Abstract
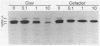
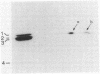
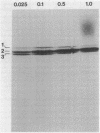
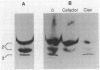
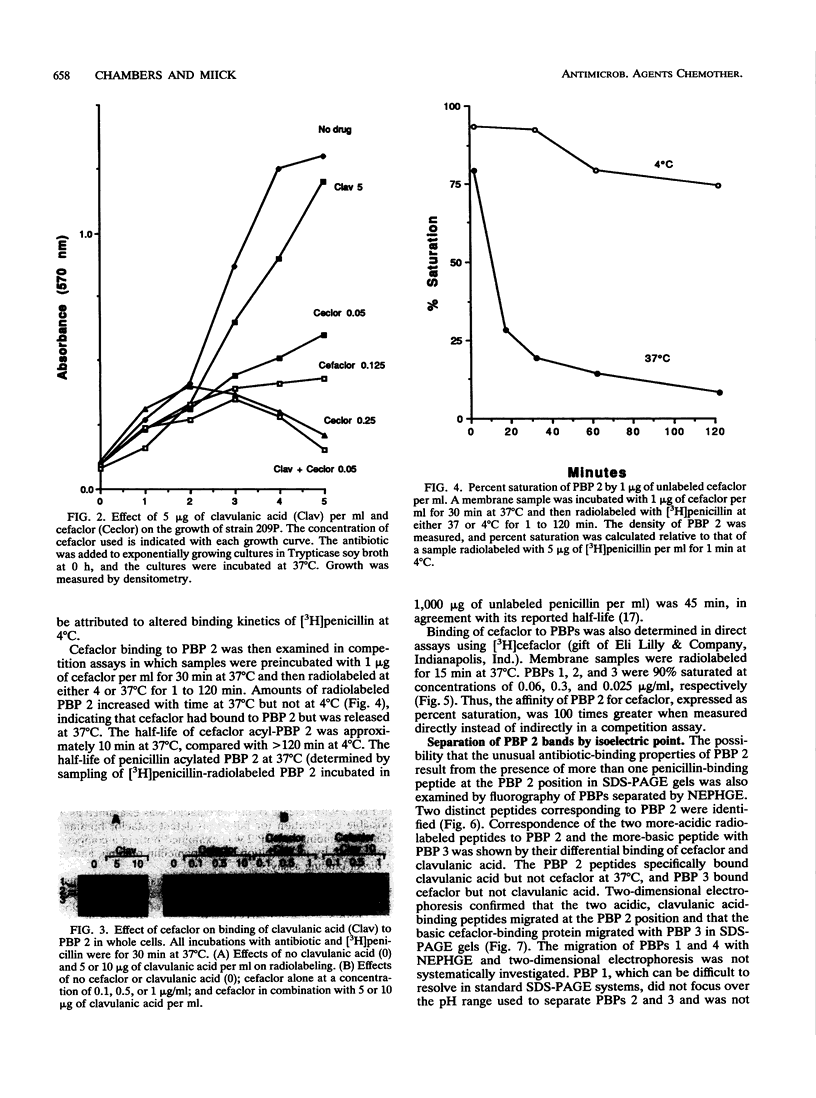
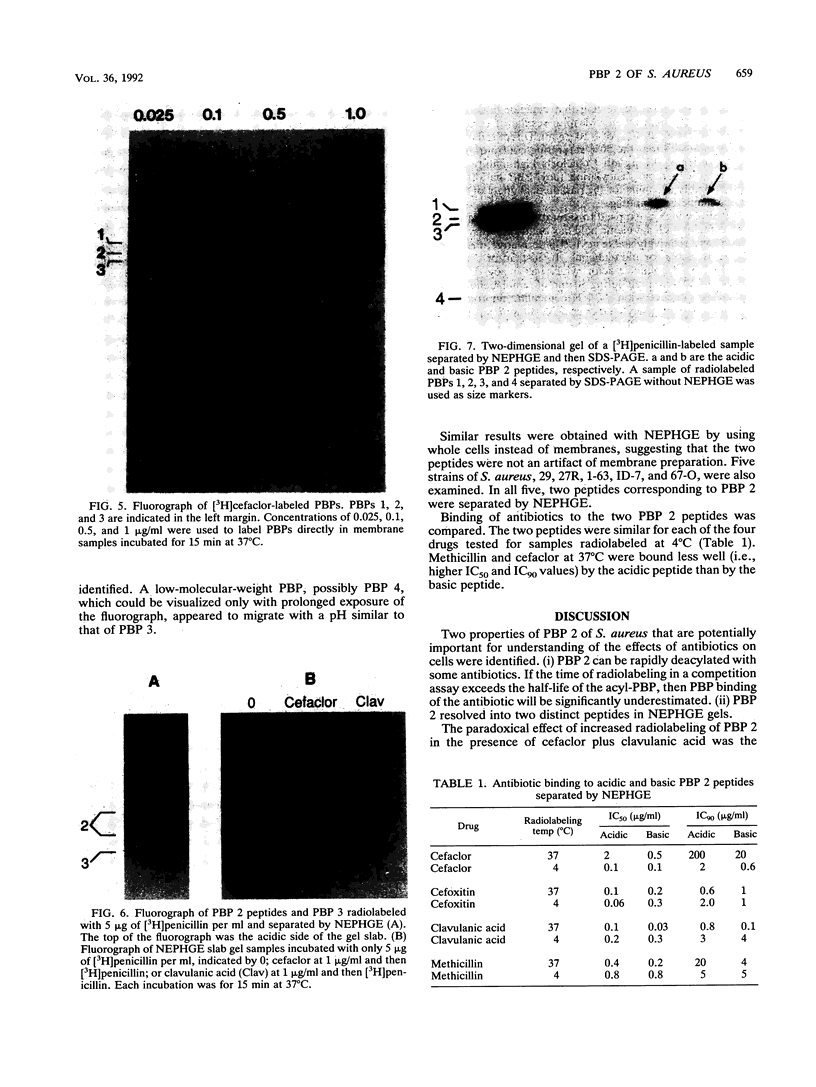
Penicillin-binding protein (PBP) 2 is the major PBP of five that have been identified in susceptible strains of Staphylococcus aureus. Beta-lactam antibiotic binding to PBP 2 is important for the antibacterial effect. Antibiotic binding to PBP 2 in strain 209P was examined with sodium dodecyl sulfate-polyacrylamide gel electrophoresis in competition assays using [3H]penicillin as the radiolabel. Clavulanic acid, which is specifically bound by PBP 2, and cefaclor, which is specific for PBP 3, were studied. Cefaclor, which alone appeared not to bind PBP 2, in combination inhibited PBP 2 binding of clavulanic acid. By varying the temperature during radiolabeling with [3H]penicillin in cefaclor competition assays and in direct radiolabeling assays with [3H]cefaclor, it was shown that cefaclor was bound by PBP 2 with high affinity (50% inhibitory concentration, less than or equal to 0.1 microgram/ml) and that the apparent low-affinity binding (50% inhibitory concentration, greater than 10 micrograms/ml) in competition assays performed at 37 degrees C was due to rapid deacylation. Two penicillin-binding peptides of PBP 2 also were identified in fluorographs of PBPs separated by nonequilibrium pH gradient gel and two-dimensional electrophoresis. Rapid deacylation for some antibiotics and the presence of two penicillin-binding peptides are two properties of PBP 2 that should be considered when correlating results of binding assays with effects of beta-lactam antibiotics on S. aureus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger-Bächi B., Strässle A., Kayser F. H. Natural methicillin resistance in comparison with that selected by in-vitro drug exposure in Staphylococcus aureus. J Antimicrob Chemother. 1989 Feb;23(2):179–188. doi: 10.1093/jac/23.2.179. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Archer G., Matsuhashi M. Low-level methicillin resistance in strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1989 Apr;33(4):424–428. doi: 10.1128/aac.33.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Sachdeva M. Binding of beta-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990 Jun;161(6):1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Dix B. A., Mauriz Y. R. Possible physiological functions of penicillin-binding proteins in Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Feb;29(2):333–336. doi: 10.1128/aac.29.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Binding of beta-lactam antibiotics to penicillin-binding proteins of Staphylococcus aureus and Streptococcus faecalis: relation to antibacterial activity. Antimicrob Agents Chemother. 1980 Nov;18(5):834–836. doi: 10.1128/aac.18.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., STROMINGER J. L. Mode of action of penicillin. Science. 1957 Jan 18;125(3238):99–101. doi: 10.1126/science.125.3238.99. [DOI] [PubMed] [Google Scholar]
- Robertson E. F., Dannelly H. K., Malloy P. J., Reeves H. C. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987 Dec;167(2):290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Sumita Y., Fukasawa M., Okuda T. Affinities of SM-7338 for penicillin-binding proteins and its release from these proteins in Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Mar;34(3):484–486. doi: 10.1128/aac.34.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Drugeon H. B., de Lencastre H. M., Jabes D., McDougall L., Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother. 1989 Nov;33(11):1869–1874. doi: 10.1128/aac.33.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin E., Tomasz A. Beta-lactam-specific resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Oct;30(4):577–583. doi: 10.1128/aac.30.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Cephalosporin-sensitive penicillin-binding proteins of Staphylococcus aureus and Bacillus subtilis active in the conversion of [14C]penicillin G to [14C]phenylacetylglycine. J Biol Chem. 1979 Dec 10;254(23):12056–12061. [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]