7554 | < 0·0001 | ||||||
| TNFαRI | 1115 ± 232 | 951 ± 176 | < 0·0001 | ||||||
| TNFαRII | 3209 ± 828 | 2112 ± 487 | < 0·0001 | ||||||
| sCD30 (U/ml) | 44 (18–153) | 27 (10–87) | < 0·0001 | ||||||
Table 2.
Serum cytokine concentrations in smokers and nonsmokers of patients with untreated moderately severe GO and healthy matched controls
| Smoking | Non-smoking | P | ||
|---|---|---|---|---|
| sIL-2R (pg/ml) | Patients | 1358 ± 439 | 1320 ± 408 | 0·728 |
| Controls | 969 ± 259 | 930 ± 189 | 0·507 | |
| IL-1RA (pg/ml) | Patients | 470 ± 323 | 312 ± 75 | 0·011* |
| Controls | 410 ± 203 | 356 ± 98 | 0·191 | |
| IL-6 (pg/ml) | Patients | 2·2 (0·9–23·4) | 2·0 (0·6–12·1) | 0·933 |
| Controls | 1·7 (0·5–11·7) | 1·2 (0·6–6·3) | 0·597 | |
| IL-6R (pg/ml) | Patients | 35 719 ± 7464 | 35 519 ± 8390 | 0·921 |
| Controls | 33 218 ± 7545 | 27 464 ± 6441 | 0·002* | |
| TNFαRI (pg/ml) | Patients | 1139 ± 237 | 1089 ± 228 | 0·409 |
| Controls | 963 ± 213 | 939 ± 128 | 0·599 | |
| TNFαRII (pg/ml) | Patients | 3301 ± 813 | 3110 ± 845 | 0·368 |
| Controls | 2054 ± 581 | 2173 ± 845 | 0·340 | |
| sCD-30 (U/ml) | Patients | 44 (26–153) | 45 (18–111) | 0·949 |
| Controls | 29 (10–87) | 26 (11–61) | 0·164 |
In the patients the different serum cytokine concentrations were not related to thyroid status (fT4), methimazole use, anti-TPO antibody titre, TBII titre, or the duration of the thyroid disease. The duration of the eye disease ranged from 4 to 240 months (median 17 months). At study entrance they had a Clinical Activity Score of 3·2 ± 1·8 (mean ± s.d.). The diplopia score was 2·4 + 0·9. Proptosis ranged from 13 to 28 mm (median 19 mm), and overall severity of eye disease (Total Eye Score) from 2 to 19 (median 12). There was no correlation between cytokine levels and several ophthalmopathy parameters; nor with the duration nor with the severity or activity of the eye disease.
Correlations between the different serum cytokines
The Th1 cytokines (TNFαRI, TNFαRII, sIL-2R) correlated well with each other (P < 0·0001), as did the Th2-derived cytokines IL-6 and sCD30 (P < 0·001). Th1 cytokine sIL-2R levels did correlate well with both Th2 cytokines, IL-6 (P < 0·01) and sCD30 (P < 0·0001). Th2 cytokine sCD30 did correlate well with all Th1 cytokines and IL-6 correlated well with sIL-2R (P < 0·01) but not with the TNF-α receptors. Lastly, IL-6 did not correlate with IL-6R (data not shown). IL-1RA only correlated with TNFαRI (P < 0·0001).
Prediction of the response to radiotherapy
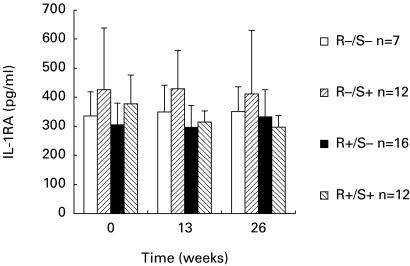
When we compared the pretreatment values in the responders to radiotherapy (supposed to have been active at baseline) with those who did not respond (presumed to have been inactive), there were no differences in any of the cytokine levels (Table 3). However, using backward logistic regression analysis, IL-6, sCD30 and sTNFαRI together predicted response. Calculating a ROC curve with IL-6, sCD30 and sTNFαRI together the AUC was 0·69. In a subgroup of 47 patients we also studied serum cytokine concentrations 3 and 6 months after radiotherapy. Serum levels did not change upon radiotherapy (Fig. 1, for data on sIL-1RA)
Table 3.
Baseline serum cytokine concentrations in patients with moderately severe Graves' ophthalmopathy (GO) who responded or did not respond to orbital irradiation
| n = | Response 34 (55%) | No response 28 (45%) | t-test or Mann– Whitney | Stepwise backward Logistic regression |
|---|---|---|---|---|
| IL-1RA (pg/ml) | 359 ± 177 | 435 ± 314 | 0·233 | |
| sIL-2R (pg/ml) | 1335 ± 416 | 1345 ± 435 | 0·922 | |
| IL-6R (pg/ml) | 35·655 ± 7272 | 35·583 ± 8657 | 0·972 | |
| TNFαRII (pg/ml) | 3174 ± 805 | 3251 ± 868 | 0·720 | |
| IL-6 (pg/ml) | 2·3 (0·6–23·4) | 1·9 (0·9–10) | 0·502 | 0·10 |
| TNFαRI (pg/ml) | 1084 ± 181 | 1151 ± 281 | 0·283 | 0·04 |
| sCD30 (U/ml) | 45 (17·5–153) | 43 (21·8–92·4) | 0·262 | 0·10 |
Fig 1.
IL-1RA in 47 moderately severe Graves’; ophthalmopathy (GO) patients measured before and 13 and 26 weeks after orbital radiotherapy. Patients are divided into responders (R+) and non-responders (R−) and smokers (S+) and non-smokers (S−).
Discussion
In this study we measured a panel of cytokines in sera of 62 patients with moderately severe GO and in 62 matched healthy subjects. We found that almost all cytokines were increased in the patients compared with the controls. This was true for both Th1- and Th2-derived cytokines, as well as for stimulatory cytokines. IL-1RA was the only exception, with similar levels in patients and controls. The question thus arises whether increased levels in the patients reflect the immune response in orbital Graves' disease. All patients, except for one, who had euthyroid Graves' disease, also had a clinical history of Graves' hyperthyroidism. However, it seems unlikely that the thyroid disease alone caused the observed elevated cytokine levels. First, all patients were euthyroid for at least 2 months prior to enrolment in our study. Second, no correlations were found between cytokine and fT4 levels. Third, we could not find any correlation between cytokine levels and parameters of autoimmune thyroid disease, like TPO antibodies or TBII. Thus the elevated serum cytokines did not seem to be related just to the thyroid disease itself. Therefore it seems that at least some cytokines might reflect the presence of the eye disease, although we can not rule out the possibility that these increased cytokine levels reflect a heightened overall activation of the immune system in Graves' disease patients. Another explanation might be that our GO patients have a different genetic background in term of MHC haplotypes compared with our controls. For, there are differences in cytokine production in vitro in subjects with a HLA-B8, DR3 haplotype compared with others [33]. We could not find a correlation between serum cytokines and the duration, severity or activity of the eye disease, but using backward logistic regression analysis, IL-6, sCD30 and TNFαRI together had some value for predicting a response to radiotherapy. This suggests that elevation of the cytokines indeed reflects active eye disease at baseline, although this should be tested for clinical relevance in a second study.
There is controversy in the literature about GO being a Th1 (cell-mediated), or Th2 (humoral) autoimmune phenomenon, or both [15,34,35]. We found that all cytokines, except for IL-1RA, were elevated, both Th1- and Th2-derived cytokines and stimulating cytokines. It is likely that these findings indicate a reflection of the delicate cytokine network in the orbit, though it remains difficult to determine their pathogenic role and place in the cascade.
We also found that smoking tended to have some effect on serum cytokine levels. Il-1RA and IL-6R were significantly higher in smokers than in non-smokers. Also some of the other cytokines tended to be higher in smokers, though this did not reach statistical significance. Smoking is strongly associated with GO [36–38]. The mechanism by which smoking might aggravate GO is not known. There might be a role for hypoxaemia, as was shown in an in vitro study by Metcalfe et al. [39], or smoking might induce antibodies like hsp antibodies, as was found by our group [12].
To our knowledge this is the first study to compare various cytokines between ophthalmopathy patients and controls. There are only data on sIL-2R and IL-6 R levels in GO patients and our study confirms that these two cytokines are elevated in GO. There are no data on the other cytokines, with the exception of IL-1RA. Hofbauer et al. [23] measured serum IL-1RA levels in 25 GO patients and found that IL-1RA was decreased in 18 smoking patients compared with seven non-smoking patients. Increased baseline IL-1RA levels were associated with a response to radiotherapy. We could not confirm these findings. We used the same assay and actually found higher IL-1RA levels in smokers than in non-smokers. It might be that this discrepancy is due to the larger sample size of our study, or to a different patient selection. We included patients on the basis of disease severity, not activity, and consequently many appeared to have had inactive disease. Hofbauer et al. included active GO patients with < 4 months disease duration. Our patients reflect usual clinical practice in presenting with disabling ophthalmopathy in whom the activity phase still has to be determined.
We conclude that sIL-2R, IL-6, IL-6R, TNFαRI and II and sCD30 are elevated in GO patients compared with healthy controls. This was not related to parameters of thyroid autoimmune disease. However, IL-6, sCD30 and TNFαRI had some value for predicting therapeutic outcome to retrobulbar radiotherapy in GO, and probably reflect active eye disease.
References
- 1.Weetman AP, Cohen S, Gatter KC, Fells P, Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989;75:222–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Burch HB, Wartofsky L. Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747–93. doi: 10.1210/edrv-14-6-747. [DOI] [PubMed] [Google Scholar]
- 3.Hufnagel TJ, Hickey WF, Cobbs WH, Jakobiec FA, Iwamoto T, Eagle RC. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves' disease. Ophthalmology. 1984;91:1411–9. doi: 10.1016/s0161-6420(84)34152-5. [DOI] [PubMed] [Google Scholar]
- 4.Bahn RS, Heufelder AE. Retroocular fibroblasts: important effector cells in Graves' ophthalmopathy. Thyroid. 1992;2:89–94. doi: 10.1089/thy.1992.2.89. [DOI] [PubMed] [Google Scholar]
- 5.Weetman AP. Thyroid-associated eye disease: pathophysiology. Lancet. 1991;338:25–28. doi: 10.1016/0140-6736(91)90013-f. [DOI] [PubMed] [Google Scholar]
- 6.Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989;10:366–91. doi: 10.1210/edrv-10-3-366. [DOI] [PubMed] [Google Scholar]
- 7.Korducki JM, Loftus SJ, Bahn RS. Stimulation of glycosaminoglycan production in cultured human retroocular fibroblasts. Invest Ophthalmol Vis Sci. 1992;33:2037–42. [PubMed] [Google Scholar]
- 8.Heufelder AE, Bahn RS. Modulation of Graves' orbital fibroblast proliferation by cytokines and glucocorticoid receptor agonists. Invest Ophthalmol Vis Sci. 1994;35:120–7. [PubMed] [Google Scholar]
- 9.Tan GH, Dutton CM, Bahn RS. Interleukin-1 (IL-1) receptor antagonist and soluble IL-1 receptor inhibit IL-1-induced glycosaminoglycan production in cultured human orbital fibroblasts from patients with Graves' ophthalmopathy. J Clin Endocrinol Metabolism. 1996;81:449–52. doi: 10.1210/jcem.81.2.8636247. [DOI] [PubMed] [Google Scholar]
- 10.Heufelder AE. Pathogenesis of Graves' ophthalmopathy: recent controversies and progress. Eur J Endocrinol. 1995;132:532–41. doi: 10.1530/eje.0.1320532. [DOI] [PubMed] [Google Scholar]
- 11.Heufelder AE, Bahn RS. Graves' immunoglobulins and cytokines stimulate the expression of intercellular adhesion molecule-1 (ICAM-1) in cultured Graves' orbital fibroblasts. Eur J Clin Invest. 1992;22:529–37. doi: 10.1111/j.1365-2362.1992.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 12.Prummel MF, Van Pareren Y, Bakker O, Wiersinga WM. Anti-heat shock protein (hsp)72 antibodies are present in patients with Graves' disease (GD) and in smoking control subjects. Clin Exp Immunol. 1997;110:292–5. doi: 10.1111/j.1365-2249.1997.tb08330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heufelder AE, Smith TJ, Gorman CA, Bahn RS. Increased induction of HLA-DR by interferon-gamma in cultured fibroblasts derived from patients with Graves' ophthalmopathy and pretibial dermopathy. J Clin Endocrinol Metabolism. 1991;73:307–13. doi: 10.1210/jcem-73-2-307. [DOI] [PubMed] [Google Scholar]
- 14.Bahn RS, Heufelder AE. Pathogenesis of Graves' ophthalmopathy. N Engl J Med. 1993;329:1468–75. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 15.McLachlan SM, Prummel MF, Rapoport B. Cell-mediated or humoral immunity in Graves' ophthalmopathy? Profiles of T-cell cytokines amplified by polymerase chain reaction from orbital tissue. J Clin Endocrinol Metabolism. 1994;78:1070–4. doi: 10.1210/jcem.78.5.8175962. [DOI] [PubMed] [Google Scholar]
- 16.Heufelder AE, Bahn RS. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves' ophthalmopathy. Eur J Clin Invest. 1993;23:10–17. doi: 10.1111/j.1365-2362.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 17.Chow CC, Lai KN, Leung JC, Chan JC, Cockram CS. Serum soluble interleukin 2 receptor in hyperthyroid Graves' disease and effect of carbimazole therapy. Clin Endocrinol (Oxf) 1990;33:317–21. doi: 10.1111/j.1365-2265.1990.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilson R, McKillop JH, Buchanan LM, Bradley H, Smith WE, Thomson JA. The effect of carbimazole therapy on interleukin 2, interleukin 2 receptors and free radicals. Autoimmunity. 1990;8:3–7. doi: 10.3109/08916939008998426. [DOI] [PubMed] [Google Scholar]
- 19.Celik I, Akalin S, Erbas T. Serum levels of interleukin 6 and tumor necrosis factor-alpha in hyperthyroid patients before and after propylthiouracil treatment. Eur J Endocrinol. 1995;132:668–72. doi: 10.1530/eje.0.1320668. [DOI] [PubMed] [Google Scholar]
- 20.Salvi M, Girasole G, Pedrazzoni M, et al. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves' disease. J Clin Endocrinol Metabolism. 1996;81:2976–9. doi: 10.1210/jcem.81.8.8768861. [DOI] [PubMed] [Google Scholar]
- 21.Okumura M, Hidaka Y, Kuroda S, Takeoka K, Tada H, Amino N. Increased serum concentration of soluble CD30 in patients with Graves' disease and Hashimoto's thyroiditis. J Clin Endocrinol Metabolism. 1997;82:1757–60. doi: 10.1210/jcem.82.6.4000. [DOI] [PubMed] [Google Scholar]
- 22.Prummel MF, Wiersinga WM, Van der Gaag R, Mourits MP, Koornneef L. Soluble IL-2 receptor levels in patients with Graves' ophthalmopathy. Clin Exp Immunol. 1992;88:405–9. doi: 10.1111/j.1365-2249.1992.tb06462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofbauer LC, Muhlberg T, Konig A, et al. Soluble interleukin-1 receptor antagonist serum levels in smokers and nonsmokers with Graves ophthalmopathy undergoing orbital radiotherapy. J Clin Endocrinol Metabolism. 1997;82:2244–7. doi: 10.1210/jcem.82.7.4068. [DOI] [PubMed] [Google Scholar]
- 24.Spinas GA, Keller U, Brockhaus M. Release of soluble receptors for tumor necrosis factor (TNF) in relation to circulating TNF during experimental endotoxinemia. J Clin Invest. 1992;90:533–6. doi: 10.1172/JCI115891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:4845–9. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- 27.Del Prete G, De Carli M, D'elios MM, et al. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp Med. 1995;182:1655–61. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Prete G, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995;9:81–86. [PubMed] [Google Scholar]
- 29.Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med. 1998;338:73–78. doi: 10.1056/NEJM199801083380201. [DOI] [PubMed] [Google Scholar]
- 30.Gerding MN, Prummel MF, Kalmann R, Koornneef L, Wiersinga WM. The use of colour slides in the assessment of changes in soft-tissue involvement in Graves' ophthalmopathy. J Endocrinol Invest. 1998;21:459–62. doi: 10.1007/BF03347327. [DOI] [PubMed] [Google Scholar]
- 31.Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–44. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prummel MF, Mourits MP, Berghout A, et al. Prednisone and cyclosporine in the treatment of severe Graves' ophthalmopathy. N Engl J Med. 1989;321:1353–9. doi: 10.1056/NEJM198911163212002. [DOI] [PubMed] [Google Scholar]
- 33.Ratanachaiyavong S, Fleming D, Janer M, et al. HLA-DPB1 polymorphisms in patients with hyperthyroid Graves' disease and early onset myasthenia gravis. Autoimmunity. 1994;17:99–104. doi: 10.3109/08916939409014664. [DOI] [PubMed] [Google Scholar]
- 34.De Carli M, D'elios MM, Mariotti S, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves' ophthalmopathy. J Clin Endocrinol Metabolism. 1993;77:1120–4. doi: 10.1210/jcem.77.5.8077301. [DOI] [PubMed] [Google Scholar]
- 35.Pappa A, Calder V, Ajjan R, et al. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO) Clin Exp Immunol. 1997;109:362–9. doi: 10.1046/j.1365-2249.1997.4491347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shine B, Fells P, Edwards OM, Weetman AP. Association between Graves' ophthalmopathy and smoking. Lancet. 1990;335:1261–3. doi: 10.1016/0140-6736(90)91315-2. [DOI] [PubMed] [Google Scholar]
- 37.Tellez M, Cooper J, Edmonds C. Graves' ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf) 1992;36:291–4. doi: 10.1111/j.1365-2265.1992.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 38.Prummel MF, Wiersinga WM. Smoking and risk of Graves' disease. JAMA. 1993;269:479–82. [PubMed] [Google Scholar]
- 39.Metcalfe RA, Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: possible role in thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf) 1994;40:67–72. doi: 10.1111/j.1365-2265.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]