Abstract
Levels of platelet-derived microparticles (PMPs), platelet activation markers (P-selectin expressed on, or annexin V binding to, platelets (plt:P-selectin or plt:annexin V, respectively)), chemokines (IL-8, monocyte chemotactic peptide-1 (MCP-1), and regulated on activation normally T-cell expressed and secreted (RANTES)), and soluble P- and E-selectins were compared in peripheral blood from diabetic and control patients in order to develop a better understanding of their potential contribution to diabetic vascular complications. Significant increases were found for PMPs, plt:P-selectin, MCP-1, RANTES and soluble P- and E-selectins in diabetic individuals, whereas IL-8 levels were similar. Furthermore, after ticlopidine treatment, most of these factors receded to baseline levels observed in non-diabetic patients. Our findings indicate that ticlopidine might be able to prevent or reduce vascular complications in diabetic patients.
Keywords: platelet-derived microparticle, diabetes, P-selectin, chemokine, ticlopidine
INTRODUCTION
More than 75% of diabetic patients die of vascular complications associated with the disease [1,2]. Although diabetes itself is a risk factor for these complications [3], diabetes is often also associated with a hypercoagulable state [4,5]; increased platelet adhesion and aggregation have been reported in patients with diabetes [6]. Abrams et al. [7] reported the detection of platelet activation-dependent antigens, such as P-selectin, by using MoAbs and flow cytometry and, using this technique, it has been directly demonstrated that the number of P-selectin-positive platelets (plt:P-selectin) is increased in diabetic patients [8].
Platelet-derived microparticles (PMPs) are produced by either platelet activation or physical stimulation under various conditions [9–12]. PMPs contain inner granules, membranous microvesicles released from activated platelets and membranous fragments produced by mechanical destruction [9], and have coagulative activity [10]. A few studies on the potential role of PMPs in diabetic complications can be found in the literature [7,8,11,12].
Levels of cell adhesion molecules have been demonstrated to be increased in both type 1 and type 2 diabetes [13] and have been implicated in the development of microvascular complications. In particular, E-selectin, which is expressed on endothelial cells and contributes to monocyte adhesion to the endothelium [14], is up-regulated at the site of atherosclerotic lesions [15], and serum levels of soluble E-selectin (sE-selectin) are elevated in patients with diabetes [13,16].
Monocytes and macrophages may play an important role in glomerular disease [17] and glomerular infiltration of macrophages is associated with diffuse glomerulosclerosis in patients with diabetic nephropathy [18]. Monocyte chemotactic peptide 1 (MCP-1) is a C-C chemokine for monocytes [19,20] and it has recently been reported that MCP-1 may contribute to glomerular monocyte infiltration in diabetic nephropathy [21]. Another member of the C-C chemokine family, regulated on activation normally T-cell expressed and secreted (RANTES) [22], is a potent chemoattractant for memory T lymphocytes, monocytes, eosinophils, and basophils. RANTES may also activate specific effector cell populations of these cells [22,23]. To our knowledge however, no in vivo findings exist concerning RANTES levels in diabetic patients. We therefore measured and compared the levels of platelet activation markers (plasma PMPs, annexin V binding to platelets (plt:annexin V) and plt:P-selectin), chemokines (IL-8, MCP-1, and RANTES), and soluble P- and E-selectins, in order to develop a better understanding of their potential contribution to diabetic vascular complications. Furthermore, we investigated the effects of the anti-platelet drug ticlopidine on these markers in diabetic patients.
MATERIALS AND METHODS
Patients
All 20 healthy volunteers and 37 diabetic patients gave their informed consent to participate in this study, according to the Declaration of Helsinki. All of the diabetic patients had type 2 diabetes. Table 1 shows the characteristics of both patients and controls.
Table 1.
Clinical characteristics of patients and healthy controls
| Control (n = 20) | Type 2 diabetes (n = 37) | P | |
|---|---|---|---|
| Men/women (no.) | 13/7 | 15/22 | |
| Age (years) | 48 ± 6 | 56 ± 5 | NS |
| BMI (kg/m2) | 23·3 ± 1·0 | 25·4 ± 1·2 | NS |
| Leucocytes (/μl) | 4970 ± 752 | 5650 ± 824 | NS |
| PLT ( × 104/μl) | 23·1 ± 4·7 | 23·5 ± 5·2 | NS |
| HbA1c (%) | 4·8 ± 0·3 | 7·8 ± 0·5 | < 0·001 |
| T-CHOL (mg/dl) | 198 ± 35 | 214 ± 29 | NS |
| TG (mg/dl) | 143 ± 37 | 162 ± 44 | NS |
Data are shown as the means ± s.e.m.
NS, Not significant; BMI, body mass index; PLT: platelet count; HbA1c, haemoglobin A1c; T-CHOL, total cholesterol; TG, triglycerides.
Flow cytometry of activated platelets and microparticles
Blood was collected into tubes containing 3·8% sodium citrate (9:1 v/v). Platelet-rich plasma (PRP) was prepared by centrifugation at 200 g for 10 min at room temperature, and the supernatant (the PRP) was then centrifuged at 1400 g for 10 min at room temperature. The resultant platelet pellet was washed twice with washing buffer (9 mmol/l Na2EDTA, 140 mmol/l NaCl, and 26 mmol/l Na2HPO4, pH 7·2) and resuspended in HEPES-Tyrode's buffer (129 mmol/l NaCl, 8·9 mmol/l NaHCO3, 0·8 mmol/l KH2PO4, 0·8 mmol/l MgCl2, 5·6 mmol/l glucose, and 10 mmol/l HEPES, pH 7·4). A equal volume of 2% paraformaldehyde was added to the washed platelets, and the mixture incubated for 15 min at room temperature. The platelets were washed twice, resuspended in stock solution (9 mmol/l Na2EDTA, 26·4 mmol/l Na2HPO4 2H2O, 140 mmol/l NaCl, 0·1% NaN3, and 2% fetal bovine serum (FBS), pH 7·2), and stored at 4°C until analysis. Analysis always took place within 48 h of platelet collection.
PMPs were detected using a modified version of a previously reported method [11,12,24]. Ten microlitres of the platelet suspension (3 × 108/ml) were added to 100 μl of HEPES-Tyrode's buffer containing 5 nmol/l EGTA, and both intact and aggregated platelets were removed by centrifugation at 1000 g for 15 min to yield a supernatant containing microparticles only. Next, 10 μl of washed intact platelets (3 × 108/ml) were added to the supernatant and incubated with 5 μg/ml KMP-9 (the FITC-labelled anti-platelet GPIX MoAb) [25] for 30 min in the dark at room temperature. After incubation, samples were diluted 1:10 with HEPES-Tyrode's buffer containing 5 mmol/l EGTA and analysed using an Ortho Cytoron Absolute analyser (Ortho Diagnostic Systems, Tokyo, Japan). Only the cells and particles positive for GPIX were gated to distinguish platelets and PMPs from electronic noise. To differentiate between platelets and PMPs, the lower limit of the platelet gate was set at the left-hand border of the forward-scatter profile of resting platelets. Ten thousand FITC-positive particles in the PMP gate were then counted to determine the number of PMPs released per 10 000 platelets.
As an index of platelet activation, plt:P-selectin expression was quantified by staining with anti-P-selectin (CLB-thromb/6; Immunotech, Marseille, France) MoAbs. Fixed platelets were washed twice with washing buffer, and incubated with FITC–P-selectin antibody for 30 min in the dark at room temperature. After incubation, samples were diluted with HEPES-Tyrode's buffer containing 2 mm EDTA and analysed using the Ortho Cytoron Absolute analyser. This instrument was calibrated for fluorescence and side scatter using 2·0 μm beads. In forward and side scatter levels, the gating area was set for the platelet population only, excluding aggregating platelets, debris, and machine noise. The setting of the negative line was for unstained platelets and platelets incubated with fluorescence-labelled mouse IgG. Assessment of platelet levels of glycoprotein (GP)IIb/IIIa and GPIb was performed using anti-GPIIb/IIIa (NNKY1-32) [24,26] and anti-GPIb (NNKY5-5) [27] MoAbs.
Assessment of PMPs and plt:P-selectin in whole blood
In order to eliminate the problems associated with platelet washing, a whole blood flow cytometric assay was also designed. The saturating concentration of FITC–P-selectin was predetermined on the basis of platelet activation by 2 μm A23187 (Sigma Chemical Co., St Louis, MO). Blood was collected into tubes containing 3·8% sodium citrate (9:1 v/v). Immediately after exposure to A23187 for 5 min, 10 μl of the collected whole blood were added to 100 μl of HEPES-Tyrode's buffer containing 5 mmol/l EGTA. Next, an equal volume of 2% paraformaldehyde was added and the sample incubated for 15 min at room temperature. Fixed samples were incubated with FITC–P-selectin antibody for 30 min. Flow cytometric analysis was determined as described in the previous section.
Assessment of annexin V binding to platelets
Suspensions of platelets were incubated with FITC-labelled annexin V (150 nmol/l) for 10 min at room temperature, diluted with 500 μl HEPES-Tyrode's buffer, and analysed using an Ortho Cytoron Absolute analyser as described earlier.
Measurement of chemokines and soluble factors
Blood samples were collected from patients and healthy controls into plastic tubes. Aliquots were distributed to tubes containing either sodium citrate or without any anticoagulant. Blood was allowed to clot at room temperature for a minimum of 1 h, and serum or citrated plasma was collected after centrifugation for 20 min at 1000 g (4°C), and was then stored at −30°C until assayed. The serum levels of chemokines and soluble factors were measured using commercial kits according to the manufacturers' instructions. Human MCP-1 and RANTES ELISA kits were from Toyobo Diagnostic, Inc. (Tokyo, Japan). IL-8 was measured using IMMULIZER (Diagnostic Products Corp., Los Angeles, CA). Levels of soluble (s)P-selectin and sE-selectin were measured using MoAb-based ELISA kits from R&D Systems (Toray Fuji Bionics Inc., Tokyo, Japan). Soluble thrombomodulin (sTM) levels were determined by enzyme immunoassay (Teijin Diagnotics, Tokyo, Japan). As positive controls, recombinant chemokines, as well as the standard solutions provided with the commercial kits, were used in each assay.
Anti-thrombosis with ticlopidine
Twenty-four of the diabetic patients had complications. We administered ticlopidine 200 mg/day for 8 weeks to 15 of these 24 patients, who gave their informed consent for the use of the drug. We did not change the other therapeutic drugs used for diabetes management during ticlopidine treatment. The patients included seven with neuropathy, four with retinopathy, four with nephropathy (without renal dysfunction), and one with retinopathy and nephropathy. In addition, we assessed seven non-diabetic patients (three with cerebral infarction, two with unstable angina, one with myocardial infarction, and one with Raynaud's disease). We compared the levels of the measured variables before and after 8 weeks administration of ticlopidine.
Statistical analysis
Statistical analysis was performed using Student's t-test, and P < 0·05 was considered significant. Comparison between patients with type 2 diabetes and healthy controls was performed using an unpaired t-test. In contrast, the paired t-test was used for comparison of data in the same patient before and after ticlopidine administration. A correction factor was applied for multiple comparisons.
RESULTS
We first examined the levels of PMPs and plt:P-selectin in washed platelets and whole blood (Table 2). Both the extent of plt:P-selectin staining and the number of PMPs in washed platelets were higher than those in whole blood, but the differences were not statistically significant. In fact, the levels were significantly correlated (plt:P-selectin: r = 0·9815, P < 0·001; PMPs: r = 0·8646, P < 0·05).
Table 2.
Platelet-derived microparticle (PMP) and P-selectin-positive platelet (plt:P-selectin) levels measured by A23187 in whole blood and washed platelet suspensions
| Sample | plt:P-selectin | PMP (/104 platelets) |
|---|---|---|
| Washed platelet suspension | 35·8 ± 7·3 (%) | 1320 ± 215 |
| Whole blood | 32·4 ± 6·6 (%) | 1195 ± 227 |
| P < 0·001 | P < 0·05 |
Data are shown as the mean ± s.d. (n = 5). P values compare the levels of plt:P-selectin or PMPs between whole blood and washed platelet suspensions.
Table 3 shows the levels of PMPs and platelet activation markers in the control and diabetic groups. There were no differences in the binding of the anti-GPIIb/IIIa and anti-GPIb MoAbs between the two groups; however, there were significant differences in the levels of PMPs and platelet activation markers (PMPs: 576 ± 71 versus 338 ± 45, P < 0·05; plt:P-selectin: 9·4 ± 1·3% versus 4·5 ± 0·8%, P < 0·01 for diabetic versus control groups, respectively).
Table 3.
Relationship between platelet-derived microparticle (PMP)/platelet activation markers in controls and diabetic patients
| Control (n = 20) | Diabetic patients (n = 37) | P | |
|---|---|---|---|
| plt:gpIIb/IIIa (%) | 94·3 ± 3·4 | 95·6 ± 3·5 | NS |
| plt:gpIb (%) | 95·7 ± 4·3 | 93·4 ± 4·0 | NS |
| plt:P-selectin (%) | 4·5 ± 0·8 | 9·4 ± 1·3 | < 0·01 |
| plt:annexin V (%) | 5·2 ± 0·8 | 10·9 ± 1·9 | < 0·001 |
| PMP (/104 plt) | 338 ± 45 | 576 ± 71 | < 0·05 |
Values are the means ± s.e.m. NS, Not significant.
Table 4 shows the levels of chemokines and soluble selectins. There were no differences in IL-8 levels between the control and diabetic groups; however, the levels of MCP-1, RANTES, and soluble factors were higher in the diabetic patients compared with the controls (MCP-1: 345 ± 19 versus 234 ± 17, P < 0·05; RANTES: 112 ± 25 versus 41 ± 5, P < 0·01).
Table 4.
Relationship between chemokines/soluble selectins in controls and diabetic patients
| Control (n = 20) | Diabetic patients (n = 37) | P | |
|---|---|---|---|
| IL-8 (pg/ml) | 7·2 ± 0·9 | 10·1 ± 2·5 | NS |
| MCP-1 (pg/ml) | 234 ± 17 | 345 ± 19 | < 0·05 |
| RANTES (ng/ml) | 41 ± 5 | 112 ± 25 | < 0·01 |
| sP-selectin (ng/ml) | 124 ± 15 | 196 ± 28 | < 0·01 |
| sE-selectin (ng/ml) | 43·2 ± 3·9 | 68·4 ± 5·5 | < 0·01 |
Values are the means ± s.e.m. NS, Not significant.
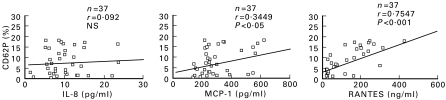
Figure 1 shows the relationship between the levels of plt:P-selectin and chemokines in diabetic patients. The IL-8 level did not significantly correlate with that of plt:P-selectin, but the MCP-1 and RANTES levels were positively correlated (P < 0·05 and P < 0·001, respectively).
Fig. 1.
Correlation between P-selectin-positive platelet (plt:P-selectin) and chemokine levels. Monocyte chemotactic peptide-1 (MCP-1) and regulated on activation normally T-cell expressed and secreted (RANTES), but not IL-8, levels were positively correlated with plt:P-selectin levels. n = 37. NS, Not significant.
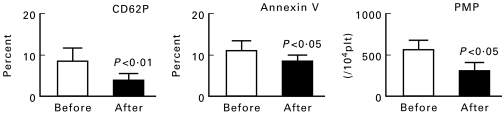
Table 5 shows the clinical and biochemical characteristics of the diabetic patients who received ticlopidine. PMPs and platelet activation markers before and after ticlopidine treatment are shown in Fig. 2. Levels of plt:P-selectin, plt:annexin V and PMPs (per 104 platelets) were reduced from 9·3 ± 2·7% to 4·9 ± 1·8% (P < 0·01), from 11·4 ± 3·1% to 8·6 ± 2·8% (P < 0·05), and from 534 ± 98 to 394 ± 54/104 platelets (P < 0·05), respectively.
Table 5.
Clinical profile of the ticlopidine-treated patients
| No. | Name | Age/sex | FBS (mg/dl) | HBA1c (%) | T-CHOL (mg/dl) | TG (mg/dl) | HDL (mg/dl) |
|---|---|---|---|---|---|---|---|
| 1 | K.M. | 74/M | 138 | 6·0 | 172 | 51 | 80 |
| 2 | E.I. | 63/F | 164 | 8·5 | 221 | 302 | 41 |
| 3 | T.M | 64/F | 133 | 7·3 | 207 | 79 | 67 |
| 4 | Y.N. | 68/F | 145 | 8·1 | 173 | 78 | 87 |
| 5 | K.N. | 59/F | 129 | 6·5 | 225 | 59 | 60 |
| 6 | H.K. | 73/F | 150 | 6·8 | 230 | 148 | 81 |
| 7 | S.N. | 64/F | 144 | 6·1 | 215 | 198 | 53 |
| 8 | S.Y. | 68/F | 166 | 7·4 | 192 | 134 | 72 |
| 9 | Y.S. | 40/M | 135 | 7·4 | 124 | 96 | 16 |
| 10 | K.A. | 74/M | 138 | 7·6 | 170 | 134 | 51 |
| 11 | T.T. | 78/M | 122 | 6·6 | 191 | 150 | 70 |
| 12 | T.M. | 56/F | 155 | 6·6 | 186 | 92 | 72 |
| 13 | J.I. | 60/F | 170 | 7·5 | 200 | 137 | 68 |
| 14 | Y.M. | 56/F | 191 | 8·8 | 223 | 74 | 64 |
| 15 | T.S. | 54/F | 212 | 10·4 | 225 | 88 | 53 |
FBS, Fasting blood sugar; HbA1c, haemoglobin A1c; T-CHOL, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein.
Fig. 2.
Expression of P-selectin-positive platelet (plt:P-selectin), annexin V binding to platelets (plt:annexin V) and plasma platelet-derived microparticle (PMP) levels before and after ticlopidine treatment in 15 diabetic patients. Data are the mean ± s.e.m. The P values are for ‘After’ versus ‘Before’.
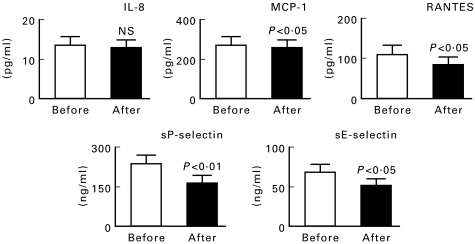
Figure 3 shows the values of chemokines and soluble selectins before and after treatment. IL-8 levels did not change significantly; however, the levels of MCP-1, RANTES, sP-selectin, and sE-selectin were significantly decreased after treatment. On the other hand, the levels of FBS and HbA1 did not differ significantly after ticlopidine treatment (data not shown). The levels of chemokines in non-diabetic patients receiving ticlopidine did not change significantly, although platelet activation markers did decrease significantly following treatment (Table 6).
Fig. 3.
Levels of chemokines and selectins before and after ticlopidine treatment in 15 diabetic patients. Data are the mean ± s.e.m. The P values are for ‘After’ versus ‘Before’.
Table 6.
Levels of chemokines and selectins before and after ticlopidine treatment in seven non-diabetic patients
| Before | After | P | |
|---|---|---|---|
| Plt:P-selectin (%) | 13·5 ± 2·9 | 8·5 ± 1·7 | < 0·05 |
| Plt:Annexin V (%) | 15·4 ± 1·7 | 12·8 ± 2·6 | NS |
| PMP (/104 plt) | 588 ± 101 | 439 ± 96 | < 0·05 |
| IL-8 (pg/ml) | 9·43 ± 1·8 | 9·7 ± 2·2 | NS |
| MCP-1 (pg/ml) | 246 ± 25 | 226 ± 21 | NS |
| RANTES (ng/ml) | 107 ± 12 | 88 ± 14 | NS |
| sP-selectin (ng/ml) | 133 ± 27 | 121 ± 33 | NS |
| sE-selectin (ng/ml) | 49·6 ± 3·6 | 45·5 ± 2·9 | NS |
Values are the means ± s.e.m. (n = 7). NS, Not significant.
Table 7 shows the changes in diabetic patients with elevated (> mean + 2 s.d. of healthy controls) sTM levels. A total of eight patients had high-sTM levels (sTM > 8·5 Teijin units (TU)/ml) and these patients included all four with nephropathy. All chemokine, selectin, and platelet activation marker levels were higher in those patients with elevated sTM levels compared with the other diabetic patients. The sTM, chemokine, soluble selectin, and platelet activation marker levels were significantly decreased after ticlopidine treatment, but the IL-8 level was not changed.
Table 7.
Levels of soluble thrombomodulin (sTM), chemokines, selectins, and platelet activation markers before and after ticlopidine treatment in diabetic patients with elevated sTM levels
| Before | After | P | |
|---|---|---|---|
| sTM (TU/ml) | 13·1 ± 1·9 | 9·5 ± 2·3 | < 0·01 |
| IL-8 (pg/ml) | 10·3 ± 1·5 | 10·1 ± 1·7 | NS |
| MCP-1 (pg/ml) | 398 ± 22 | 275 ± 31 | < 0·01 |
| RANTES (ng/ml) | 124 ± 11 | 86 ± 9 | < 0·01 |
| sP-selectin (ng/ml) | 245 ± 29 | 144 ± 23 | < 0·001 |
| sE-selectin (ng/ml) | 70·3 ± 6·1 | 45·9 ± 5·3 | < 0·01 |
| plt:P-selectin (%) | 11·2 ± 1·5 | 4·5 ± 2·1 | < 0·01 |
| plt:Annexin V (%) | 11·3 ± 1·7 | 8·6 ± 1·8 | < 0·05 |
| PMP (/104 plt) | 693 ± 75 | 420 ± 63 | < 0·05 |
Values are the means ± s.e.m. (n = 8).
NS, Not significant; MCP-1, monocyte chemotactic peptide 1; RANTES, regulated on activation normally T-cell expressed and secreted; plt:P-selectin, P-selectin-positive platelets; plt:Annexin V, annexin V binding to platelets; PMP, platelet-derived microparticles.
TU, Teijin unit.
DISCUSSION
The present study shows that the levels of two C-C chemokines, two soluble selectins, PMPs, and two platelet activation markers were elevated in patients with diabetes, and that these elevations were significantly decreased by ticlopidine treatment.
Platelets in diabetic patients often have increased sensitivity to secondary aggregation in response to agonists [28]. Hyperactive platelets may lead to capillary microembolization via the formation of microaggregates [29]. In the present study, the level of plasma PMPs was significantly higher in diabetic patients than in healthy subjects. PMPs play an important role in coagulation mechanisms [10]. Therefore, these increased levels may be a cause of hypercoagulability [7]. In addition, an increase in PMP levels has been observed in disease states where activated platelets have been detected in vivo [11,12].
The levels of plt:P-selectin were also higher in diabetic patients than in healthy subjects. An increase in P-selectin expression on platelets is known to be a marker of platelet activation [7] and flow cytometric analysis of plt:P-selectin levels has been standardized [7,30]. Using this technique, it has been directly demonstrated that elevated levels of P-selectin-positive platelets circulate in patients with coronary artery disease or those with diabetes [31]. Our findings agree with these previous reports and, in conjunction with the PMP findings, also suggest that enhanced platelet activity and related procoagulant activity in diabetes may induce hypercoagulability.
We evaluated the plasma concentrations of chemokines and soluble selectins in diabetic patients, although it is unclear whether the serum levels of P- and E-selectin reflect the tissue levels of these selectins. In the present study, the levels of the two C-C chemokines MCP-1 and RANTES were increased in diabetic patients compared with healthy subjects. Enhanced levels of C-C chemokines have been found in inflammatory and immune-mediated disease [32–34]. Thus, we believe that our findings support the notion that immunological and inflammatory processes are important features of diabetic complications. MCP-1 and RANTES are produced by a variety of leucocytes; RANTES is also produced by platelets, whereas MCP-1 is also produced by endothelial cells and fibroblasts [23,35]. In the present study, not only RANTES but also MCP-1 levels were positively correlated with the plt:P-selectin level. Activated platelets have been found to stimulate MCP-1 production in monocytes through enhanced RANTES secretion and direct platelet–monocyte contact mediated by P-selectin expression on the platelet surface [36]. This mechanism of enhancement of MCP-1 expression in leucocytes has recently been shown to be operative in patients with acute myocardial infarction [37].
We next studied the effects of the anti-platelet drug ticlopidine on the levels of PMPs, platelet activation markers, chemokines, and soluble selectins. Ticlopidine is a well-known inhibitor of platelet aggregation and it has undergone extensive clinical trials to test its effectiveness at reducing the risk of ischaemic stroke, myocardial infarction, and death from vascular disease [38]. The levels of plt:P-selectin decreased significantly after ticlopidine administration, demonstrating the anti-platelet effect of this drug. In addition, the level of PMPs, which are produced in association with platelet activation, was decreased significantly by ticlopidine administration. These results of ticlopidine on platelet activation markers were similar in non-diabetic patients.
In diabetic patients, arteriosclerosis and microangiopathy are both major complications [39] and diabetes is characterized by the premature development of micro- and macrovascular disease [40]. PMPs have procoagulant activity that is capable of generating thrombin [41] and thrombin, in turn, induces platelet adhesion to endothelial cells, resulting in vascular injury [42]. Thus, anti-platelet therapy with ticlopidine is also thought to be useful for anti-thrombin therapy, including anticoagulation therapy, since it suppresses the production of intrinsic coagulants released following platelet activation.
The levels of chemokines and soluble selectins in diabetic patients were also decreased significantly by ticlopidine. By contrast, these levels did not exhibit any significant changes in non-diabetic patients, presumably because the chemokine levels in non-diabetic patients were not greatly elevated prior to drug administration. RANTES and MCP-1 have chemotactic activity for both monocytes and lymphocytes and, in particular, MCP-1 has been postulated to be a major signal for the accumulation of mononuclear leucocytes in disease states [22]. These monocytes and lymphocytes interact and activate endothelial cells. The increased expression of endothelial adhesion molecules following activation may have a potential role in accelerating diabetic macroangiopathy. These adhesion molecules include E-selectin, which is a product of both endothelial cells, as well as P-selectin, a product of endothelial cells and platelets [43,44]. In the present study, both soluble E- and P-selectin levels were slightly increased in diabetic patients. Thus, the finding of a significant decrease in chemokine and soluble selectin levels following ticlopidine administration suggests concomitant inhibition of diabetic macroangiopathy development.
Finally, we examined sTM levels, as this might predict early nephropathy or be a marker for microvascular complications in diabetic patients [45]. In accordance with this, our patients with high sTM levels included all four patients with nephropathy. We investigated the effects of ticlopidine in all the diabetic patients with high sTM levels. Levels of chemokines (MCP-1 and RANTES), soluble selectins (P-selectin and E-selectin), and platelet activation markers (plt:P-selectin, plt:P-annexin V and PMP) all decreased significantly after ticlopidine treatment, as did sTM levels. Ticlopidine has been reported to have a direct effect on the immune system [46,47]. In particular, May et al. [47] reported that it reduced monocyte–platelet interactions. Our results appear to reflect the effect of ticlopidine on the immune system, in addition to its anti-platelet effect. The present findings thus suggest that ticlopidine may be useful for inhibiting the development of diabetic complications.
In conclusion, levels of plt:P-selectin, plasma PMPs, chemokines, and soluble selectins were significantly higher in patients with diabetes than in normal controls, and administration of ticlopidine significantly decreased these levels. These effects were more pronounced in patients with high sTM levels. These findings suggest that ticlopidine may prevent the development of complications, in which chemokines, selectins, and activated platelets participate, in diabetic patients.
Acknowledgments
We thank Mr Tatsunori Matsuzaki, Mr Mabanu Yamaoka, and Miss Misao Abe of the Department of Blood Transfusion, Kansai Medical University, for providing technical support. This study was supported in part by a Research Grant for Advanced Medical Care from the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Panzram G. Mortality and survival in type 2 (non-insulin dependent) diabetes. Diabetologia. 1987;30:123–31. doi: 10.1007/BF00274216. [DOI] [PubMed] [Google Scholar]
- 2.Songer TJ, DeBerry K, LaPorte RE, Tuomilehto J. International comparisons of IDDM mortality. Diabetes Care. 1992;15(Suppl. 1):15–21. doi: 10.2337/diacare.15.1.s15. [DOI] [PubMed] [Google Scholar]
- 3.Colwell JA, Bingham SF, Abraira C, Anderson JW, Comstock JP, Kwaan HC, Nuttall F. Veterans Administration Cooperative Study on antiplatelet agents in diabetic patients after amputation for gangrene: II. Effects of aspirin and dipyridamole on atherosclerotic vascular disease rates. Diabetes Care. 1986;9:140–8. doi: 10.2337/diacare.9.2.140. [DOI] [PubMed] [Google Scholar]
- 4.Schafer AI. The hypercoagulable states. Ann Intern Med. 1985;102:814–8. doi: 10.7326/0003-4819-102-6-814. [DOI] [PubMed] [Google Scholar]
- 5.Frade LJG, de la Calle H, Alava I, Navarro JL, Creighton LJ, Gaffney PJ. Diabetes as a hypercoagulable state: its relationship with fibrin fragments and vascular damage. Thromb Res. 1987;47:533–40. doi: 10.1016/0049-3848(87)90358-6. [DOI] [PubMed] [Google Scholar]
- 6.Colwell JA, Halushka PV. Platelet function in diabetes. Br J Haematol. 1980;44:521–6. doi: 10.1111/j.1365-2141.1980.tb08705.x. [DOI] [PubMed] [Google Scholar]
- 7.Abrams CHS, Ellison N, Budzynski AZ, Shattil SJ. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990;75:128–8. [PubMed] [Google Scholar]
- 8.Tschope D, Esser J, Schwippert B, Rosen P, Kehrel B, Nieuwenhuis HK, Gries FA. Large platelets circulate in an activated state in diabetes. Semin Thromb Haemost. 1991;17:433–9. doi: 10.1055/s-2007-1002650. [DOI] [PubMed] [Google Scholar]
- 9.George JN, Thoi LL, McManus LM, Reimann TA. Isolation of human platelet membrane microparticles from plasma and serum. Blood. 1982;60:834–40. [PubMed] [Google Scholar]
- 10.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263:18205–12. [PubMed] [Google Scholar]
- 11.Nomura S, Komiyama Y, Miyake T, et al. Amyloid β-protein precursor-rich platelet microparticles in thrombotic disease. Thromb Haemost. 1994;72:519–22. [PubMed] [Google Scholar]
- 12.Nomura S, Suzuki M, Katsura K, et al. Platelet-derived microparticles may influence the development of atherosclerosis in diabetes. Atherosclerosis. 1995;116:235–40. doi: 10.1016/0021-9150(95)05551-7. [DOI] [PubMed] [Google Scholar]
- 13.Cominacini L, Pasini AF, Garbin U, et al. Elevated levels of soluble E-selectin in patients with IDDM and NIDDM: relation to metabolic control. Diabetologia. 1995;38:1122–4. doi: 10.1007/BF00402185. [DOI] [PubMed] [Google Scholar]
- 14.Fries JWU, Williams AJ, Atkins RC, Newman W, Lipscomb MF, Collins T. Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am J Pathol. 1993;143:725–37. [PMC free article] [PubMed] [Google Scholar]
- 15.Davies MJ, Gordon JL, Gearing AJH, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM-1, and E-selectin in human atheroscrelosis. J Pathol. 1993;171:223–9. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 16.Steiner M, Reinhardt KM, Krammer B, Ernst B, Blann AD. Increased levels of soluble adhesion molecules in type 2 (non-insulin dependent) diabetes are independent of glycaemic control. Thromb Haemost. 1994;72:979–84. [PubMed] [Google Scholar]
- 17.Cattell V. Macrophages in acute glomerular inflammation. Kidney Int. 1994;45:945–52. doi: 10.1038/ki.1994.128. [DOI] [PubMed] [Google Scholar]
- 18.Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, Yoshinaga K. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21:480–5. doi: 10.1016/s0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- 19.Rollins BJ, Yoshimura T, Leonard EJ, Prober SJ. Cytokine-activated human endothelial cells synthesize and secrete a monochemoattractant, MCP-1/JE. Am J Pathol. 1990;136:1229–33. [PMC free article] [PubMed] [Google Scholar]
- 20.Colotta F, Borre A, Wang JM, Tattanelli M, Maddalena F, Polentarutti N, Peri G, Matovani A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992;148:760–5. [PubMed] [Google Scholar]
- 21.Ihm CG, Park JK, Hong SP, Lee TW, Cho BS, Kim MJ, Cha DR, Ha H. A high glucose concentration stimulates the expression of monocyte chemotactic peptide 1 in human mesangial cells. Nephron. 1998;79:33–37. doi: 10.1159/000044988. [DOI] [PubMed] [Google Scholar]
- 22.Baggiolini M, Dewald B, Moster B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokine. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 23.Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–92. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomura S, Suzuki M, Kido H, et al. Differences between platelet and microparticle glycoprotein IIb/IIIa. Cytometry. 1992;13:621–9. doi: 10.1002/cyto.990130610. [DOI] [PubMed] [Google Scholar]
- 25.Miyake T, Nomura S, Komiyama Y, et al. Effect of a new monoclonal anti-glycoprotein IX antibody, KMP-9, on high shear-induced platelet aggregation. Thromb Haemost. 1997;78:902–9. [PubMed] [Google Scholar]
- 26.Nomura S, Nagata H, Oda K, Kokawa T, Yasunaga K. Effects of EDTA on membrane glycoprotein IIb-IIIa complex: analysis using flow cytometry. Thromb Res. 1987;47:47–58. doi: 10.1016/0049-3848(87)90239-8. [DOI] [PubMed] [Google Scholar]
- 27.Yanabu M, Ozaki Y, Nomura S, et al. Tyrosine phosphorylation and p72syk activation by an anti-glycoprotein Ib monoclonal antibody. Blood. 1997;89:1590–8. [PubMed] [Google Scholar]
- 28.Winocour PD. Platelet abnormalities in diabetes. Diabetes. 1992;41(Suppl.):26–31. doi: 10.2337/diab.41.2.s26. [DOI] [PubMed] [Google Scholar]
- 29.Packham MA, Mustard JF. The role of platelets in the development and complications of atherosclerosis. Semin Hematol. 1986;23:8–19. [PubMed] [Google Scholar]
- 30.Tschope D, Spangenberg P, Esser J, Schwippert B, Kehrel B, Rosen P, Gries FA. Flow cytometric detection of surface membrane alterations and concomitant changes in the cytoskeletal actins status of activated platelets. Cytometry. 1990;11:652–6. doi: 10.1002/cyto.990110515. [DOI] [PubMed] [Google Scholar]
- 31.Murakami T, Komiyama Y, Masuda M, et al. Flow cytometric analysis of platelet activation markers CD62P and CD63 in patients with coronary artery disease. Eur J Clin Invest. 1996;26:996–1003. doi: 10.1046/j.1365-2362.1996.2360585.x. [DOI] [PubMed] [Google Scholar]
- 32.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, Pope RM, Stieter RM. Macrophage inflammatory protein-1: a novel chemokine cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994;93:921–8. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pattison J, Nelson PJ, Huie P, von Leuttichau I, Farshid G, Sibley RK, Krensky AM. RANTES chemokine expression in cell-mediated transplant rejection of the kidney. Lancet. 1994;343:209–11. doi: 10.1016/s0140-6736(94)90992-x. [DOI] [PubMed] [Google Scholar]
- 34.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 35.Holme PA, Muller F, Solum NO, Brosstad F, Froland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in HIV-1 infection. FASEB J. 1998;12:79–90. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 36.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–34. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann F-J, Marx N, Gawaz M, et al. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation. 1997;95:2387–94. doi: 10.1161/01.cir.95.10.2387. [DOI] [PubMed] [Google Scholar]
- 38.Gent M. A systematic overview of randomized trials of antiplatelet agents for the prevention of stroke, myocardial infarction, and vascular death. In: Hass WK, Easton JD, editors. Ticlopidine, platelets and vascular disease. New York: Springer-Verlag; 1993. pp. 99–116. [Google Scholar]
- 39.Report of a WHO Study Group. Diabetes. Tech Rep Series. 1985;727:58–67. [PubMed] [Google Scholar]
- 40.Graier WF, Wascher TC, Lackner L, Toplak H, Krejs GJ, Kukovetz WR. Exposure to elevated d-glucose concentrations modulates vascular endothelial cell vasodilatory response. Diabetes. 1993;42:1497–505. doi: 10.2337/diab.42.10.1497. [DOI] [PubMed] [Google Scholar]
- 41.Miyazaki Y, Nomura S, Miyake T, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88:3456–64. [PubMed] [Google Scholar]
- 42.Venturini CM, Kaplan JE. Thrombin induces platelet adhesion to endothelial cells. Semin Thromb Hemost. 1992;18:275–83. doi: 10.1055/s-2007-1002435. [DOI] [PubMed] [Google Scholar]
- 43.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 44.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Ann Rev Immunol. 1993;1:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 45.Shimano H, Takahashi K, Kawakami M, Gotoda T, Harada K, Shimada M, Yazaki Y, Yamada N. Elevated serum and urinary thrombomodulin levels in patients with non-insulin-dependent diabetes. Clin Chim Acta. 1994;225:89–96. doi: 10.1016/0009-8981(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 46.Barcellini W, Borghi MO, Lazzaroni E, Vismara A, Ferraro G, Meroni PL. Study on the interference of ticlopidine on the immune system. Int J Tiss Reac. 1988;10:183–8. [PubMed] [Google Scholar]
- 47.May AE, Neumann F-J, Gawaz M, Ott I, Walter H, Schomig A. Reduction of monocyte–platelet interaction and monocyte activation in patients receiving antiplatelet therapy after coronary stent implantation. Eur Heart J. 1997;18:1913–20. doi: 10.1093/oxfordjournals.eurheartj.a015200. [DOI] [PubMed] [Google Scholar]