Abstract
Anti-myeloperoxidase antibodies (anti-MPO) are a major type of anti-neutrophil cytoplasmic antibody (ANCA). While evaluating anti-MPO monoclonal antibodies from SCG/Kj mice, we observed several hybridomas that appeared to react with both MPO and DNA. Sera from some patients with systemic lupus erythematosus (SLE) also react with MPO and DNA. We hypothesized that the MPO binding activity is a false-positive result due to the binding of DNA, contained within the antigen binding site of anti-DNA antibodies, to the cationic MPO. Antibodies from tissue culture supernatants from ‘dual reactive’ hybridomas were purified under high-salt conditions (3 m NaCl) to remove any antigen bound to antibody. The MPO and DNA binding activity were measured by ELISA. The MPO binding activity was completely abrogated while the DNA binding activity remained. The MPO binding activity was restored, in a dose-dependent manner, by the addition of increasing amount of calf-thymus DNA (CT-DNA) to the purified antibody. Sera from six patients with SLE that reacted with both MPO and DNA were treated with DNase and showed a decrease in MPO binding activity compared with untreated samples. MPO binding activity was observed when CT-DNA was added to sera from SLE patients that initially reacted with DNA but not with MPO. These results suggest that the DNA contained within the antigen binding site of anti-DNA antibodies could bind to the highly cationic MPO used as substrate antigen in immunoassays, resulting in a false-positive test.
Keywords: ANCA, anti-MPO, anti-DNA, systemic lupus erythematosus
INTRODUCTION
Anti-neutrophil cytoplasmic antibodies (ANCA) are associated with certain forms of small vessel vasculitis such as Wegener's granulomatosis (WG), microscopic polyangiitis (MPA), and Churg–Strauss syndrome (CSS), and pauci-immune necrotizing and crescentic glomerulonephritis [1]. The necrotizing vasculitis and crescentic glomerulonephritis seen in these diseases is characterized by a paucity of immunoglobulin and complement deposition along the vessel walls. Several in vitro and in vivo studies indicate that these autoantibodies play a role in the pathogenesis of these diseases [2]. Serologic assays for ANCA are frequently performed in patients with signs or symptoms of vasculitis or glomerulonephritis. The autoantibodies are primarily directed toward myeloperoxidase (MPO) or proteinase 3 (PR3), constituents of the granules of neutrophils and monocytes [3]. In indirect immunofluorescence assays (IFA), using neutrophils as substrates, the majority of antibodies to MPO cause a perinuclear staining pattern (P-ANCA) when the substrate is fixed with ethanol and the majority of antibodies to PR3 cause a cytoplasmic pattern (C-ANCA). The P-ANCA target antigens are cytoplasmic proteins that translocate to the nuclear membrane as an artefact of fixation process used during preparation of substrate neutrophils for IFA [3].
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by the presence of a variety of autoantibodies including those directed towards DNA, chromatin, histones and ribonucleoproteins [4]. ANCA have also been detected in the serum of some patients with SLE, particularly those with drug-induced lupus [5–8]. The majority of these are P-ANCA with specificity for MPO or elastase, but the presence of antinuclear antibodies (ANA) in the sera of patients with SLE makes IFA interpretation difficult.
Mice of the MRL/lpr strain have spontaneous antibody responses to DNA as well as to various nuclear protein antigens, similarly to patients with SLE [9]. Recently, sera from some of these mice have been shown to contain anti-MPO antibodies [10]. Furthermore, anti-MPO MoAbs produced by hybridomas derived from these mice often bind to DNA as well as MPO [11].
The spontaneous crescentic glomerulonephritis/Kinjoh (SCG/Kj) mouse is an inbred strain derived from (MRL/Mp-lpr/lpr × BXSB) by F1 crossing and selecting for high frequency of glomerular crescents [12]. These mice are genetically and phenotypically very similar to the MRL mice. Some SCG/Kj mice have circulating anti-MPO antibodies [13]. We established a panel of anti-MPO antibody-producing monoclonal hybridomas from unimmunized SCG/Kj mice and found that supernatant from some of these hybridomas bound to MPO as well as DNA [14].
Determination of antibody specificity from unpurified tissue culture supernatants can be erroneous if the antigens are also present in the supernatants, because immune complexes can form and alter the reactivity of the complexed antibodies. The antigen bound to the antigen-binding site of its specific antibody can also bind to other antigens, either by charge interactions or by specific protein–protein interactions. Brinkman et al. have shown that anti-DNA MoAbs bind to DNA released from necrotic cells in tissue culture and this complex in turn binds to certain cationic substrates used in various assays [15]. More recently, Kramers et al. have shown that these non-specific interactions can also occur in vivo [16]. Purification of the MoAbs from tissue culture supernatants under dissociating conditions abrogated the polyreactivity.
The goal of the present study is to determine if the dual binding to DNA and MPO that we observed with supernatants from hybridomas derived from SCG/Kj mice was a false-positive artefact of the screening assay used. Additionally, we determined whether a similar dual reactivity to DNA and MPO occurs with human anti-DNA antibodies from patients with SLE. The results presented here indicate that the antibodies produced by the ‘dual reactive’ hybridomas are specific for only DNA and that the binding to MPO is not due to specific antigen recognition. Furthermore, the MPO binding capacity of sera from patients with SLE may be overestimated due to a similar non-specific binding of MPO to anti-DNA/DNA complexes.
MATERIALS AND METHODS
Production of hybridomas
SCG/Kj mice were obtained from a colony at the Animal Institute of the University of South Florida. All animal care and manipulation was in accordance with the guidelines of the University of North Carolina at Chapel Hill. Hybridomas were generated by fusing the splenic mononuclear cells with the P3-X63Ag8.653 murine myeloma cell line as previously described []. Tissue culture supernatants from wells with growing hybridomas were screened for anti-MPO antibodies by ELISA and cells from wells testing positive were cloned by limiting dilution.
Antibody purification
Tissue culture supernatants were harvested from bulk cultures grown in Dulbecco's modified essential medium containing 10% fetal calf serum (FCS; Gibco BRL, Gaithersburg, MD). The class, subclass, and light chain type of the antibodies were determined using the Mouse Monoclonal Antibody Isotyping Kit (Amersham, Aylesbury, UK) following the manufacturer's instructions. The antibodies were purified under disassociating high salt conditions following the method described by Brinkman et al. [15]. High salt conditions favour the dissociation of antibody–antigen complexes and subsequent dialysis removes most of the lower molecular weight antigens. Passage through a Sepharose column equilibrated with the high salt buffer allows for further purification and concentration of the free antibodies. Briefly, the supernatants were concentrated by centrifugation using filtration units (Millipore, Bedford, MA). The concentrate was dialysed extensively against a buffer containing 3 m NaCl, 1·5 m glycine–NaOH pH 8·9 (Buffer A). The dialysed material was applied to Sepharose protein G columns (Pharmacia, Piscataway, NJ) equilibrated with Buffer A. The columns were washed extensively with Buffer A and the bound antibodies were eluted with 0·1 m citric acid pH 4·0–6·0. The eluates were dialysed against PBS pH 7·4 (Sigma, St Louis, MO).
MPO-ELISA
Purified human MPO (Calbiochem, San Diego, CA), at 5 μg/ml in bicarbonate coating buffer (Sigma), was coated onto high-binding microtitre plates (Costar, Acton, MA) overnight at 4°C. The plates were blocked for 1 h at room temperature with PBS containing 0·05% Tween and 0·2% normal donkey serum (Sigma). The plates were washed three times with PBS–Tween and 100 μl of tissue culture supernatant or purified antibody at 1 μg/ml was added and incubated for 1 h at room temperature. The plates were washed and 100 μl of alkaline phosphatase-conjugated donkey anti-mouse immunoglbulin heavy and light chain (Jackson Immunoresearch, West Grove, PA) at a 1:20 000 dilution in PBS–Tween were added. The plates were incubated for an additional 1 h at room temperature followed by washing. Paranitrophenyl phosphate substrate (Sigma) was added to each well. After incubation for 1 h, the optical density (OD) was measured at 405 nm wavelength.
DNA–ELISA
The tissue culture supernatants positive for anti-MPO antibodies were tested for anti-DNA antibodies by ELISA. Phenol-chloroform-purified calf thymus DNA (Sigma) was used at 5 μg/ml in borate-buffered saline (BBS) to coat polystyrene plates overnight at 4°C. The phenol chloroform extraction ensures that the DNA used does not contain a protein antigen that might be the source of the observed cross-reactivity. After coating, the plates were blocked with BBS containing Tween-80 for 1 h followed by addition of 100 μl of culture supernatant or purified antibody and incubation at 4°C overnight. Alkaline phosphatase-conjugated goat anti-mouse IgG and IgM (Jackson Immunoresearch) were added followed by paranitrophenyl phosphate substrate (Sigma). After incubation for 1 h, the OD was read at 405 nm wavelength.
Preincubation with calf thymus DNA
Phenol chloroform-extracted calf thymus DNA was diluted in BBS and added at varying concentrations (0·5 μg/ml to 100 μg/ml) to high salt purified tissue culture supernatant. After incubation at room temperature for 1 h, the antibody/DNA mixture was transferred to ELISA plates coated with either MPO or DNA and tested for binding as described above.
Patient sera
Sera from six patients with SLE diagnosed according to the American College of Rheumatology criteria were obtained from the Nephropathology Laboratory, UNC School of Medicine. These sera were selected because they had previously been determined to contain antibodies to DNA and had tested P-ANCA-positive by IFA and anti-MPO-positive by ELISA. In addition, one serum sample was obtained from a patient with a diagnosis of MPA that also reacted with both DNA and MPO by ELISA. Five serum samples from patients with SLE that were ANA+, anti-DNA+, but anti-MPO antibody-negative, were obtained from the Clinical Immunology Laboratory, UNC Hospitals. All serum samples were diluted at 1:100 and tested for MPO binding capacity by ELISA as described above. Alkaline phosphatase-conjugated donkey anti-human IgG, IgA and IgM (Jackson Immunoresearch) were used as the secondary antibodies in these ELISAs.
DNase treatment of patient sera
The six sera from patients with SLE and one patient with MPA that tested positive for both anti-DNA and anti-MPO antibodies were treated with 40 μg/ml DNase I (Boehringer Mannheim, Indianapolis, IN) and 10 mm MgCl2 for 1 h at 37°C. The reactions were stopped with 15 mm EDTA (Sigma) and incubation at 56°C for 10 min. A final dilution of 1:100 was then tested by MPO and DNA ELISAs as described above. Samples treated with 40 μg/ml bovine serum albumin (BSA; Sigma) instead of DNase I were used as negative controls. Anti-MPO antibody-positive control serum was obtained from the Nephropathology Laboratory and anti-DNA antibody positive control serum was obtained from the Clinical Immunology Laboratory, UNC Hospitals for the MPO and DNA ELISAs, respectively.
Preincubation of patient sera with calf thymus DNA
Anti-DNA antibody-positive, but anti-MPO antibody-negative sera from five patients with SLE were incubated with chloroform-purified calf thymus DNA for 1 h at room temperature. The samples were then adjusted to a 1:100 dilution and added to ELISA plates coated with either MPO or DNA and the binding was measured as described above.
RESULTS
Hybridomas
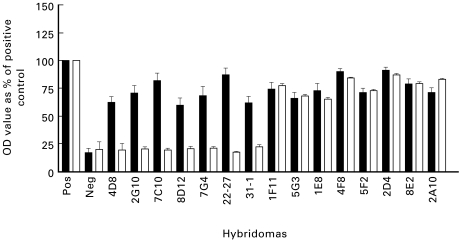
Tissue culture supernatants from a total of 20 monoclonal hybridomas, generated from five unimmunized SCG/Kj mice, bound to MPO in the screening ELISA (Fig. 1) Supernatants from seven of these hybridomas also bound to DNA by ELISA. None of these supernatants reacted with PR3 or BSA (data not shown). The immunoglobulin fraction from bulk cultures of the hybridomas was purified by the high salt method described by Brinkman et al. [15].
Fig. 1.
Myeloperoxidase (MPO)ELISA of supernatants (▪) and high salt purified (□) antibodies from hybridomas derived from SCG/Kj mice (data from three independent experiments). OD, Optical density.
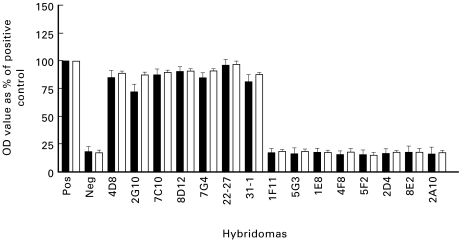
Upon high salt purification, the MPO binding activity of all seven anti-DNA/anti-MPO antibody-positive supernatants that originally bound to both MPO and DNA was abrogated while the MPO binding activity of the supernatants from hybridomas that produced anti-MPO only antibodies remained (Figs 1 and 2). The purification procedure did not diminish the DNA binding activity of those supernatants that initially bound only DNA. In fact, with some supernatants, the purification increased the DNA binding capacity, possibly because of an increased concentration of immunoglobulin compared with bulk culture supernatants or freeing of antigen binding sites by removal of complexed DNA.
Fig. 2.
DNA-ELISA of supernatants (▪) and high salt purified antibodies (□) from hybridomas derived from SCG/Kj mice (data from three separate experiments). OD, Optical density.
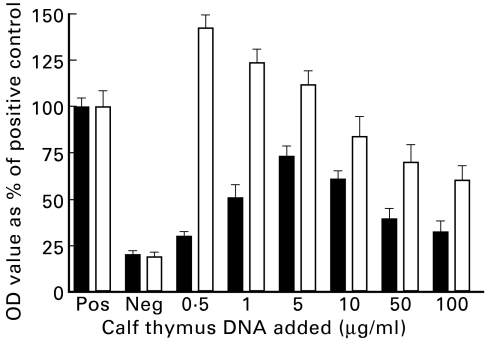
To determine if the binding of anti-DNA antibodies to MPO was due to DNA/anti-DNA complexes, purified antibody from one hybridoma was incubated with varying concentrations of phenol chloroform-purified calf thymus DNA (Fig. 3). The addition of exogenous DNA resulted in an increase in the MPO binding capacity of the purified anti-DNA antibody in a dose-dependent manner. At low concentration of added DNA, the binding was primarily to DNA and with increasing amounts of added DNA, the MPO binding increased. The maximum MPO binding capacity was observed at 5 μg/ml added DNA. At concentrations above this, the MPO binding decreased, presumably because of inhibition of complex formation due to excess antigen. The DNA binding ability of the purified antibody incubated with the highest concentration of calf thymus DNA (100 μg/ml) was decreased to less than half that of pure antibody alone. This was probably due to inhibition of binding to solid-phase DNA on the ELISA plate by the fluid-phase exogenous DNA.
Fig. 3.
Myeloperoxidase (MPO)- (▪) and DNA-ELISA (□) of high salt purified antibody from hybridoma 22-27 preincubated with varying amounts of calf thymus DNA (data from three separate experiments). OD, Optical density.
SLE patient sera
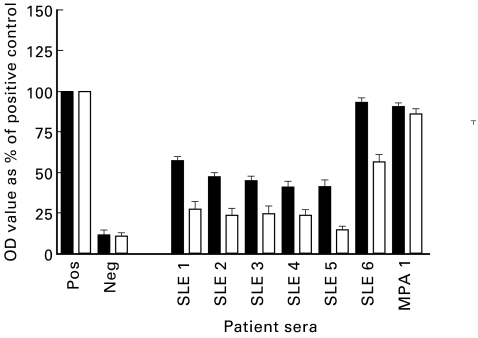
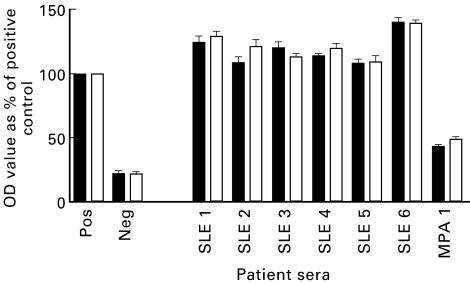
Sera from six patients with SLE (SLE nos 1–6) and one patient with MPA (MPA no. 1) that had initially tested P-ANCA+ by IFA and anti-MPO antibody-positive by ELISA were obtained. All seven patients had previously tested positive for ANA using Hep-2 slides and for anti-dsDNA by Crithidia luciliae assay. The sera were incubated with either DNase I or with BSA at 37°C for 1 h in the presence of MgCl2 to digest DNA contained within the antigen binding sites of anti-DNA antibodies. The MPO binding capacity of the DNase-treated sera was compared with that of sera treated with BSA (Fig. 4.) The BSA-treated sera showed MPO binding capacity ranging from 40% to 95% that of the assay positive control, which was similar to the ELISA value prior to BSA treatment. Upon treatment with DNase I, there was a decrease in MPO binding capacity ranging from 30% to 60% compared with BSA-treated sera. DNase treatment of the serum sample from the MPA patient did not decrease the MPO or the DNA binding capacity. Furthermore, DNase treatment did not diminish the DNA binding capacity of any sera (Fig. 5).
Fig. 4.
Myeloperoxidase (MPO)-ELISA of systemic lupus erythematosus (SLE) and microscopic polyangiitis (MPA) patient sera with (□) and without (▪) DNase treatment (data from three separate experiments). OD, Optical density.
Fig. 5.
DNA-ELISA of systemic lupus erythematosus (SLE) and microscopic polyangiitis (MPA) patient sera with (□) and without (▪) DNase treatment (data from three separate experiments). OD, Optical density.
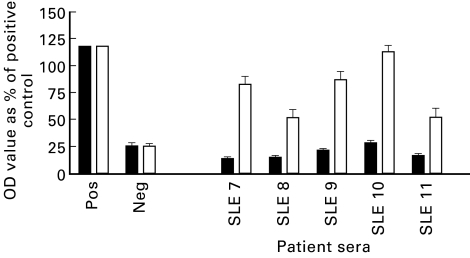
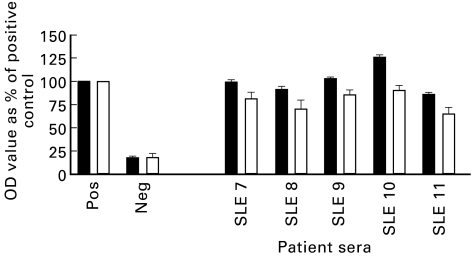
To determine whether MPO binding can be artificially induced in anti-DNA antibody containing sera, we preincubated five anti-DNA antibody-positive (but anti-MPO-negative) sera with calf thymus DNA. The MPO binding ability of all five sera prior to the addition of exogenous DNA was < 20% that of the assay positive control (Fig. 6). Upon addition of calf thymus DNA, the MPO binding capacity increased, ranging from 50% to 115% of the assay positive control. The anti-DNA antibody activity of the sera decreased after the addition of exogenous DNA (Fig. 7). This is consistent with inhibition by the soluble DNA added.
Fig. 6.
Myeloperoxidase (MPO)-ELISA of systemic lupus erythematosus (SLE) patient sera with (□) and without (▪) the addition of exogenous calf thymus DNA (CT-DNA) (data from three separate experiments). OD, Optical density.
Fig. 7.
DNA-ELISA of systemic lupus erythematosus (SLE) patient sera with (□) and without (▪) the addition of exogenous calf thymus DNA (CT-DNA) (data from three separate experiments).OD, Optical density.
DISCUSSION
WG, MPA, and CSS are forms of small vessel vasculitis that are most often associated with circulating antibodies to neutrophil cytoplasmic constituents (ANCA) [1]. The necrotizing vasculitis seen in these diseases is characterized by lack of substantial immunoglobulin or complement deposition in the vessel wall, in contrast to the fine linear staining of the basement membranes seen with anti-glomerular basement membrane (GBM) antibodies or the granular staining seen with immune complex deposition [2]. There is evidence that ANCA are pathogenic in WG, MPA and CSS [18–20].
SLE is a systemic autoimmune disease characterized by the presence of autoantibodies to a variety of nuclear antigens including DNA and various ribonucleoproteins such as the Sm complex [4]. Sera from some patients with SLE have been reported also to contain ANCA [5–8]. While the frequency of ANCA in SLE is generally low, ANCA is quite common in patients with the drug-induced form of lupus [21]. The staining pattern seen by IFA is P-ANCA, though the results of this assay must be interpreted carefully because of the presence of antinuclear antibodies. Confirmatory enzyme immunoassays using purified antigens show that some of the ANCA detected in the sera of patients with SLE bind MPO. The nuclear antigens targeted in SLE have physiologic roles in synthesis and processing of nucleic acids. The association of MPO and DNA appears to be artefactually induced by the fixation procedure used in preparing IFA slides that causes MPO to translocate to the nucleus.
The MRL/Mp-lpr/lpr mouse strain spontaneously develops lymphoproliferation (lpr) due to a defect in programmed cell death (apoptosis) that results in excessive accumulation of T and B cells [9]. These mice characteristically have multiple autoantibodies including anti-DNA, anti-ribonucleoproteins, and rheumatoid factors. While the polyclonal activation of B cells could be a reason for the presence of the various autoantibodies, the responses to some antigens appear to be driven by antigen [22–25].
According to Harper et al., approximately 20% of MRL mice, which are closely related to SCG/Kj mice, also have circulating anti-MPO antibodies [10]. They suggest that histologically, the vasculitis seen in the anti-MPO antibody-positive MRL mice, who also have circulating anti-DNA antibodies, resembles that seen in patients with MPA [11]. This is different from the disease seen in the majority of MRL mice and in patients with SLE. Interestingly, the frequency of anti-MPO antibodies in the MRL mice is similar to that seen with other nuclear antigens such as the Sm antigen [22]. Additionally, while the antibodies to DNA appear early in these mice, the response to MPO occurs in the late phases, which is also when the responses to other nuclear antigens are seen [25]. Harper et al. also generated seven hybridomas that secreted anti-MPO antibodies from two MRL mice [11]. They found that all seven were also ANA+ and reacted with DNA by ELISA. Furthermore, in ELISA cross-inhibition studies, increasing the salt concentrations of the buffers inhibited binding to MPO. They did not use high salt conditions during purification of the culture supernatants, and therefore the polyreactivity seen may be artefactual.
In their analysis of a panel of polyreactive anti-DNA antibody producing hybridomas from MRL mice, Brinkman et al. showed that the cross-reactivity was due to DNA released from dead cells in tissue culture binding to the anti-DNA antibodies [15]. They showed that the negatively charged DNA contained within the antigen binding site of the anti-DNA antibody can interact with cationic substrates. Purification under conditions that favour the dissociation of antigen–antibody complexes, such as high salt buffers, abrogates polyreactivity. Furthermore, when they added back exogenous DNA to these purified antibodies, the polyreactivity returned in a dose-dependent manner. They show that this false positivity is seen not only in ELISAs, but also in assays where non-reduced, non-denatured native cationic antigens are used, such as by Western blot.
More recently, Kramers et al. have shown that anti-histone MoAbs derived from MRL mice can bind to DNA released from necrotic cells via histones, also released from dead cells, binding to the anti-histone antibodies [16]. Using PAGE, they showed that antibodies from the tissue culture supernatants contained histones and DNA after purification with Sepharose columns equilibrated with PBS, but not after purification under high salt conditions.
The SCG/Kj mouse strain is derived by crossing two lupus-prone strains, the MRL and the BSXB mice [12]. These mice develop spontaneous crescentic glomerulonephritis and some have circulating anti-MPO antibodies [13]. While evaluating anti-MPO MoAbs from these mice by ELISA, unpurified culture supernatants from seven hybridomas appeared to bind to both MPO and DNA, whereas those of 13 bound to MPO only. The original hypothesis was that this ‘dual reactivity’ represented a stage in the maturation of the anti-MPO response in these mice [14].
In the present study, we determined that the ‘dual reactivity’ observed in the SCG/Kj-derived hybridomas was due to the binding of DNA/anti-DNA complexes in the crude hybridoma culture supernatants to MPO by a non-specific charge interaction rather than true dual antigen specificity. We purified culture supernatants under high salt conditions and tested these preparations for MPO and DNA binding by ELISA. The MPO binding capacity of all seven dual reactive hybridomas was abrogated upon purification, while the DNA binding capacity remained. Furthermore, the addition of exogenous DNA back to the purified antibody resulted in MPO binding in a dose-dependent manner. This is strong evidence that the apparent anti-MPO antibody activity of the hybridoma supernatants that also bind DNA is a false-positive reactivity.
Purification of the supernatants that bound MPO only did not alter the MPO binding capacity, suggesting that these are MPO-specific hybridomas and that the purification procedure does not alter MPO specificity. Analysis of the expressed V region gene usage by these antibodies indicates that there are differences between the MPO and the DNA binding antibodies (Jethwa et al., J Immunol, submitted). The V regions utilized by the anti-DNA antibody producing hybridomas are very similar to those of other anti-DNA antibodies, yet different from those of the anti-MPO antibody producing hybridomas. The anti-MPO antibody producing hybridomas show a restriction in the light chain V gene usage whereas the anti-DNA producing hybridomas use diverse light chain V region genes. These observations also provide strong evidence that the anti-DNA and anti-MPO antibodies in SCG/Kj mice are completely distinct from each other.
To determine whether the prevalence of anti-MPO antibodies in patients with SLE is overestimated because of false-positive binding between MPO and anti-DNA/DNA complexes, we analysed the MPO binding capacity of sera from patients with SLE. Circulating anti-DNA antibodies in these patients could bind to DNA released from tissue destruction and this complex in turn could bind to MPO. Sera from six SLE patients that were positive for ANCA by IFA and anti-MPO antibody-positive by ELISA were treated with DNase to digest bound DNA and dissociate complexes. Due to the limited amount of patient sera, the immunoglobulin fraction was not purified with high salt but with DNase treatment. In their analysis of anti-DNA antibodies from MRL mice, Brinkman et al. showed that DNase treatment could abrogate cross-reactivity [15]. After treatment, all six SLE sera showed a decrease in the MPO binding capacity by ELISA, whereas the DNA binding remained unchanged.
The MPO binding was not fully abolished after DNase treatment as it was in the cross-reactive tissue culture supernatants after high salt purification. This may be due to protection from digestion of some DNA by the bound antibody. Alternatively, there may be some true MPO binding antibodies in these sera. However, the MPO binding after treatment of five of the six SLE fell within the normal reference range, and thus could be considered serologically negative for MPO-ANCA.
We also analysed serum from a patient with MPA, which had also tested positive for anti-MPO and anti-DNA antibodies. DNase treatment of this serum sample did not alter the MPO binding capacity. Perhaps, in this patient with vasculitis, there are circulating complexes of anti-MPO/MPO and that the MPO contained within the antigen binding sites can in turn bind to the DNA used as substrates in the ELISA. However, the presence of circulating immune complexes in ANCA-associated vasculitis has not been determined, neither has the prevalence of anti-DNA antibody positivity in vasculitic patients with circulating anti-MPO antibodies. Thus, some patients with ANCA-vasculitis may have both types of circulating autoantibodies.
To determine if anti-DNA antibody-containing sera can acquire MPO binding activity, sera from five patients with SLE and circulating anti-DNA antibodies but no anti-MPO antibodies were tested for MPO binding after the addition of calf thymus DNA. The addition of exogenous DNA resulted in the acquisition of MPO binding to varying degrees by all five sera.
The availability of DNA to anti-DNA antibody-containing sera may explain why not all anti-DNA antibody-containing sera are also anti-MPO antibody-positive. Perhaps the dual reactivity is seen only when the disease is accompanied by the release of DNA from tissue destruction. Alternatively, the complexes may be forming in vitro after blood collection due to DNA being released from necrotic cells in the blood collection tubes. Another explanation may be that the cross-reactivity is only seen in a subset of anti-DNA antibodies that are able to form more stable complexes.
DNase treatment of sera from SLE patients positive for both anti-DNA and anti-MPO antibodies can be a valuable tool to ascertain the nature of the ‘dual reactivity’ in clinical and research specimens. Our data suggest that this treatment can be especially useful for those sera that show borderline or weakly positive anti-MPO activity in the presence of anti-DNA antibodies. Additional prospective studies are planned that will analyse the clinical utility of routine DNase treatment and/or high salt purification of ‘dual reactive’ sera from SLE patients.
In conclusion, the results of this study suggest that anti-MPO MoAbs that also bind to DNA are in fact anti-DNA antibodies, and that the MPO binding of these samples is a false-positive artefact due to impure antibody preparations that contain anti-DNA/DNA complexes. Furthermore, the MPO binding capacity of some sera from patients with SLE may be overestimated due to a similar false positivity. Additional studies will be required to determine the in vivo consequences, if any, of the cross-reactivity between antibodies to DNA and MPO in both mice and humans.
REFERENCES
- 1.Jennette JC, Falk RJ. Antineutrophil cytoplasmic autoantibodies: discovery, specificity, disease associations, and pathogenic potential. Adv Pathol Lab Med. 1995;8:363–78. [Google Scholar]
- 2.Jennette JC, Falk RJ. Pathogenesis of the vascular and glomerular damage in ANCA-positive vasculitis. Neph Dial Transpl. 1998;13(Suppl. 1):16–20. doi: 10.1093/ndt/13.suppl_1.16. [DOI] [PubMed] [Google Scholar]
- 3.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 4.Elkon K. Autoantibodies in systemic lupus erythematosus. Curr Opin Rheumatol. 1995;7:384–8. doi: 10.1097/00002281-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Spronk PE, Bootsma H, Horst G, et al. Antineutrophil cytoplasmic antibodies in systemic lupus erythematosus. Br J Rheumatol. 1996;35:625–31. doi: 10.1093/rheumatology/35.7.625. [DOI] [PubMed] [Google Scholar]
- 6.Schnabel A, Hauschild S, Gross WL. Anti-neutrophil cytoplasmic antibodies in generalized autoimmune diseases. Int Arch Allergy Immunol. 1995;109:201–6. doi: 10.1159/000237238. [DOI] [PubMed] [Google Scholar]
- 7.Schnabel A, Csernok E, Isenberg DA, et al. Antineutrophil cytoplasmic antibodies in systemic lupus erythematosus. Prevalence, specificities, and clinical significance. Arthritis Rheum. 1995;38:633–7. doi: 10.1002/art.1780380509. [DOI] [PubMed] [Google Scholar]
- 8.Pauzner R, Urowitz M, Gladman D, et al. Antineutrophil cytoplasmic antibodies in systemic lupus erythematosus. J Rheumatol. 1994;21:1670–3. [PubMed] [Google Scholar]
- 9.Chan O, Madaio MP, Shlomchik MJ. The roles of B cells in MRL/lpr murine lupus. Ann NY Acad Sci. 1997;815:75–87. doi: 10.1111/j.1749-6632.1997.tb52046.x. [DOI] [PubMed] [Google Scholar]
- 10.Harper JM, Lockwood CM, Cooke A. Anti-neutrophil cytoplasm antibodies in MRL-lpr/lpr mice. Clin Exp Immunol. 1993;93(Suppl.1):22. [Google Scholar]
- 11.Harper JM, Thiru S, Lockwood CM, et al. Myeloperoxidase autoantibodies distinguish vasculitis mediated by anti-neutrophil cytoplasm antibodies from immune complex disease in MRL/Mp-lpr/lpr mice: a spontaneous model for human microscopic angiitis. Eur J Immunol. 1998;28:2217–26. doi: 10.1002/(SICI)1521-4141(199807)28:07<2217::AID-IMMU2217>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Kinjoh K, Kyogoku M, Good RA. Genetic selection for crescent formation yields mouse strain with rapidly progressive glomerulonephritis and small vessel vasculitis. Proc Natl Acad Sci USA. 1993;90:3413–7. doi: 10.1073/pnas.90.8.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinjoh K, Good RA, Nemoto K, et al. SCG/Kj mice develop crescentic glomerulonephritis (CGN), vasculitis and MPO-specific P-ANCA. Clin Exp Immunol. 1995;101(Suppl. 1):53. [Google Scholar]
- 14.Nachman PH, Clarke SH, Kinjoh K, et al. Cross-reactivity of murine monoclonal autoantibodies to DNA and MPO. J Am Soc Neph. 1995;6:845. [Google Scholar]
- 15.Brinkman K, Termaat RM, de Jong J, et al. Cross-reactive binding patterns of monoclonal antibodies to DNA are often caused by DNA/anti-DNA immune complexes. Res Immunol. 1989;140:595–612. doi: 10.1016/0923-2494(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 16.Kramers C, Hylkema MN, van Bruggen MC, et al. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest. 1994;94:568–77. doi: 10.1172/JCI117371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney JF, Radbrush A, Liesegang B, et al. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979;123:1548–50. [PubMed] [Google Scholar]
- 18.Heeringa P, Brouwer E, Cohen-Tervaert JW, et al. Animal models of anti-neutrophil cytoplasmic antibody associated vasculitis. Kidney Int. 1998;53:253–63. doi: 10.1046/j.1523-1755.1998.00743.x. [DOI] [PubMed] [Google Scholar]
- 19.Falk RJ, Terrell RS, Charles LA, et al. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder AHL, Heeringa P, Brouwer E, et al. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a FcγRII-dependent process. Clin Exp Immunol. 1994;98:270–8. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cambridge G, Wallace H, Bernstein RM, et al. Autoantibodies to myeloperoxidase in idiopathic and drug-induced systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994;33:109–14. doi: 10.1093/rheumatology/33.2.109. [DOI] [PubMed] [Google Scholar]
- 22.Bloom DD, Davignon DJ, Retter MW, et al. V region gene analysis of anti-Sm hybridomas from MRL/Mp-lpr/lpr mice. J Immunol. 1993;150:1591–610. [PubMed] [Google Scholar]
- 23.Retter MW, Cohen PL, Eisenberg RA, et al. Both Sm and DNA are selecting antigens in the anti-Sm B cell response in autoimmune MRL/lpr mice. J Immunol. 1996;156:1296–306. [PubMed] [Google Scholar]
- 24.Retter MW, Eisenberg RA, Cohen PH, et al. Sm and DNA binding by dual reactive B cells requires distinct VH, Vê, and CDR3 structures. J Immunol. 1995;55:2248–57. [PubMed] [Google Scholar]
- 25.Bloom DD, Davignon J, Cohen PH, et al. Overlap of the anti-Sm and anti-DNA responses of MRL/Mp-lpr/lpr mice. J Immunol. 1993;150:1579–90. [PubMed] [Google Scholar]
- 26.Pisetsky DS, McCarty GA, Peters DV. Mechanisms of autoantibody production in autoimmune MRL mice. J Exp Med. 1980;152:1302–10. doi: 10.1084/jem.152.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]