Abstract
Differences have been shown between HLA characteristics of patients with different courses of alveolar echinococcosis (AE). Notably the HLA B8, DR3, DQ2 haplotype was associated with more severe forms of this granulomatous parasitic disease. We compared IL-10, IL-5, interferon-gamma (IFN-γ) and tumour necrosis factor (TNF) secretion by peripheral blood mononuclear cells (PBMC) isolated from eight HLA-DR3+, DQ2+, B8+ AE patients and from 10 HLA-DR3−, DQ2−, B8− patients after non-specific mitogenic and specific Echinococcus multilocularis antigenic in vitro stimulation. PBMC from seven HLA-DR3+, DQ2+, B8+ healthy subjects and nine HLA-DR3−, DQ2−, B8− subjects were also studied as controls. PBMC from AE patients with HLA DR3+, DQ2+ haplotype secreted higher levels of IL-10 without any stimulation and after specific antigenic stimulation than did patients without this haplotype. Higher levels of IL-5 and IFN-γ were also produced by these patients' PBMC after stimulation with non-purified parasitic antigenic preparations; however, the specific alkaline phosphatase antigen extracted from E. multilocularis induced only Th2-type cytokine secretion. A spontaneous secretion of TNF by HLA DR3+, DQ2+ B8+ AE patients was also found. These results suggest that HLA characteristics of the host can influence immune-mediated mechanisms, and thus the course of AE in humans; specific antigenic components of E. multilocularis could contribute to the preferential Th2-type cytokine production favoured by the genetic background of the host.
Keywords: IL-5, IL-10, interferon-gamma, tumour necrosis factor, alveolar echinococcosis, cellular immunity
INTRODUCTION
Alveolar echinococcosis (AE) is a chronic and severe parasitic disease [1,2]. The lesions resulting from infection by the helminth parasite Echinococcus multilocularis seem mainly to be caused by the host's inappropriate reaction to parasite oncospheres that are carried into the portal venous system, trapped in the liver and then develop as a cancer-like tumour. In the past 5 years studies of patients with AE and of experimental models of larval E. multilocularis infection have demonstrated that Th2 responses, especially IL-10 secretion, might be associated with the progressive form of AE, and that Th1 responses are associated with resistance [3–5]. Abortive AE as described by Rausch et al. [6] associates hepatic calcification and a positive E. multilocularis serology. It has been shown that peripheral blood mononuclear cells (PBMC) from abortive AE patients secreted significantly lower levels of IL-5 and IL-10 than PBMC from progressive AE patients without any stimulation; PBMC from abortive AE patients also produced significantly lower levels of IL-10 after non-specific mitogenic stimulation and specific antigenic exposure [7].
Differences between HLA phenotype of patients with AE and healthy subjects from the same endemic area, and between AE patients with different evolutive courses, have been shown in a recent European study of 151 patients [8]. The HLA B8, DR3, DQ2 haplotype, in particular, was more frequent in patients with the most severe forms of AE. This observation suggests that MHC class II alleles could play a role in determining the clinical course of an infection by E. multilocularis in humans through the preferential induction of Th2 cytokine response in those patients with the most active parasitic growth.
In this study therefore we compared IL-10, IL-5, interferon-gamma (IFN-γ) and tumour necrosis factor (TNF) secretion by PBMC isolated from eight HLA-DR3+, DQ2+, B8+ AE patients and from 10 HLA-DR3−, DQ2−, B8− patients after non-specific mitogenic and specific E. multilocularis antigenic stimulation in vitro. PBMC from seven HLA-DR3+, DQ2+, B8+ healthy subjects and nine HLA-DR3−, DQ2−, B8− subjects were also studied as controls.
PATIENTS and METHODS
Blood samples
Blood samples were collected from 18 patients with progressive AE who had medium-sized, stable lesions at the time of the study. The eight patients with the HLA-DR3+, DQ2+, B8+ haplotype are referred to as ‘DR3+ patient group’, and the 10 patients without this haplotype constitute the ‘DR3− patient group’. None of these 18 patients had undergone a curative hepatectomy or a liver transplantation before the study. PBMC were also isolated from 16 healthy control donors from the same geographical area: seven subjects with the HLA-DR3+, DQ2+, B8+ haplotype (‘DR3+ control group’) and nine subjects without this haplotype (‘DR3− control group’). All patients and control subjects gave their informed consent, and the study was approved by the Regional Ethical Committee, as required by French law (Comité Consultatif pour la Protection des personnes en Recherche Biomédicale de Franche-Comté; decision on Jan 6, 1995).
Antigenic preparations
Crude extract antigen (Emc Ag) was made from a homogenate of E. multilocularis metacestodes (‘Jura vert’ isolate, maintained on Meriones unguiculatus) as previously described (Laboratory of Parasitology, Créteil, France) [4]. Vesicular fluid antigen (Emf Ag) free of host protein contamination was prepared according to the method of Hemphill et al. (Parasitology Institute, Bern, Switzerland) [9]. Metacestode alkaline phosphatase (EmAp Ag; Laboratory of Parasitology, Faculty of Pharmacy, Lyon, France) extraction was done as follows (all procedures were carried out at 4°C): the metacestodes were washed three times with 0·9% NaCl and homogenized in 100 mm Tris–HCl buffer pH 7·6, 100 mm NaCl, 1 mm MgCl2, 1 mm CaCl2, 0·02 mm ZnCl2 using a scissors homogenizer for 10 min, a grinder for an additional 10 min, and then sonicated for 5 min (15 W). The homogenate was mixed with an equal volume of 1-butanol, cooled to −20°C, and shaken slowly for 3 h at 4°C. The mixture was then centrifuged at 9000 g for 30 min in a refrigerated Sorvall centrifuge (Rotor S34). The aqueous layer was removed. The supernatant fraction and the pellet were again mixed, an equal volume of 1-butanol was added, then shaken overnight at 4°C and then centrifuged at 9000 g for 30 min. The butanol was eliminated, the supernatant fraction was dialysed against 0·9% NaCl for 12 h and then concentrated using a sodium salt of carboxymethylcellulose (Aquacid II; Calbiochem, La Jolla, CA). Using this procedure, and compared with that previously described, EmAp Ag was concanavalin A-free, and thus could not interfere with PBMC proliferation as a non-specific mitogen. The sample was stored at −80°C. The total amount of protein in the three antigenic preparations was determined by the BioRad protein assay (BioRad, Ivry‐sur‐Seine, France).
Cell cultures
PBMC were isolated from 35 ml heparinized blood of AE patients and healthy subjects by centrifugation over Ficoll (Eurobio, Les Ulis, France) gradient as previously described [3]. PBMC were washed twice using Hanks' solution (Eurobio) and resuspended at a concentration of 5 × 105 cells/ml in supplemented RPMI medium (Eurobio, Les Ulis, France) as described by Sturm et al. [3]. Cells were cultured at 106 cells in 2 ml of medium with one of the antigenic preparations or the non-specific mitogen phytohaemagglutinin (PHA; Biosepra S.A., Villeneuve‐la‐Garenne, France), at the optimal concentrations and time length determined previously [4]. The following concentrations were selected for the antigenic preparations: 5 μg/culture of Emc antigen, 2·3 μg/culture of Emf antigen, 3·5 μg/culture of EmAp antigen and 7 μg/culture for the PHA as described previously [4]. Cultures were incubated at 37°C with 5% CO2.
Lymphocyte proliferation assay
The cells were cultured in 96-well curved-bottom plates (Polylabo, Strasbourg, France) at 105 cells in 200 μl. Each antigen and the PHA were tested in triplicate and three wells were included with no stimulus. The proliferative responses were assessed by measuring the 3H-thymidine (Amersham, Paris, France) incorporated into DNA [4]. After 96 h of stimulation, cells were pulse-labelled with 0·5 μCi of 3H-thymidine and harvested onto fibreglass filters (Polylabo) 18 h afterwards. The incorporated radioactivity was counted in a liquid scintillation counter. Results are expressed as the mean ct/min of triplicate samples ± s.d.
Cytokine assays
Supernatants of PBMC were collected after 48 h of culture with PHA and after 72 h of culture with antigens. Supernatants from cultures which were mitogen- or antigen-free were also studied as controls. Cytokine productions were measured using commercial kits for ELISA (Quantikine Immunoassay IL-5 D500, R&D Systems, Abingdon, UK; 80-3549-00 Predicta R Human IL-10 ELISA kit, 80-3932-00 Human IFNγ Duoset, 80-3933-00 Human TNFα Duoset, Genzyme, Cambridge, UK). Values < 3·0 pg/ml for IL-5 and IFN-γ, 5·0 pg/ml for IL-10 and 4·4 pg/ml for TNF were below the sensitivity of the assays and recorded as 0. Results were expressed in pg/ml of the cytokine secreted in culture supernatants.
Detection and measurement of cytokine mRNA by semiquantitative reverse transcriptase-polymerase chain reaction
PBMC were harvested 24 h after culture whatever the stimulation. Total mRNA were immediately extracted with Trizol reagent (Gibco, BRL Life Technologies, Cergy‐Pontoise, France) and stored at −70°C for study of the expression of IL-10, IL-5 and IFN-γ mRNA by reverse transcriptase-polymerase chain reaction (RT-PCR). cDNA were obtained by reverse transcription. Specific cDNA sequences were amplified by PCR with a DNA thermal cycler (Minicycler PTC 150; Prolabo) in the presence of β-actin and cytokine sets of sense and antisense primers as previously described by Godot et al. [4]. Cytokine mRNA was quantified by a densitometric technique using the Molecular Imager system (Gel Doc 1000 UV; BioRad) as described by Godot et al. [4]. Results were expressed as a ratio: percentage of cytokine mRNA expression to 100% of β-actin mRNA expression.
Statistical analysis
The differences between non-stimulated and stimulated cells in each group were analysed by the non-parametric paired Wilcoxon signed rank test. Unpaired (Mann–Whitney) tests were used to compare differences in cytokine secretion and cytokine mRNA expression by PBMC from DR3+ and DR3− AE patients on the one hand, and DR3+ and DR3− control subjects on the other hand. In both tests, P < 0·05 was considered significant.
RESULTS
Proliferation of PBMC was not impaired in any of the four groups studied, since we observed a significantly elevated lymphocyte proliferation upon PHA stimulation. Lymphocyte proliferation, after either antigen or PHA stimulation, was not significantly different in DR3+ and DR3− AE patients (data not shown).
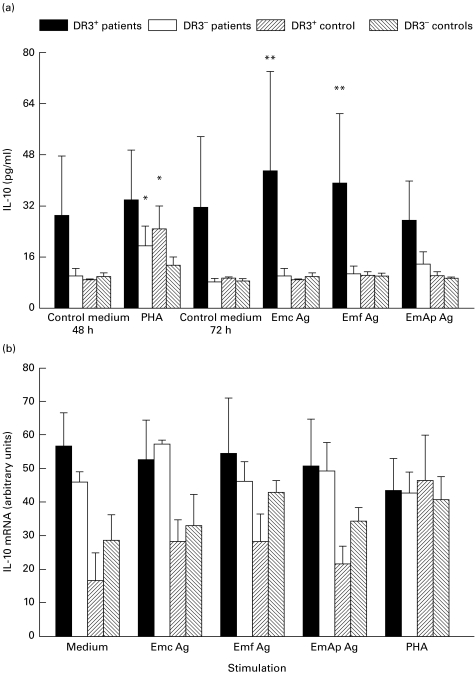
The basal secretion of IL-10 by PBMC from DR3+ AE patients after 48 h or 72 h of culture without any stimulation was higher than that observed in DR3− AE patients and in both control groups (Fig. 1a). It was not enhanced by PHA stimulation, unlike IL-10 secretion by PBMC from DR3− AE patients (P = 0·01) and DR3+ controls (P = 0·01) (Fig. 1a). IL-10 mRNA expression was markedly induced by PHA in PBMC from DR3+ control subjects (Fig. 1b). It was always high in patients' PBMC whatever the stimulation. IL-10 secretion by PBMC from DR3+ AE patients, stimulated by Emc and Emf antigens, was significantly higher than that observed in similarly stimulated PBMC from DR3− AE patients (Fig. 1a, P = 0·02 and P = 0·03, respectively).
Fig. 1.
Secretion of IL-10 (a) and IL-10 mRNA expression (b) in peripheral blood mononuclear cell (PBMC) cultures. IL-10 protein and mRNA levels were assessed in PBMC cultures from alveolar echinococcosis (AE) patients and healthy subjects with or without the HLA-DR3, DQ2, B8 haplotype, after cell stimulation with phytohaemagglutinin (PHA) or Echinococcus multilocularis antigens. Cultures without any stimulation served as controls. (a) Cytokine secretion was measured using an ELISA assay. Values are means ± SEM. (b) IL-10 mRNA expression was semiquantified by densitometric analysis after polymerase chain reaction amplification of cDNA with specific primer pairs. Results are expressed as arbitrary units as described in PATIENTS and METHODS. *P < 0·05: statistically significant difference for cytokine protein or cytokine mRNA level between non-stimulated and stimulated cells in each group (Wilcoxon signed rank test); **P < 0·05: statistically significant difference for cytokine protein or mRNA level between cell cultures from DR3+ and DR3− AE patients (Mann–Whitney U-test). Control medium, no stimulation; Emc Ag, stimulation with the crude extract antigen of E. multilocularis; Emf Ag, stimulation with the vesicular fluid purified antigen; EmAp Ag, stimulation with the alkaline phosphatase of E. multilocularis; PHA, stimulation with the non-specific mitogen, phytohaemagglutinin.
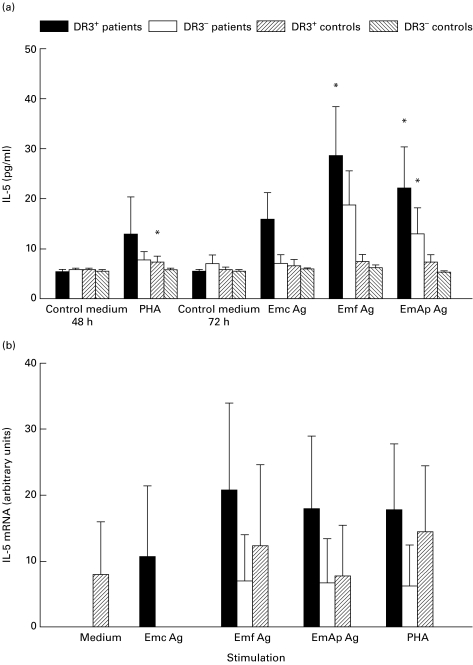
In the two groups of patients, as well as in DR3− control subjects, the levels of IL-5 were not significantly elevated upon PHA exposure (Fig. 2a). Emf and EmAp antigens significantly induced the secretion of IL-5 in the PBMC culture supernatants from DR3+ AE patients (P = 0·02 and P = 0·04, respectively). The secretion of IL-5 was significantly enhanced only by EmAp in PBMC supernatants from DR3− AE patients (P = 0·04). Whatever the stimulation, no expression of IL-5 mRNA in PBMC isolated from the DR3− control subjects was observed, but it was present in the PBMC from DR3+ control subjects (Fig. 2b). All antigenic preparations induced a marked IL-5 mRNA expression in the PBMC from DR3+ AE patients, which was always higher than that observed in PBMC from DR3− AE patients and both groups of controls.
Fig. 2.
Secretion of IL-5 (a) and IL-5 mRNA expression (b) in peripheral blood mononuclear cell (PBMC) cultures. IL-5 protein and mRNA levels were assessed in PBMC cultures from alveolar echinococcosis (AE) patients and healthy subjects with or without the HLA-DR3, DQ2, B8 haplotype, after cell stimulation with phytohaemagglutinin (PHA) or Echinococcus multilocularis antigens. Cultures without any stimulation served as controls. (a) Cytokine secretion was measured using an ELISA assay. Values are means ± s.e.m. (b) IL-5 mRNA expression was semiquantified by densitometric analysis after polymerase chain reaction amplification of cDNA with specific primer pairs. Results are expressed as arbitrary units as described in PATIENTS and METHODS. *P < 0·05: statistically significant difference for cytokine protein or cytokine mRNA level between non-stimulated and stimulated cells in each group (Wilcoxon signed rank test); **P < 0·05: statistically significant difference for cytokine protein or mRNA level between cell cultures from DR3+ and DR3− AE patients (Mann–Whitney U-test). Control medium, no stimulation; Emc Ag, stimulation with the crude extract antigen of E. multilocularis; Emf Ag, stimulation with the vesicular fluid purified antigen; EmAp Ag, stimulation with the alkaline phosphatase of E. multilocularis; PHA, stimulation with the non-specific mitogen, phytohaemagglutinin.
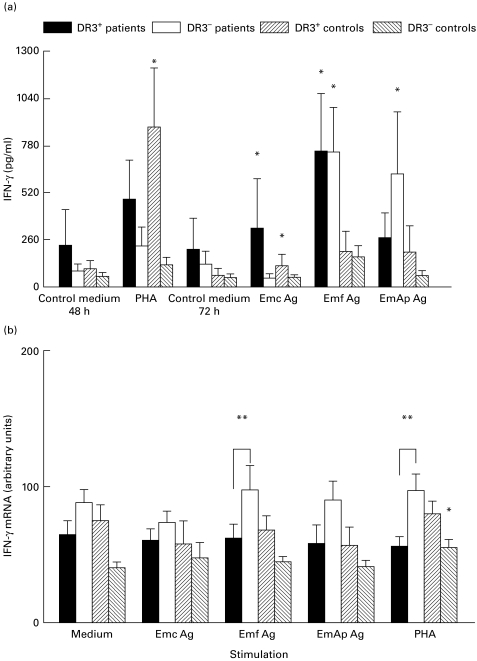
PHA induced a significant increase in IFN-γ secretion by PBMC from DR3+ control subjects (P = 0·02 versus non-stimulated cells, Fig. 3a). Emf antigen was the most powerful inducer of IFN-γ in both DR3+ and DR3− AE patients' PBMC supernatants (P = 0·02 in both cases), while EmAp-related IFN-γ secretion was significant only in DR3− AE patients (P = 0·04 versus non-stimulated cells). IFN-γ mRNA expression was significantly more enhanced by PHA and Emf in the PBMC from DR3− AE patients than in those from DR3+ AE patients (Fig. 3b, P = 0·01 and P = 0·04, respectively).
Fig. 3.
Secretion of IFN-γ (a) and IFN-γ mRNA expression (b) in peripheral blood mononuclear cell (PBMC) cultures. IFN-γ protein and mRNA levels were assessed in PBMC cultures from alveolar echinococcosis (AE) patients and healthy subjects with or without the HLA-DR3, DQ2, B8 haplotype, after cell stimulation with phytohaemagglutinin (PHA) or Echinococcus multilocularis antigens. Cultures without any stimulation served as controls. Cytokine secretion was measured using an ELISA assay. Values are means ± s.e.m. IFN-γ mRNA expression was semiquantified by densitometric analysis after polymerase chain reaction amplification of cDNA with specific primer pairs. Results are expressed as arbitrary units as described in PATIENTS and METHODS. *P < 0·05: statistically significant difference for cytokine protein or cytokine mRNA level between non-stimulated and stimulated cells in each group (Wilcoxon signed-rank test); **P < 0·05: statistically significant difference for cytokine protein or mRNA level between cell cultures from DR3+ and DR3− AE patients (Mann–Whitney U-test). Control medium, no stimulation; Emc Ag, stimulation with the crude extract antigen of E. multilocularis; Emf Ag, stimulation with the vesicular fluid purified antigen; EmAp Ag, stimulation with the alkaline phosphatase of E. multilocularis; PHA, stimulation with the non-specific mitogen, phytohaemagglutinin.
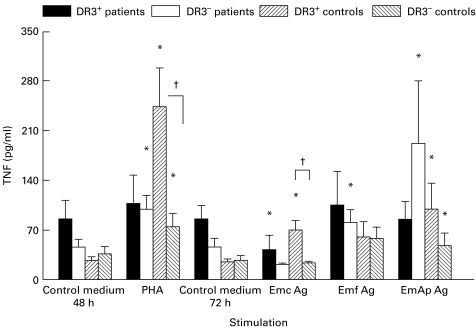
Without any stimulation, the basal secretion of TNF by PBMC from DR3+ AE patients was spontaneously high, compared with that of PBMC from all other groups (Fig. 4). However, as for IFN-γ, a differential effect of crude versus purified antigenic preparations was observed; a markedly increased production of TNF was induced by the purified alkaline phosphatase in DR3− patients. A significant increase was observed upon PHA stimulation in DR3+ healthy subjects (P = 0·03).
Fig. 4.
Secretion of tumour necrosis factor (TNF) in peripheral blood mononuclear cell (PBMC) cultures. TNF protein was assessed in PBMC cultures from alveolar echinococcosis (AE) patients and healthy subjects with or without the HLA-DR3, DQ2, B8 haplotype after cell stimulation with phytohaemagglutinin (PHA) or Echinococcus multilocularis antigens. Cultures without any stimulation served as controls. Cytokine secretion was measured using an ELISA assay. Values are means ± s.e.m. *P < 0·05: statistically significant difference for cytokine protein level between non-stimulated and stimulated cells in each group (Wilcoxon signed-rank test); †P < 0·05: statistically significant difference for cytokine protein level between cell cultures from DR3+ and DR3− healthy control subjects (Mann–Whitney U-test). Control medium, no stimulation; Emc Ag, stimulation with the crude extract antigen of E. multilocularis; Emf Ag, stimulation with the vesicular fluid purified antigen; EmAp Ag, stimulation with the alkaline phosphatase of E. multilocularis; PHA, stimulation with the non-specific mitogen, phytohaemagglutinin.
DISCUSSION
Larval growth of E. multilocularis varies between, and even within, strains of rodents or laboratory mice [10]. Many studies have shown that these differences in sensitivity to E. multilocularis might be attributed to differences in the host immune response: both the course of DTH and the cell subpopulations which infiltrate the lesions vary in ‘susceptible’ and ‘resistant’ mice [11,12]. It has also been shown that the periparasitic fibrosis induced by the effector immune response could play a major role in the ‘resistance status’[13].
The importance of the host's genetic background as a determinant of protective immune responses against infections is well established [14,15]. Recently it has been suggested that there was an association between the severity of human AE and genetic markers of the MHC. A preliminary investigation indicated that HLA-DR13 might be associated with an increased propensity for AE development [16]. Eiermann et al. provided evidence that HLA-DR11 might reduce the risk of developing the disease after E. multilocularis infection, and that the severe forms of AE were significantly associated with HLA-DR3 and HLA-DQ2 in patients [8]. In this study, we examined the role of MHC class II genes in selecting Th1- versus Th2-type profile in human AE. The HLA-DR3, DQ2 and B8 haplotype has been associated with various autoimmune diseases [17]. The immune status of healthy individuals with this common haplotype seems characterized by an impairment in cellular immunity [17–20]. Our results revealed that, in DR3+ control subjects in vitro IL-10, IL-5, IFN-γ and TNF secretions were significantly enhanced upon PHA exposure. However, after PHA stimulation a lower production of Th2-type cytokine was observed in PBMC from the DR3− control group. In DR3+ AE patients, PBMC stimulation with both crude extract and vesicular fluid antigens (the less purified antigenic preparations) promoted high IL-10, IL-5 and TNF secretion levels, but also elevated levels of IFN-γ. Conversely, PBMC isolated from DR3+ AE patients, and stimulated with the purified alkaline phosphatase of E. multilocularis, produced high levels of IL-10 and IL-5, but no increase in IFN-γ and TNF. This could be due to the presence of a mixture of epitopes with dual effects on MHC–peptide T cell interactions in the less purified antigenic preparations, even though, like Emf Ag, they were not contaminated by host antigens. The alkaline phosphatase of the parasite is an antigen particularly involved in the humoral immune response against E. multilocularis [21]; it could belong to those parasitic antigens able to drive the cytokine profile towards the Th2-type, and thus could participate in the evasion mechanisms.
The current study revealed that DR3+ patients secreted higher levels of IL-10 without stimulation, and after antigenic stimulation, than did DR3− patients. It is interesting to note that, in a parallel study, no IL-10 secretion was observed by non-stimulated PBMC obtained from AE patients with abortive lesions and, after PHA and E. multilocularis antigen exposure, their IL-10 production was significantly lower than in AE patients with active lesions [7]. Our findings seem thus to confirm the role of IL-10 in the progression of human AE and suggest that the influence of the host's genetic background on the immunopathology of the infection could be partially mediated by IL-10, known to be one of the major anti-inflammatory cytokines implicated in tolerance and used as a therapeutic agent because of its effects on antigen presentation, cell co-operation and effector cell functions [22,23]. An elevated production of IL-10 was similarly observed in systemic lupus erythematosus, a disease typically associated with the HLA DR3, DQ2 haplotype [24].
Many studies have reported the efficacy of the proinflammatory cytokine TNF against pathogens, particularly parasites [25–28]. In some cases disease was also associated with the detection of TNF in serum [29]. Elevated secretion of TNF in normal subjects seems to be associated with HLA-DR3 expression. Abraham et al. reported a significantly higher production of TNF in DR3+ than in DR3− individuals and this difference was also observed in our control subjects [30]. In all AE patients with active lesions, macrophages located at the extreme periphery of the periparasitic granuloma expressed TNF mRNA, whereas this expression was detected at the border of the parasitic vesicle only in the lesions from a patient with a more severe form of the disease, in macrophages exhibiting the CD11− CD25+ phenotype [31]. This suggests that the periparasitic liver necrosis could be favoured by activated TNF-producing cells. However, Amiot et al. [32] have observed that Lymphotoxin-TNF-deficient mice infected by E. multilocularis harboured a significantly higher parasite burden than did the wild-type mice, suggesting a major effector role for this cytokine. We observed a spontaneously elevated secretion of TNF in non-stimulated PBMC from DR3+ patients, which could not be further increased upon antigen or PHA stimulation. In severe cases of human AE, many clinical symptoms such as fever, wasting, cachexia, fatigue, and extended hepatic tissue necrosis suggest a role for TNF. Our findings seem to support this role, but this does not preclude a protective function which, at the initial stages of larval development, could be beneficial to the host.
Although the biochemical mechanisms mediated by IL-10 and TNF in severe AE have not yet been fully identified, this study provides evidence that HLA-B8, DR3, DQ2 could be a host property which might influence the course of AE in humans through immune-mediated mechanisms.
Acknowledgments
We wish to thank Professors Danièle Lenys, and Jean-Philippe Miguet, Drs Martine Liance and Jacqueline Chabod for their contribution to this study, Lois Rose for the preparation of the manuscript and Sabeha Biichlé for care and management of the healthy control subjects. We would like to express our gratitude to the patients, the healthy control subjects and nursing staff without whom this study would not have been possible. This work was supported by the French Ministry of Health and Besançon University Hospital (PHRC 8, 1994 UF-1452), and by the European Commission (InterReg II grant).
REFERENCES
- 1.Bresson-Hadni S, Vuitton DA, Lenys D, et al. Cellular immune response in Echinococcus multilocularis infection in humans. (1) Lymphocyte reactivity to Echinococcus antigens in patients with alveolar echinococcosis. Clin Exp Immunol. 1989;78:61–66. [PMC free article] [PubMed] [Google Scholar]
- 2.Miguet JP, Bresson-Hadni S, Vuitton DA. Echinococcosis of the liver. In: McIntyre B, Benhamou JP, Bircher J, Rizetto M, Rodes J, editors. Oxford textbook of clinical hepatology. Oxford: Oxford University Press; 1991. p. 721. [Google Scholar]
- 3.Sturm D, Menzel J, Gottstein B, et al. Interleukin-5 is the predominant cytokine produced by peripheral blood mononuclear cells in alveolar echinococcosis. Infect Immun. 1995;63:1688–97. doi: 10.1128/iai.63.5.1688-1697.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godot V, Harraga S, Deschaseaux M, et al. Increased basal production of interleukin-10 by peripheral blood mononuclear cells in human alveolar echinococcosis. Eur Cytokine Netw. 1997;8:401–8. [PubMed] [Google Scholar]
- 5.Emery I, Liance M, Deriaud E, et al. Characterization of T-cell immune response of Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol. 1996;18:1–10. doi: 10.1111/j.1365-3024.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 6.Rausch RL, Wilson JF, Schantz PM, et al. Spontaneous death of Echinococcus multilocularis: cases diagnosed serologically (by EM2 ELISA) and clinical significance. Am J Trop Med. 1987;36:576–85. doi: 10.4269/ajtmh.1987.36.576. [DOI] [PubMed] [Google Scholar]
- 7.Godot V, Harraga S, Beurton I, et al. Resistance/susceptibility to Echinococcus multilocularis infection and cytokine profile in humans. I. Comparison of patients with progressive and abortive lesions. Clin Exp Immunol. 2000;121:484–90. doi: 10.1046/j.1365-2249.2000.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiermann T, Bettens F, Tiberghien P, et al. HLA and alveolar echinococcosis. Tissue Antigens. 1998;52:124–9. doi: 10.1111/j.1399-0039.1998.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 9.Hemphill A, Gottstein B. Immunological and morphological studies on the proliferation of in vivo cultivated Echinococcus multilocularis metacestode. Parasitol Res. 1995;81:605–14. doi: 10.1007/BF00932028. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita J, Ohbayashi M, Doi R. Studies on Echinococcosis XV. Secondary multilocular echinococcosis by intrahepatic inoculation. Jap J Vet Res. 1963;4:125–8. [Google Scholar]
- 11.Liance M, Bresson-Hadni S, Meyer JP, et al. Cellular immunity in experimental Echinococcus multilocularis infection. I. Sequential and comparative study of specific in vivo delayed-type hypersensitivity against Echinococcus multilocularis antigens in resistant and sensitive mice. Clin Exp Immunol. 1990;82:373–7. doi: 10.1111/j.1365-2249.1990.tb05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bresson-Hadni S, Liance M, Meyer JP, et al. Cellular immunity in experimental Echinococcus multilocularis infection. II. Sequential and comparative phenotypic study of the periparasitic mononuclear cells in resistant and sensitive mice. Clin Exp Immunol. 1990;82:378–83. doi: 10.1111/j.1365-2249.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerret S, Vuitton DA, Liance M, et al. Echinococcus multilocularis: relationship between susceptibility/resistance and liver fibrogenesis in experimental mice. Parasitol Res. 1998;84:657–67. doi: 10.1007/s004360050466. [DOI] [PubMed] [Google Scholar]
- 14.Meyer CG, Gallin M, Erttmann KD, et al. HLA-D alleles associated with generalized disease, localized disease, and putative immunity in Onchocerca volvulus infection. Proc Natl Acad Sci USA. 1994;91:7515–9. doi: 10.1073/pnas.91.16.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill AVS, Allsopp CEM, Kwiatkowski D, et al. Common West African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 16.Gottstein B, Bettens F. Association between HLA-DR13 and susceptibility to alveolar echinococcosis. J Infect Dis. 1994;169:1416–7. doi: 10.1093/infdis/169.6.1416. [DOI] [PubMed] [Google Scholar]
- 17.Modica MA, Di Lorenzo G, Galluzzo A, et al. Soluble interleukin-2 receptor defect in vitro in HLA-B8, DR3 positive subjects. Autoimmunity. 1990;7:87–96. doi: 10.3109/08916939008993381. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari JL, Terasaki PI. Berlin: Springer-Verlag; 1985. HLA and disease associations. [Google Scholar]
- 19.McCombs CC, Michalski JP. Lymphocyte abnormality associated with HLA-B8 in healthy young adults. J Exp Med. 1982;156:936–41. doi: 10.1084/jem.156.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Candore G, Cigna D, Todaro M, et al. T-cell activation in HLA-B8, DR3-positive individuals—early antigen expression defect in vitro. Hum Immunol. 1995;42:289–94. doi: 10.1016/0198-8859(94)00103-w. [DOI] [PubMed] [Google Scholar]
- 21.Sarciron ME, Bresson-Hadni S, Mercier M, et al. Antibodies against Echinococcus multilocularis alkaline phosphatase as markers for the specific diagnosis and the serological monitoring of alveolar echinococcosis. Parasite Immunol. 1997;19:61–68. doi: 10.1046/j.1365-3024.1997.d01-183.x. [DOI] [PubMed] [Google Scholar]
- 22.Maureen H, O'garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 23.Lebeaut A, Garaud JJ. Clinical development of interleukin 10. Eur Cytokine Netw. 1997;8:303–4. [PubMed] [Google Scholar]
- 24.Viallard JF, Pellegrin JL, Ranchin V, et al. Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1999;115:189–95. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander HR, Sheppard BC, Jensen LC. Treatment with recombinant human tumor necrosis factor alpha protects rats against the lethality, hypotension and hyperthermia of Gram negative sepsis. J Clin Invest. 1991;88:34–39. doi: 10.1172/JCI115298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tite JP, Dougan G, Chatfiels SN. The involvement of tumor necrosis factor in immunity to Salmonella infection. J Immunol. 1991;147:3161–4. [PubMed] [Google Scholar]
- 27.Titus R, Sherry B, Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989;170:2097–104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong GHW, Goeddel DV. Tumor necrosis factor α and β inhibit virus replication and synergize with interferons. Nature. 1986;323:819–22. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 29.Vassali P. The physiopathology of tumor necrosis factor. Annu Rev Immunol. 1992;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 30.Abraham LJ, French MA, Drawkins RL. Polymorphic MHC ancestral haplotypes affect the activity of tumor necrosis factor-alpha. Clin Exp Immunol. 1993;1:14–18. doi: 10.1111/j.1365-2249.1993.tb05940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bresson-Hadni S, Petitjean O, Monnot-Jacquard B, et al. Cellular localisations of interleukin-1β, interleukin-6 and tumor necrosis factor-α mRNA in a parasitic granulomatous disease of the liver, alveolar echinococcosis. Eur Cytokine Netw. 1994;5:461–8. [PubMed] [Google Scholar]
- 32.Amiot F, Vuong P, Desfontaines M, et al. Secondary alveolar echinococcosis in lymphotoxin-α and tumor necrosis factor-α deficient mice: exacerbation of Echinococcus multilocularis larval growth is associated with cellular changes in the periparasitic granuloma. Parasite Immunol. 1999;21:475–83. doi: 10.1046/j.1365-3024.1999.00245.x. [DOI] [PubMed] [Google Scholar]