Abstract
The aim of this study was to examine the kinetic profile of bioactive TNF levels in aqueous humour of rabbit eyes undergoing corneal allograft rejection and to investigate the effect of locally blocking TNF activity after corneal transplantation. In a rabbit corneal transplantation, endothelial allograft rejection was identified and correlated with increase in central graft thickness. Samples of aqueous humour obtained on alternate days following transplantation were tested for TNF mRNA and bioactive TNF protein. To investigate the effect of locally blocking TNF activity in allograft recipients, the fusion protein TNFR-Ig was administered by injections into the anterior chamber after transplantation. Pulsatile increases in levels of this cytokine were found in 14 of 15 allograft recipients. Peaks of TNF bioactivity preceded by varying intervals the observed onset of rejection in allograft recipients. TNF levels were not elevated in aqueous humour from corneal autograft recipient controls or in serum of allografted animals. mRNA levels were elevated before onset of and during clinically observed allograft rejection. In three of seven animals receiving TNFR-Ig injections on alternate days from day 8 to day 16 post-transplant, clear prolongation of corneal allograft survival was demonstrated. Bioactive TNF is present in aqueous humour following rabbit corneal allotransplantation. Rather than correlating directly with endothelial rejection onset, pulsatile peak levels of TNF precede and follow the observed onset of endothelial rejection. Blockade of TNF activity prolongs corneal allograft survival in some animals, indicating that this cytokine may be a suitable target in local therapy of corneal allograft rejection.
Keywords: cornea, transplantation, cytokine, TNF, aqueous humor
INTRODUCTION
Even though corneal allografts are the most successful transplants, rejection is still an important clinical problem in many patients. Allograft rejection is the commonest cause of corneal graft failure, accounting for 33% [1] to 42% [2] of failed transplants. In cases at high risk of rejection in whom the recipient corneal bed is vascularized or who have a previously rejected graft, graft survival is <30% at 4 years [3]. Most of the available information on pathology of graft rejection is available from animal models, largely rabbit [4], rat [5,6] and mouse [7] in which allograft rejection is characterized by infiltration of T cells and macrophages into the cornea and aqueous humour. There have been few studies investigating the role of cytokines in corneal allograft rejection. These have reported findings on cytokine mRNA [8] and protein, including TNF measured by ELISA [9,10], in homogenized cornea following corneal transplantation in the rat and mouse, respectively. One disadvantage of assessing cytokines by mRNA detection using reverse transcriptase-polymerase chain reaction (RT-PCR) is that message does not necessarily correlate with protein production, on account of possible post-transcriptional regulation or post-translational modification. Another experimental constraint in studies on small rodents is that cytokines can be quantified at only one time point following transplantation if homogenization of the corneal transplant is required for cytokine analysis. The study presented here reports kinetic profiles of bioactive TNF in sequential samples from individual animals without removing the graft.
Up-regulation of TNF has been demonstrated in several models of allograft rejection, but TNF is known to induce gene products involved in diverse types of inflammatory response. It has a key role in the cytokine network, mediating both early and late events involved in inflammation. TNF was sequenced and the gene encoding it cloned in the mid 1980s [11–13]. TNF exists in a secreted mature form and a membrane-bound precursor form, and is produced largely by macrophages, neutrophils and activated lymphocytes. After membrane cleavage by metalloproteinases it forms a biologically active trimer [14]. Its effects are transmitted via cross-linking of the membrane-bound receptor molecules p55 and p75 [15]. One of the principal effects of TNF is to trigger the release of a series of cytokines that amplify and extend the effects of TNF alone. These include IL-1, IL-6, IL-8, interferon-gamma (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), nerve growth factor (NGF), transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF).
The anatomical simplicity of corneal grafts and relative ease with which rejection processes can be visualized and monitored at the cellular and molecular levels compared with transplants of other tissues are major advantages of this model of allograft rejection. Moreover, corneal transplantation is the only model in which local cytokine production can be assessed without tissue disruption, because the anatomy of the anterior chamber (AC) allows sampling of a fluid in direct contact with target donor endothelial cells. Corneal graft rejection in the rabbit model used here shows close similarity to the human, in particular patients with vascularized recipient corneal beds at high risk of rejection. Animals can be easily examined on a slit-lamp microscope and endothelial rejection lines, analogous to those seen in humans, can be directly visualized. In this study sequential levels of bioactive TNF were measured in aqueous humour samples from the anterior chamber before, during and after onset of allograft rejection; TNF levels were related to the clinically observed onset of endothelial rejection and to the corneal graft thickening caused by loss of graft endothelial pump function. In order to study the effect on rejection of blocking the activity of TNF in the anterior chamber in vivo, a recombinant TNF receptor blocking molecule was injected intracamerally.
MATERIALS AND METHODS
Rabbit corneal transplantation
Female outbred New Zealand White (NZW) rabbits of >2·5 kg body weight were used as recipients of corneal grafts from adult female Dutch Belted (DB) strain donors. Rabbits were obtained from Harlan Olac Ltd (Bicester, UK). Animals were cared for in accordance with Home Office regulations. Transplants were performed on the right eye only. On account of the very low incidence of rejection in normal, avascular recipient corneal beds, to study rejection, vascularization was induced in the recipient cornea by insertion of 8/0 silk sutures approximately 3 weeks before transplant [16]. Vascularization was scored by assessing vessel growth across the cornea as 1–4 per quadrant with 1, 2, 3 and 4 indicating 25%, 50%, 75% and 100% of radius, respectively. All NZW corneas had a vascularization score of at least 8, with a score of at least 2 in each quadrant before transplantation. Full thickness 8 mm diameter donor corneal grafts were transplanted into prevascularized NZW corneas, using a continuous 10/0 nylon suture [17] and a recipient corneal trephine diameter of 7·5 mm. Autografts, to control for inflammation caused by surgical trauma and alterations in TNF levels caused by removal of aqueous samples, were performed on prevascularized NZW corneas as for an allograft by removal of a corneal disc of 8·0 mm diameter, 180° rotation and then suturing it in position. Chloramphenicol ointment was applied for the first 3 days after grafting in all animals.
Post-operative assessment and diagnosis of rejection
Following corneal transplantation rabbits were examined on alternate days with an examining slit-lamp. Grafts were scored for the degree of clarity, oedema, vascularization, and examined for an endothelial rejection line [18]. Endothelial rejection onset was taken as the first day on which the endothelial rejection line was observed.
Pachymetry
Corneal thickness is an indirect measure of the action of the corneal endothelial pump mechanism, and as such an objective measure of corneal endothelial function [19]. In a subgroup of rabbits, at each examination central corneal graft thickness was recorded using an ultrasonic pachymeter (Humphrey 855; Carl Zeiss, Oberkochen, Germany). Only topical anaesthesia was required—one drop of amethocaine 1% (Chauvin Pharmaceuticals, Romford, UK) was applied immediately prior to pachymetry. The mean of three readings was calculated for analysis.
Anterior chamber paracentesis
Aqueous humour samples from the anterior chamber were obtained at 2–3-day intervals following transplantation. The eye was anaesthetized using only two drops of amethocaine 1%. A 30 G needle was used to aspirate 150–200 μl of rabbit aqueous from the graft recipient eye. Care was taken to enter the anterior chamber without intersecting corneal vessels in order to ensure that there was no contamination of the aqueous humour sample with blood. Samples were kept in heparinized microtest tubes (Eppendorf, Hamburg, Germany), using 2 μl of heparin sodium (1000 U/ml; CP Pharmaceuticals, Wrexham, UK). All specimens were tested on the day of sampling, as it was found that any duration of storage at room temperature or following freezing reduced the measured bioactive TNF levels (data not shown).
TNF bioassay
The L929 mouse fibrosarcoma cell line (kindly donated by Dr T. Evans, Imperial College School of Medicine, London, UK) was used to assess TNF activity by bioassay [20]. Cells were maintained in full L929 medium (RPMI with 10% fetal calf serum (FCS), 2 mm glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin, all obtained from Gibco BRL (Paisley, UK)). L929 cells were suspended at a concentration of 3 × 105/ml and 100 μl/well plated out onto a 96-well plate (Nunclon, Nalg Nunc International, Roskilde, Denmark). After incubation overnight at 37°C and 5% CO2 the cells were sensitized with 25 μl actinomycin D (Sigma Chemical Co., Poole, UK) at a concentration of 8 μg/ml. This reagent blocks mRNA synthesis and renders the L929 cells highly sensitive to TNF-mediated lysis. Rabbit aqueous samples were diluted 1:2 in full L929 medium and 75 μl of each sample added to each well. Samples were tested in triplicate or quadruplicate. After a further overnight incubation at 37°C and 5% CO2, the medium was discarded and the plate washed with 200 μl RPMI without additives per well. Cells were fixed in 10% formyl saline for 10 min, using 50 μl/well. The fixative was discarded and the cells stained with Gram's crystal violet solution (BDH Laboratory Supplies, Poole, UK). The absorbance at 560 nm was recorded. A standard curve was constructed from dilutions of recombinant rat TNF (Serotec, Oxford, UK), and TNF levels in the aqueous test samples were calculated. To assess the effect of normal rabbit aqueous humour on the bioassay, a standard curve was performed with and without 75 μl of 1:2 dilution of normal rabbit aqueous humour. The standard curve with rabbit aqueous showed little alteration from that with L929 medium and TNF alone. As an additional control, normal rabbit aqueous containing the TNF blocking molecule TNFR-Ig at 10 ng/ml, but no TNF, did not cause L929 cell lysis. This demonstrates that at different TNF concentrations the presence of the other components of aqueous in the bioassay does not significantly affect the level of lysis. Rabbit aqueous from normal ungrafted eyes did not induce L929 cell lysis from complement or any other factors.
Measurement of serum TNF required complement removal by heat inactivation at 56°C for 30 min, as complement-induced L929 cell lysis was found in serum samples tested. All test serum samples showed no L929 cell lysis after heat treatment. TNF levels were not altered by heat inactivation, as demonstrated by heat treating serum containing a known amount of added recombinant TNF, which gave identical readings on the bioassay to untreated medium containing TNF (data not shown). The heat-inactivated serum samples containing recombinant TNF showed complete inhibition of lysis on addition of TNFR-Ig.
RNA isolation and reverse transcription from aqueous samples
Aqueous humour samples (100 μl) (see above) were collected and centrifuged at 10 g in a microfuge at 4°C for 10 min. The supernatant was discarded and the cells snap-frozen and stored at −70°C. Cellular mRNA from each aliquot of cells was co-extracted with 10 μg transfer RNA (from Escherichia coli: Roche Diagnostics Ltd, Lewes, UK) using RNAzol B (AMS Biotechnology Ltd, Abingdon, UK). The mRNA was reverse transcribed using M-MLV reverse transcriptase (Gibco BRL) in the reverse transcriptase buffer and incubated at 37°C for 40 min. Another 2 μl of M-MLV reverse transcriptase were added and incubated for a further 40 min at 37°C. To stop the reaction, the samples were heated at 70°C for 10 min.
PCR using GAPDH primers
All PCR reactions were carried out for 40 cycles using an Omnigene Thermal cycler (Hybaid, London, UK). Each cycle consisted of 94°C for 30 s, 58°C for 40 s and 72°C for 1 min. The 20-μl reaction volume consisted of 0·5 U Taq polymerase (Qiagen, Hilden, Germany), 1× PCR reaction buffer (including 1·5 mm MgCl2; Qiagen), 0·2 mm of each dNTP (Pharmacia, Milton Keynes, UK), 1 μm of each GAPDH primer, 2 μl of cDNA sample, and was overlaid with 30 μl of mineral oil (Sigma). The reaction was started with 94°C for 2 min to denature all cDNA samples. Ten microlitres of PCR product were visualized by agarose gel electrophoresis and ethidium bromide staining, and analysed using a Gel Doc 1000 video gel documentation system (BioRad, Hemel Hempstead, UK). Phytohaemagglutinin (PHA; 1 μg/ml; Sigma)/phorbol myristate acetate (PMA; 10 ng/ml; Sigma)-stimulated (18 h) rabbit spleen cells were used as a positive control (data not shown).
PCR using TNF primers
PCR reactions were carried out as above on each cDNA sample, using TNF primers, 1·5 mm MgCl2 and 5 μl cDNA. Each cycle consisted of 94°C for 30 s, 60°C for 40 s and 72°C for 1 min. The samples were heated at 94°C for 2 min to denature all cDNA samples.
Primer sequences
All primers were obtained from MWG-biotech (Milton Keynes, UK): GAPDH sense primer 5′-GCT GAA CGG GAA ACT CAC TG-3′, GAPDH antisense primer 5′-TCC ACC ACC CTG TTG CTG TA-3′, PCR product 307 kb; TNF sense primer 5′-GCT CAC GGA CAA CCA GCT-3′, TNF antisense primer 5′-TCC CAA AGT AGA CCT GCC C-3′, PCR product 332 kb.
TNFR-Ig
Specificity of the bioassay for aqueous and serum samples was confirmed by complete blocking of lysis by a recombinant TNF receptor molecule, TNFR-Ig. This is a bivalent fusion protein comprising the extracellular domain of the human p55 TNF receptor joined to the hinge, CH2 and CH3 domains of human IgG1. Recombinant protein was expressed in Chinese hamster ovary cells and purified by affinity chromatography on a column of Protein-A-fast flow Sepharose, followed by ion exchange chromatography on S-Sepharose fast flow using a Biopilot chromatography system (Pharmacia) [21]. Complete blocking of 5 ng/ml of both rabbit and recombinant TNF was achieved by 10 ng/ml TNFR-Ig.
Intracameral injections of TNFR-Ig
Anterior chamber injections of TNFR-Ig were administered in groups of allograft recipients at specified times after grafting. PBS (100 μl) containing 0·1 mg of TNFR-Ig, or human IgG1 in controls, was injected into the anterior chamber of the eye using a 30 G needle on a 1-ml syringe and using topical anaesthesia only. Two groups of animals were studied using different time points for injection of the TNFR-Ig: the first group comprised seven rabbits which received five injections of 0·1 mg of TNFR-Ig or human IgG1 on alternate days from day 8 to 16 post-grafting, and a second group of three rabbits which received intracameral injections from days 14 to 22 post-graft. The study groups were assessed by sequential examination and pachymetry as described above.
RESULTS
Corneal allografts were performed as described in 15 NZW rabbits for measurement of TNF levels in 200 μl aqueous samples. In a subgroup of five of these, a total of 300 μl of aqueous humour was aspirated: 200 μl were tested by bioassay for TNF and 100 μl of the same specimen were processed by RT-PCR for TNF mRNA (see Materials and methods). Rotational autografts were performed in five NZW rabbits as a control group. In another group of graft recipients, the effect on graft survival of intracameral injections of TNFR-Ig was examined.
Interval to onset of rejection
All grafts attained normal transparency by the first post-operative day. In the untreated allograft recipients the median time for appearance of the endothelial rejection line was 22 days (Fig. 1a). Autografts did not show evidence of rejection, although there was post-operative inflammation which settled over the week following grafting. Pachymetry in the allografts showed marked corneal thickening following appearance of the rejection line: this thickening demonstrates the reduction of endothelial cell function associated with injury from allogeneic inflammation (Fig. 1b). Increased thickness was accompanied by increased graft opacity scores (data not shown). Autografts did not show evidence of rejection: transplanted corneas retained normal thickness and transparency (Fig. 1b).
Fig. 1.
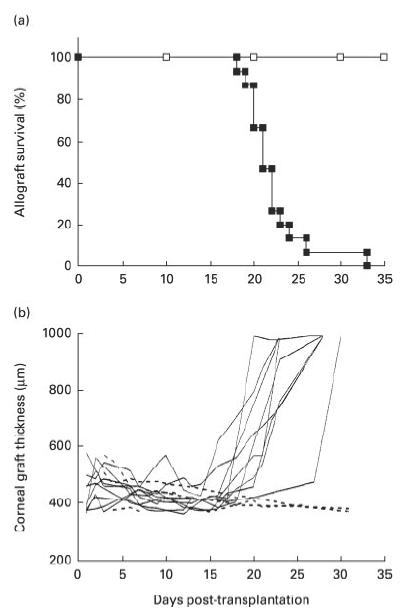
(a) Onset of endothelial rejection line in untreated allograft (▪) and autograft (□) corneas. Corneal allografts were examined every 2 days for evidence of an endothelial rejection line. All 15 allografts rejected, with a median time to appearance of the endothelial line of 22 days. None of five autografts showed evidence of endothelial rejection. (b) Pachymetry of corneal allografts and autografts. Corneal thickness measurements (pachymetry) on nine of the allografts (——) and in three of five autografts (- - - - -) in (a). All the allografts showed increased thickness and opacity associated with endothelial rejection, due to failure of the corneal endothelial pump function. The autografts did not undergo rejection and remained thin and transparent.
Levels of TNF in aqueous humour and serum
In 14 out of 15 allograft recipients, TNF levels were clearly elevated in aqueous samples, with in some animals peaks of at least 10 ng/ml TNF recorded. Peaks did not clearly correlate with rejection onset and high levels were evident both before (n = 10) and after (n = 4) rejection episodes (typical patterns are shown in Fig. 2a–c). In several animals, successive samples showed a fluctuating pattern of elevation and diminution of TNF levels to background levels even when intraocular inflammation associated with rejection was observed on examination. Oscillations in TNF levels were not due to leakage of TNF from serum into the anterior chamber at the time of testing as an artefact of the sampling technique, because (i) serum TNF was not elevated at any time tested, (ii) many allograft aqueous samples contained low TNF levels, and (iii) absent or very low levels of bioactive TNF were detected in all five autograft eyes (Fig. 2d). Samples from autografts were tested over a 15-day period correlating with rejection in allografted eyes. Comparison of the profile of TNF levels in the two groups of animals showed that TNF levels were significantly elevated in the allograft group (Mann–Whitney U-test, P = 0·0014). Normal rabbit aqueous did not contain any bioactive TNF.
Fig. 2.
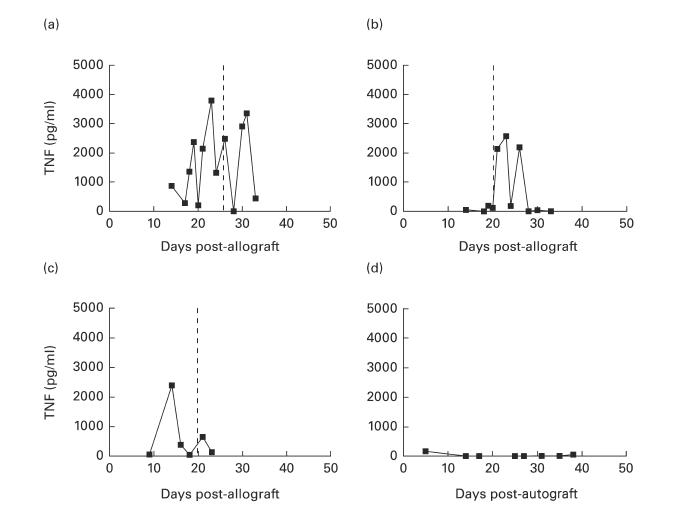
(a–c) Bioactive TNF profile in aqueous humour from allograft recipients. Three typical TNF profiles from aqueous samples from rabbits which had received a corneal allograft on day 0, showing high fluctuations in TNF levels measured by bioassay. Broken line indicates endothelial rejection onset. (d) Bioactive TNF profile in aqueous from an autograft recipient. Typical TNF profile from aqueous samples from a rabbit which had undergone a rotational corneal autograft procedure on day 0, showing negligible TNF levels measured by bioassay.
Extraction of mRNA for TNF from aqueous samples
In a group of five allograft recipients, the cellular component of samples of 100 μl of aqueous was extracted to assess the content of mRNA encoding both TNF and GAPDH, a housekeeping gene which reflects the overall cell content of the sample. Normal rabbit aqueous was used as a control. Figure 3 shows the levels of mRNA for GAPDH and TNF, in two representative animals, and the corresponding bioactive TNF levels in the same aqueous sample. TNF mRNA was never detected before day 13 post-transplantation. In two animals TNF protein was detected before mRNA for TNF was detected in aqueous. Although TNF mRNA did not correlate in timing or intensity with the clinical appearance of the corneal endothelial rejection line, in all rabbits there was detectable mRNA for TNF present around the time that acute rejection was underway. All animals showed gradual increase in mRNA for GAPDH before and during rejection, which diminished after rejection, and this alteration in GAPDH mRNA reflected the increase and then decrease of aqueous cells seen clinically in these eyes over the rejection process. Increases in RNA assayed from inflammatory cells in the AC probably account for the increases in TNF mRNA observed, rather than up-regulation in TNF at the cellular level. No mRNA was detected in samples from normal eyes, although these sometimes contained low levels of mRNA for GAPDH.
Fig. 3.
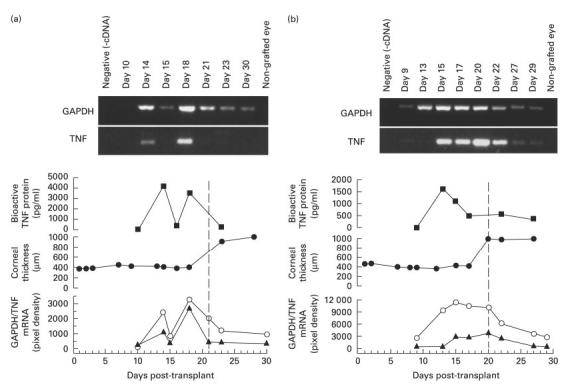
Temporal correlation of sequential aqueous humour levels of bioactive TNF protein, TNF mRNA, GADPH mRNA and corneal graft thickness from two representative rabbit corneal allograft recipients. (a) Rabbit 1. (b) Rabbit 2. Abscissas for all panels are standardized as day post-transplant. Vertical dotted line indicates observed onset on endothelial rejection in each rabbit. Panels from top: mRNA for GAPDH (polymerase chain reaction (PCR) product 307 kb) and TNF (PCR product 332 kb); TNF protein measured on bioassay; central corneal graft thickness; relative mRNA levels for TNF (▴) and GAPDH (circles) in sequential samples from each rabbit.
Treatment with intracameral injections of TNFR-Ig
As day 8 following transplantation was the earliest time at which high levels of bioactive TNF were measured in the anterior chamber, intracameral injection of the soluble receptor fusion protein was commenced at this time. In the group treated with intracameral TNFR-Ig on days 8–16, prolongation of graft survival, indicated by pachymetry and by graft clarity on examination, was found in three of the seven animals (Fig. 4). Two of the remaining four animals in this treatment group showed a small protective effect on endothelium compared with contemporaneous untreated controls, in that graft thickening was delayed. Three control animals which received intracameral human IgG1 injections on alternate days 8–16 post-transplantation rejected grafts on days 20, 22 and 22, the same tempo as untreated animals. Statistical analysis of corneal graft survival assessed by pachymetry in the seven rabbits treated with TNFR-Ig compared with those controls treated with human IgG1 was highly suggestive of a protective effect, although not statistically significant (Mann–Whitney U-test, P = 0·06). A further group of three rabbits treated with TNFR-Ig on days 14–22 showed no difference from untreated controls (data not shown). No local toxic effect resulting from TNFR-Ig injections was evident.
Fig. 4.
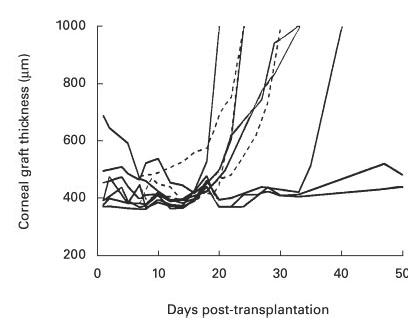
Pachymetry in treated and control corneal allograft recipients, demonstrating effect of TNFR-Ig on graft survival. Corneal allograft function was assessed by pachymetry, increase in thickness correlating with observed onset of endothelial rejection. Solid lines show rabbits which received five intracameral injections, each of 0·1 mg of TNFR-Ig on alternate days 8–16 following corneal transplantation (n = 7); early post-grafting thickness did not correlate with graft survival. Broken lines show pachymetry on allograft controls treated with 0·1 mg human IgG1 on days 8–16 post-transplantation (n = 3). The data are highly suggestive of a protective effect of the TNFR-Ig injections, but do not reach statistical significance (Mann–Whitney U-test, P = 0·06).
DISCUSSION
The role of cytokines in allograft rejection has been extensively studied in many organ systems. A generalization is that elevated Th1-type cytokines are more likely to be associated with rejection and Th2 with tolerance [22], but there are many studies in allotransplantation in which this division of cytokine function is not seen [23]. TNF as a cytokine largely derived from the monocyte/macrophage lineage is classified as a proinflammatory rather than a Th1 cytokine, but its actions are analogous to T cell-derived cytokines in the Th1 subset. TNF levels at the time of allograft rejection appear to be elevated in some organ transplants but not in others. In human heart transplantation, serum TNF is elevated but levels do not correlate specifically with allograft rejection [24–26]. Immunohistochemical studies on local production in clinical biopsy specimens from cardiac allografts undergoing rejection have shown that TNF protein levels do not relate to the grade of cardiac rejection [26], although blocking TNF activity using anti-TNF serum in a rat model slightly prolongs cardiac allograft survival [27]. Serum TNF is elevated following bone marrow transplantation in some types of graft versus host disease [28,29] and following renal transplantation at the time of rejection [30]. Biopsies of rejecting renal transplants also show increased TNF expression using immunohistochemistry [31]. During acute liver rejection serum levels of TNF are elevated [32,33].
These studies clearly demonstrate that both TNF mRNA and protein are locally elevated during corneal allograft rejection in the rabbit. Bioactive protein was allospecific as the elevation was not seen in autograft eyes. Although not an inbred model, the rabbit is in other respects the animal of choice to demonstrate the profile of the TNF response in individual graft recipients, for the following reasons: (i) rejection onset can be clearly identified, and (ii) the anterior chamber has sufficient volume to allow aqueous humour samples to be removed without requiring the animal to be killed, and thus it is feasible to obtain sequential samples. It was thus possible to correlate the kinetic levels of bioactive protein in the aqueous humour with onset in that animal of rejection of the endothelial cell layer of the allograft, which is in direct contact with the aqueous humour. High TNF levels did not correlate with the observed onset of endothelial rejection, but were found in all allograft recipients except one. TNF may be involved as a mediator in corneal allograft rejection or directly in graft injury, although whether it is intrinsic to the rejection process or a by-product thereof is not clear. Of interest is the intermittent peaking of TNF levels seen, with very high readings followed by low levels within 2 days. This may reflect fluctuations in the complex interactions between TNF and its soluble receptor, which antagonizes TNF activity—one possibility is that high levels of TNF are actually present at times when the bioassay detects low levels, but the presence of soluble TNF receptor blocks its cytotoxicity on the bioassay. Another possible explanation is negative feedback on cellular TNF secretion by counteracting cytokines. Autocrine regulation of the later phases of macrophage activation by macrophage inhibitory cytokine 1, itself up-regulated by TNF, has been proposed by Bootcov et al. [34]. An additional possibility is regulation of TNF mRNA stability and translation by regulatory sequences such as AU-rich elements (ARE). These sequences have been demonstrated in macrophages to be actively involved in biphasic positive–negative TNF regulatory loops [35,36]. It is possible that aqueous removal from the rabbit anterior chamber induces local inflammation and breakdown of the blood–aqueous barrier. However, elevated levels of TNF protein were not found in specimens from autograft recipients, indicating that the sampling procedure itself did not induce TNF release. We believe that oscillations in TNF levels are not directly adaptive or an artefact, but rather reflect inherent properties of a system evolved to produce a rapid response to pathogens. A mathematical model for the regulation of TNF activity in the anterior chamber which incorporates negative feedback and amplification pathways is presented elsewhere [37,38].
The source of TNF in aqueous is likely to be macrophages or monocytes present in the aqueous humour itself or in the iris vasculature. Indeed, some of the TNF activity detected may derive from cytokine released by these cells during the bioassay. The fact that TNF protein is in some animals present in aqueous before its mRNA in the aqueous cellular component suggests that initially TNF may be released from cells within the iris, before breakdown of the blood/aqueous barrier which then releases cells producing TNF into the anterior chamber. Elevated macrophage migration inhibitory factor (MIF) has been found in aqueous humour in a rabbit model of graft rejection, indicating a local mechanism for maintaining a macrophage population in the anterior chamber [39,40].
This is the first reported kinetic study of cytokine protein levels in corneal transplantation. Most studies to date in other solid organ allografts have used ELISA or radioimmunoassay (RIA) to measure protein levels rather than bioactivity, and serum levels rather than local levels have been studied. One study which did investigate local TNF bioactivity, by Demeester [41], showed a bimodal elevation in this cytokine during rejection in a rat lung allotransplantation model, a finding consistent with ours. Torres et al. detected elevated mRNA for TNF at the time of rejection in homogenized corneal allografts, but not in autografts, also consistent with our findings [8]. Sano et al. found raised TNF, in homogenized mouse corneas, both in allo- and syngeneic grafts before onset of rejection, and in rejected and unrejected allografts at a later time point, but not in normal mouse cornea [9]. Zhu et al. found higher levels of TNF in allogeneic than syngeneic recipient mouse corneas at 7 and 14 days following transplantation, therefore also finding TNF in early stages following grafting, if not directly correlating it to the timing of rejection [10]. These authors also noted recipient peripheral cornea as the principal source. This finding is consistent with our detection of TNF protein in the aqueous humour—another interface of recipient with allogeneic tissue, and alloreactive cells infiltrate the recipient peripheral cornea as they do the aqueous. Our finding of bioactive protein in the anterior chamber before and following onset of rejection correlates with detection by ELISA of TNF in homogenized corneas by these investigators, but the functional role of TNF may be more complex than suggested in those studies. Moreover, detection of TNF mRNA and protein in aqueous humour may not accurately reflect levels in the corneal tissue itself.
As we had found active TNF in the aqueous humour and demonstrated a blocking effect of TNFR-Ig in the bioassay, the rationale for the treatment study was to examine the effect of exogenous soluble receptor in the compartment in which we had detected bioactive cytokine. Direct blockade of the local effect of TNF in the anterior chamber by using intracameral injections of TNFR-Ig had a protective effect on endothelial function and in the prolongation of graft survival in some animals. There are limitations in the technique of administration of the TNFR-Ig, as the short half-life of aqueous humour means that blockade of TNF function is almost certainly incomplete. However, these results do suggest a role for antagonism of the action of this cytokine in the treatment of corneal allograft rejection, and demonstrate that TNFR-Ig has minimal ocular toxicity in this model, notably in an animal in which, like humans, corneal endothelial cells have a low capacity for endothelial cell response to injury by mitosis.
Our data support a possible role for TNF in the pathophysiology of corneal allograft rejection. Both the p55 and the p75 TNF receptors have been demonstrated on corneal endothelial cells [42]. However, the majority of cells in the body express one or both receptors, implying that receptor presence itself is not sufficient to account for the diversity of cell type-specific responses to TNF. TNF has been demonstrated to increase MHC class II and intercellular adhesion molecule-1 (ICAM-1) expression on vascular endothelial cells, and in vitro has been shown to increase expression of inducible nitric oxide synthase in bovine corneal endothelial cells [43]. These effects of TNF suggest a possible proinflammatory role in donor cell injury in corneal allograft rejection: the rejection-associated corneal endothelial cell damage shown by graft thickening on pachymetry may be related in part to exposure to high levels of TNF. The prolongation of allograft survival in some animals by TNFR-Ig might be due to reduction in adhesion molecule expression, preventing cell trafficking. This would explain why the temporal relationship between TNF production and rejection onset is not exact: TNF is facilitating rather than mediating rejection. On the other hand, a number of observations in TNF-deficient and TNFR-deficient mice suggest that TNF has a critical role in limiting and reversing inflammation and tissue injury [44]. In the corneal transplantation model reported here, this may limit injury to recipient tissues in the anterior chamber caused by effector cells in the allogeneic response. There may not only be different sources of TNF but also differential functions of this cytokine at different times with respect to onset of rejection. Of particular interest in this respect is the recent report by Yamada and colleagues that the combined deletion of p55 and p75 TNF receptors had no effect on survival of MHC or minor alloantigen-disparate murine corneal grafts [45].
Although TNF levels in the aqueous at any specific time point do not directly correlate with the onset of endothelial corneal graft rejection, the levels of this cytokine in aqueous humour are clearly elevated following corneal allotransplantation compared with autograft controls. Locally delivered antagonists of the action of TNF in the aqueous humour may hold potential in the future therapy of resistant corneal allograft rejection.
Acknowledgments
This work was supported by project grants from The Iris Fund for Prevention of Blindness, Action Research (S/P/3090) and Hammersmith Hospital Locally Organized Research Scheme (SG/96/0029).
REFERENCES
- 1.Vail A, Gore SM, Bradley BA, Easty DL, Rogers CA, Armitage WJ. Influence of donor and histocompatibility factors on corneal graft outcome. Transplantation. 1994;58:1210–7. [PubMed] [Google Scholar]
- 2.Williams KA, Roder D, Esterman A, Muehlberg SM, Coster DJ. Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology. 1992;99:403–14. doi: 10.1016/s0161-6420(92)31960-8. [DOI] [PubMed] [Google Scholar]
- 3.Hill JC. Systemic cyclosporine in high-risk keratoplasty. Short- versus long-term therapy. Ophthalmology. 1994;101:128–33. doi: 10.1016/s0161-6420(13)31253-6. [DOI] [PubMed] [Google Scholar]
- 4.Williams KA, Standfield SD, Wing SJ, Barras CW, Mills RA, Comacchio RM, Coster DJ. Patterns of corneal graft rejection in the rabbit and reversal of rejection with monoclonal antibodies. Transplantation. 1992;54:38–43. doi: 10.1097/00007890-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Katami M. Corneal transplantation—immunologically privileged status. Eye. 1991;5:528–48. doi: 10.1038/eye.1991.96. [DOI] [PubMed] [Google Scholar]
- 6.Larkin DFP, Calder VL, Lightman SL. Identification and characterization of cells infiltrating the graft and aqueous humour in rat corneal allograft rejection. Clin Exp Immunol. 1997;107:381–92. doi: 10.1111/j.1365-2249.1997.279-ce1171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonoda Y, Sano Y, Ksander B, Streilein JW. Characterization of cell-mediated immune responses elicited by orthotopic corneal allografts in mice. Invest Ophthalmol Vis Sci. 1995;36:427–34. [PubMed] [Google Scholar]
- 8.Torres PF, De Vos AF, van der Gaag R, Martins B, Kijlstra A. Cytokine mRNA expression during experimental corneal allograft rejection. Exp Eye Res. 1996;63:453–61. doi: 10.1006/exer.1996.0135. [DOI] [PubMed] [Google Scholar]
- 9.Sano Y, Osawa H, Sotozono C, Kinoshita S. Cytokine expression during orthotopic corneal allograft rejection in mice. Invest Ophthalmol Vis Sci. 1998;39:1953–7. [PubMed] [Google Scholar]
- 10.Zhu S, Dekaris I, Duncker G, Dana MR. Early expression of proinflammatory cytokines interleukin-1 and tumor necrosis factor-α after corneal transplantation. J Interferon Cytokine Res. 1999;19:661–9. doi: 10.1089/107999099313811. [DOI] [PubMed] [Google Scholar]
- 11.Pennica D, Nedwin GE, Hayflick JS, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312:724–9. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by γ-interferon. Nature. 1985;318:665–7. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- 13.Shirai T, Yamaguchi H, Ito H, Todd CW, Wallace RB. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. Nature. 1985;313:803–6. doi: 10.1038/313803a0. [DOI] [PubMed] [Google Scholar]
- 14.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 15.Bazzoni F, Alejos E, Beutler B. Chimeric tumor necrosis factor receptors with constitutive signaling activity. Proc Natl Acad Sci USA. 1995;92:5376–80. doi: 10.1073/pnas.92.12.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams KA, Mann TS, Lewis M, Coster DJ. The role of resident accessory cells in corneal allograft rejection in the rabbit. Transplantation. 1986;42:667–71. doi: 10.1097/00007890-198612000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Williams KA, Johnstone EW, Guymer RH, Larkin DF, Yates RM, Coster DJ. Corneal transplantation in small animals. In: Green MK, Mandel TE, editors. Experimental transplantation models in small animals. Chur: Harwood Academic Publishers; 1995. pp. 107–32. [Google Scholar]
- 18.Cho BJ, Gross SJ, Pfister DP, Holland EJ. In vivo confocal microscopic analysis of corneal allograft rejection in rabbits. Cornea. 1998;17:417–22. doi: 10.1097/00003226-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 19.McDonnell PJ, Enger C, Stark WJ, Stulting RD. Corneal thickness changes after high-risk penetrating keratoplasty. Collaborative Corneal Transplantation Study Group. Arch Ophthalmol. 1993;111:1374–81. doi: 10.1001/archopht.1993.01090100082032. [DOI] [PubMed] [Google Scholar]
- 20.Fomsgaard A, Worsaae H, Bendtzen K. Detection of tumour necrosis factor from lipopolysaccharide-stimulated human mononuclear cells by enzyme-linked immunosorbent assay and cytotoxicity bioassay. Scand J Immunol. 1988;27:143–7. doi: 10.1111/j.1365-3083.1988.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 21.Dick A, Duncan L, Hale G, Waldmann H, Isaacs J. Neutralizing TNF-α activity modulates T-cell phenotype and function in experimental autoimmune uveoretinitis. J Autoimmun. 1998;11:255–64. doi: 10.1006/jaut.1998.0197. [DOI] [PubMed] [Google Scholar]
- 22.Dallman MJ. Cytokines and transplantation: Th1/Th2 regulation of the immune response to solid organ transplants in the adult. Curr Opin Immunol. 1995;7:632–8. doi: 10.1016/0952-7915(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 23.Bishop GA, Sun J, DeCruz DJ, Rokahr KL, Sedgwick JD, Sheil AG, Gallagher ND, McCaughan GW. Tolerance to rat liver allografts. III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J Immunol. 1996;156:4925–31. [PubMed] [Google Scholar]
- 24.Grant SC, Guy SP, Lamb WR, Brooks NH, Brenchley PE, Hutchinson IV. Expression of cytokine messenger RNA after heart transplantation: relationship with rejection and serum cytokines. Transplantation. 1996;62:910–6. doi: 10.1097/00007890-199610150-00007. [DOI] [PubMed] [Google Scholar]
- 25.Chang DM, Ding YA, Kuo SY, Chang ML, Wei J. Cytokines and cell surface markers in prediction of cardiac allograft rejection. Immunol Invest. 1996;25:13–21. doi: 10.3109/08820139609059287. [DOI] [PubMed] [Google Scholar]
- 26.Azzawi M, Grant SD, Hasleton PS, Yonan N, Campbell CS, Deiraniya AK, Rahman A, Hutchinson IV. TNF alpha mRNA and protein in cardiac transplant biopsies: comparison with serum TNF alpha levels. Cardiovasc Res. 1996;32:551–6. [PubMed] [Google Scholar]
- 27.Coito AJ, Binder J, Brown LF, de Sousa M, Van de Water L, Kupiec Weglinski JW. Anti-TNF-alpha treatment down-regulates the expression of fibronectin and decreases cellular infiltration of cardiac allografts in rats. J Immunol. 1995;154:2949–58. [PubMed] [Google Scholar]
- 28.Abdallah AN, Boiron JM, Attia Y, Cassaigne A, Reiffers J, Iron A. Plasma cytokines in graft vs host disease and complications following bone marrow transplantation. Hematol Cell Ther. 1997;39:27–32. doi: 10.1007/s00282-997-0027-2. [DOI] [PubMed] [Google Scholar]
- 29.Hagglund H, Bostrom L, Remberger M, Ljungman P, Nilsson B, Ringden O. Risk factors for acute graft-versus-host disease in 291 consecutive HLA-identical bone marrow transplant recipients. Bone Marrow Transplant. 1995;16:747–53. [PubMed] [Google Scholar]
- 30.Dorge SE, Roux Lombard P, Dayer JM, Koch KM, Frei U, Lonnemann G. Plasma levels of tumor necrosis factor (TNF) and soluble TNF receptors in kidney transplant recipients. Transplantation. 1994;58:1000–8. doi: 10.1097/00007890-199411150-00005. [DOI] [PubMed] [Google Scholar]
- 31.Noronha IL, Eberlein-Gonska M, Hartley B, Stephens S, Cameron JS, Waldherr R. In situ expression of tumor necrosis factor-alpha, interferon-gamma, and interleukin-2 receptors in renal allograft biopsies. Transplantation. 1992;54:1017–24. doi: 10.1097/00007890-199212000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Imagawa DK, Millis JM, Seu P, et al. The role of tumor necrosis factor in allograft rejection. III. Evidence that anti-TNF antibody therapy prolongs allograft survival in rats with acute rejection. Transplantation. 1991;51:57–62. doi: 10.1097/00007890-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Imagawa DK, Millis JM, Olthoff KM, et al. The role of tumor necrosis factor in allograft rejection. I. Evidence that elevated levels of tumor necrosis factor-α predict rejection following orthotopic liver transplantation. Transplantation. 1990;50:219–25. doi: 10.1097/00007890-199008000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–9. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-α production by tristetraprolin. Science. 1998;281:1001–5. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 36.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 37.Chan CCW, Stark J, George AJT. Analysis of cytokine dynamics in corneal allograft rejection. Proc R Soc Lond B. 1999;266:2217–23. doi: 10.1098/rspb.1999.0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callard R, George AJT, Stark J. Cytokines, chaos and complexity. Immunity. 1999;11:507–13. doi: 10.1016/s1074-7613(00)80125-9. [DOI] [PubMed] [Google Scholar]
- 39.Sher NA, Doughman DJ, Mindrup E, Minaai LA, Foon KA. Macrophage migration inhibition factor activity in the aqueous humor during experimental corneal xenograft and allograft rejection. Am J Ophthalmol. 1976;82:858–65. doi: 10.1016/0002-9394(76)90061-1. [DOI] [PubMed] [Google Scholar]
- 40.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Role of macrophage migratory inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693–6. [PubMed] [Google Scholar]
- 41.DeMeester SR, Rolfe MW, Kunkel SL, Swiderski DL, Lincoln PM, Deeb GM, Strieter RM. The bimodal expression of tumor necrosis factor-α in association with rat lung reimplantation and allograft rejection. J Immunol. 1993;150:2494–505. [PubMed] [Google Scholar]
- 42.Cunningham ET, Stalder A, Sanna PP, Liu SS, Bloom FE, Howes EL, Campbell IL, Margolis TP. Localization of tumor necrosis factor receptor messenger RNA in normal and herpes simplex virus-infected mouse eyes. Invest Ophthalmol Vis Sci. 1997;38:9–15. [PubMed] [Google Scholar]
- 43.Dighiero P, Behar Cohen F, Courtois Y, Goureau O. Expression of inducible nitric oxide synthase in bovine corneal endothelial cells and keratocytes in vitro after lipopolysaccharide and cytokine stimulation. Invest Ophthalmol Vis Sci. 1997;38:2045–52. [PubMed] [Google Scholar]
- 44.Marino MW, Dunn A, Grail D, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada J, Streilein JW, Dana MR. Role of tumor necrosis factor receptors TNFR-I (P55) and TNFR-II (P75) in corneal transplantation. Transplantation. 1999;68:944–9. doi: 10.1097/00007890-199910150-00008. [DOI] [PubMed] [Google Scholar]


