Abstract
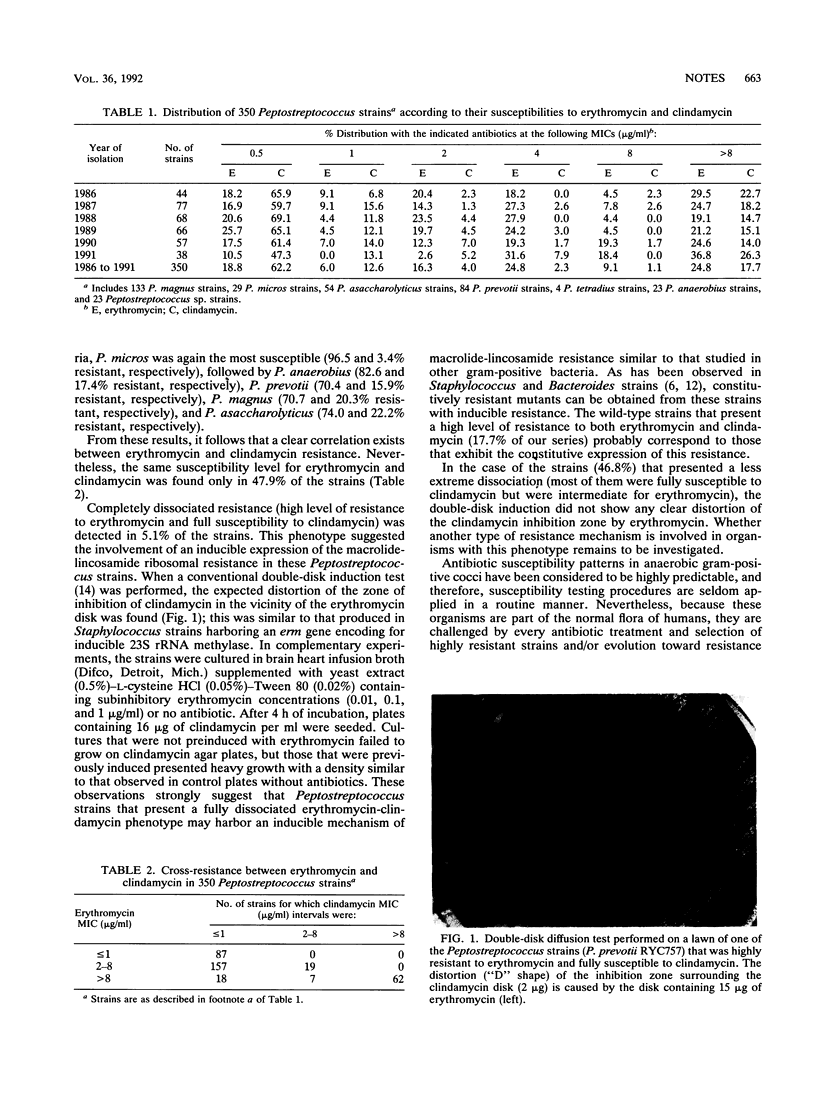
The activities of erythromycin and clindamycin against 350 Peptostreptococcus strains were studied during a 5-year period (1986 to 1991). In 5.1% of the Peptostreptococcus strains, which presented dissociated resistance (clindamycin MIC, less than or equal to 1 microgram/ml; erythromycin MIC, greater than 8 micrograms/ml), evidence of inducible macrolide-lincosamide resistance was shown. A total of 17.7% of the strains presented a constitutive phenotype; the clindamycin and erythromycin MICs for these strains were greater than 8 micrograms/ml.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubreuil L., Devos J., Neut C., Romond C. Susceptibility of anaerobic bacteria from several French hospitals to three major antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):764–766. doi: 10.1128/aac.25.6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G. B., Ayers O. M. Antimicrobial susceptibilities of anaerobic bacteria isolated from female genital tract infections. Antimicrob Agents Chemother. 1985 Mar;27(3):324–331. doi: 10.1128/aac.27.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. The control region for erythromycin resistance: free energy changes related to induction and mutation to constitutive expression. Mol Gen Genet. 1981;182(2):341–348. doi: 10.1007/BF00269681. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Lindmark A., Person I. Susceptibility of anaerobic bacteria to FCE 22101. Antimicrob Agents Chemother. 1987 May;31(5):831–833. doi: 10.1128/aac.31.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm-Smith M. J., Sweet R. L., Hadley W. K. Occurrence of clindamycin-resistant anaerobic bacteria isolated from cultures taken following clindamycin therapy. Antimicrob Agents Chemother. 1986 Jul;30(1):11–14. doi: 10.1128/aac.30.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm-Smith M. J., Sweet R. L. In vitro activity of cefmetazole, cefotetan, amoxicillin-clavulanic acid, and other antimicrobial agents against anaerobic bacteria from endometrial cultures of women with pelvic infections. Antimicrob Agents Chemother. 1987 Sep;31(9):1434–1437. doi: 10.1128/aac.31.9.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig M., Baquero F. Selection of constitutively resistant mutants of inducible clindamycin-resistant Bacteroides vulgatus. Eur J Clin Microbiol Infect Dis. 1989 Aug;8(8):711–715. doi: 10.1007/BF01963757. [DOI] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Susceptibility of anaerobic bacteria to 23 antimicrobial agents. Antimicrob Agents Chemother. 1976 Oct;10(4):736–752. doi: 10.1128/aac.10.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Siddhikol C., Lai C. J., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: requirements for induction. J Bacteriol. 1971 Jun;106(3):835–847. doi: 10.1128/jb.106.3.835-847.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H. M., Finegold S. M. In vitro activity of cefoperazone plus sulbactam compared with that of other antimicrobial agents against anaerobic bacteria. Antimicrob Agents Chemother. 1988 Mar;32(3):403–406. doi: 10.1128/aac.32.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]



