Abstract
We investigated the role of inflammatory cytokines in a mouse model of chronic Pseudomonas aeruginosa infection mimicking diffuse panbronchiolitis (DPB), and determined the effects of clarithromycin therapy on the production of these cytokines. The concentrations of IL-1β, IL-2, IL-4, IL-5, interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) were measured serially in the lungs of mice with experimentally induced chronic respiratory P. aeruginosa infection until 60 days after inoculation. The concentrations of these cytokines during the course of the disease were significantly higher than baseline (before inoculation, P < 0·01 for all cytokines). Clarithromycin significantly inhibited the production of IL-1β and TNF-α in the lung (P < 0·01). The same treatment also reduced the levels of other cytokines, albeit insignificantly. Treatment with anti-TNF-α antibody significantly reduced the number of pulmonary lymphocytes and concentration of IL-1β in the lung (P < 0·01), but did not change the number of viable bacteria. Our findings resemble those detected in bronchoalveolar lavage fluid of patients with DPB and indicate that inflammatory cytokines play an important role in chronic P. aeruginosa lung infection. Our results also show that macrolides modulated the production of these cytokines, ultimately reducing lymphocyte accumulation in the lung. Our data suggest that anti-TNF-α antibody might be a useful new strategy for the treatment of chronic respiratory P. aeruginosa infection.
Keywords: inflammatory cytokines, chronic respiratory infection, Pseudomonas aeruginosa, macrolide antibiotics, anti-TNF-α antibody
INTRODUCTION
Diffuse panbronchiolitis (DPB) is a clinicopathological disease entity characterized by thickening of the walls of respiratory bronchioles with accumulation of lymphocytes, plasma cells, and histiocytes [1]. In addition, bronchus-associated lymphoid tissue (BALT) hyperplasia is occasionally observed more often in DPB than in other respiratory diseases [2]. These findings suggest that lymphocytes are an important cellular component of bronchial inflammation in DPB.
In 1984, Kudoh et al. [3] first reported that low-dose and long-term treatment with erythromycin was effective in chronic lower respiratory tract infections, including DPB. In a series of studies, we previously investigated the mechanisms of chronic inflammation in DPB patients and the effect of macrolide antibiotics [4,5]. These studies showed no clear mechanism for the inflammatory response in DPB and no definite mode of action of macrolide antibiotics. More recently, we reported the presence of high concentrations of inflammatory cytokines, including IL-1β and IL-8 in the bronchoalveolar lavage fluid (BALF) of patients with DPB compared with control normal subjects [6], and that long-term macrolide treatment significantly reduced the level of these cytokines. We also demonstrated that erythromycin inhibits IL-8 production by 1α.25-dihydroxyvitamin D3-stimulated THP-1 cells [7]. These results suggested that inflammatory cytokines are associated not only with the development of chronic respiratory infection but also with the response to macrolides.
We have recently established a new murine model of chronic Pseudomonas aeruginosa respiratory tract infection mimicking DPB, and reported that the total number of lymphocytes in the lung increased significantly after inoculation of the bacteria, and that macrolide antibiotics could alter the initial phase of lymphocyte accumulation [8]. In the present study, we postulated that inflammatory cytokines such as IL-1β, IL-2, IL-4, IL-5, interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) can modulate the accumulation of lymphocytes in the lung and that macrolides can regulate the production of such cytokines. To investigate these issues, we used our newly established murine model of chronic bronchial infection and determined the serial changes in cytokine concentrations in the lung and the effect of macrolide antibiotic on the production of these cytokines.
MATERIALS AND METHODS
Laboratory animals
Male, ddY, 7-week-old, 30–35 g body weight, specific pathogen-free mice were purchased from Shizuoka Agricultural Cooperative Association Laboratory Animals (Shizuoka, Japan). All animals were housed in a pathogen-free environment and received sterile food and water in the Laboratory Animal Centre for Biomedical Science at Nagasaki University. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation at our Institution.
Bacterial strain
Mice were infected with mucoid P. aeruginosa NUS10, a clinical isolate from sputum of patients at Nagasaki University Hospital. The bacteria were stored at −70°C in brain heart infusion broth (BBL Microbiology Systems, Cockeysville, MD) supplemented with 10% (v/v) glycerol and 5% (w/v) skim milk (Yukijirushi Co., Tokyo, Japan) until use.
Experimental model of infection
Disposable sterile plastic cut-down intravenous catheters with 3 Fr. (1 mm) outer diameter (Atom Co., Tokyo, Japan) were used for incubation. The tube was 3·0 mm long with a few slits made at the proximal end to prevent blockage by oral secretions. To prepare the inoculum, P. aeruginosa was cultured on trypticase soy agar plate for 24 h. The bacteria were suspended in saline, harvested by centrifugation (3000 g, 4°C, 10 min), resuspended in sterile saline and adjusted to 1–2 × 109 colony-forming units (CFU)/ml as estimated by turbidimetry. The incubation tube was then immersed in the bacterial saline suspension for 3 days at 37°C. Bacterial counts on these tubes 3 days after incubation just before intubation were 6·19 ± 0·35 (log10 CFU/ml, mean ±s.d., n = 10). To detach bacteria from a tube, the tube suspended in saline was shaken vigorously, and bacterial number in saline was counted and expressed as CFU/ml. The method used for inducing infection has been described previously [8]. Briefly, the incubation tube harbouring the bacteria was attached to the blunted tip of the needle of an intravenous catheter (Angiocath; Becton Dickinson Vascular Access, Sandy, UT). The needle-tube was inserted through the oral cavity, and then advanced through the vocal cords. When the tip of the tube was in the trachea, the needle/catheter was pulled out and the outer sheath was pushed gently to place the precoated tube into the main bronchus. The outer sheath (tube) had been kept for the period of the experiment.
Bacteriological examination
Both lungs were homogenized and cultured separately. Bacterial enumeration was performed by serial dilution on trypticase soy agar in NAC agar plates (BBL).
Clarithromycin treatment
The treatment protocol was similar to that described previously [8]. Treatment commenced 7 days after intubation by oral administration of clarithromycin (10 mg/kg per day), or saline for control mice. The antibiotic was administered once a day for 10 days by gavage in the awake mouse.
Preparation of pulmonary intraparenchymal lymphocytes
Pulmonary intraparenchymal lymphocytes were prepared as described previously [8,9]. Briefly, mice were killed by cervical dislocation. After thoracotomy, the lung vascular bed was flushed by injecting 2–3 ml of chilled physiological saline into the right ventricle. The lungs were then excised and washed in physiological saline. The lungs were teased with a stainless steel mesh and incubated in RPMI 1640 (Gibco BRL, Grand Island, NY) with 5% fetal calf serum (FCS), 100 U/ml penicillin G, 100 μg/ml streptomycin, 10 mm HEPES, 50 μm 2-mercaptoethanol, and 2 mm l-glutamine, containing 20 U/ml collagenase (Sigma Chemical Co., St Louis, MO) and 1·0 μg/ml DNase I (Sigma). A volume of 10 ml was used for each set of lungs. After incubation for 60 min at 37°C with vigorous shaking, the tissue fragments and most dead cells were removed by passing through a 100-μm nylon mesh. After centrifugation, the cell pellet was resuspended in 4 ml of 40% (v/v) Percoll (Pharmacia, Uppsala, Sweden) and layered onto 4 ml of 80% (v/v) Percoll. After centrifugation at 600 g for 20 min at 15°C, the cells at the interface were collected, washed twice and counted using a haemocytometer. Approximately 4 × 104 cells were centrifuged onto a glass slide at 160 g for 2 min using Cytospin 2 (Shandon Instruments, Sewickly, PA) and stained by May–Giemsa staining. At least 300 cells were examined for differentiation of cellular fraction by photo microscopic observation.
Cytokine assays
The concentrations of TNF-α, IL-1β, IFN-γ, IL-2, IL-4 and IL-5 in the aqueous lung extract were assayed by mouse cytokine ELISA test kits (Amersham, Aylesbury, UK). To prepare aqueous lung extracts, the lungs were homogenized in 1·0 ml PBS using a glass tissue homogenizer (Takashima, Tokyo, Japan) for 60 s in an ice-cold water bath. The homogenates were then centrifuged at 2000 g for 60 min at 4°C. The supernatants were collected and used for the assay [10].
Treatment with anti-TNF-α antibody
Mice were treated by i.p. injection of 1 ml of anti-mouse TNF-α antibody (neutralization activity of 1·0 × 105 U/ml; Genzyme, Cambridge, MA). Treatment commenced 7 days after intubation of the precoated tube with mucoid-type P. aeruginosa. Anti-mouse TNF-α antibody was administered once a day for 10 days. Control mice were injected with normal rabbit serum.
Statistical analysis
Data were expressed as mean ± s.d. Differences between groups were examined for statistical significance using the unpaired Student's t-test. P <0·05 denoted the presence of a statistically significant difference.
RESULTS
Serial changes in cytokine concentrations in aqueous lung extract
As shown in Table 1, the levels of IFN-γ, IL-2, IL-4 and IL-5 were below the detection limit at baseline (prior to experimentally induced infection), although the presence of detectable levels of TNF-α and IL-1β was noted at baseline. The concentration of each cytokine significantly increased at day 7 after inoculation compared with the baseline (P < 0·01). High levels were persistently present even at day 60 post-infection. The concentrations of TNF-α and IL-1β in lung extracts were approximately 60–100-fold and 300–360-fold higher than those at baseline, respectively. The peak concentrations of IFN-γ and IL-2 were noted at day 60. There was no reasonable time point for highest concentration of IL-4 and IL-5 (Table 1).
Table 1.
The serial change in the level of cytokines in the aqueous lung extract*
| Days | TNF-α | IL-1β | IFN-γ | IL-2 | IL-4 | IL-5 |
|---|---|---|---|---|---|---|
| Before inoculation | 17·5 ± 5·7 | 6·5 ± 1·7 | ND | ND | ND | ND |
| 7 | 1274·0 ± 157·2** | 1929·7 ± 649·8** | 35·2 ± 19·4** | 35·0 ± 23·6** | 75·2 ± 16·5** | 19·7 ± 4·3** |
| 30 | 1117·0 ± 218·9** | 2382·8 ± 724·1** | 30·2 ± 8·8** | 40·0 ± 22·8** | 80·3 ± 11·9** | 27·4 ± 9·1** |
| 60 | 1393·5 ± 325·3** | 2041·8 ± 547·3** | 120·8 ± 30·1** | 91·2 ± 10·6** | 63·5 ± 13·4** | 23·0 ± 11·2** |
Values expressed as mean ±s.d.
Each group consisted of four animals; ND, not detectable.
P < 0·01 versus before inoculation.
The experiments were reproducible (n = 3).
Effect of clarithromycin on cytokine production in aqueous lung extracts
Treatment with oral clarithromycin reduced cytokine concentrations in aqueous lung extracts, although the effect was significant only for TNF-α and IL-1β (P < 0·01, Table 2). Clarithromycin did not produce a significant fall in the number of bacteria isolated from the lung after 10 days of treatment.
Table 2.
The effect of clarithromycin on the level of cytokines in the aqueous lung extract*
| Cytokine concentration, pg/ml | ||
|---|---|---|
| Cytokines | Treatment with clarithromycin (n = 5) | Control (treatment with saline, n = 5) |
| TNF-α | 276·2 ± 119·6** | 1289·9 ± 276·9 |
| IL-1β | 554·5 ± 157·7** | 1956·0 ± 356·9 |
| IFN-γ | 22·4 ± 7·4 | 33·9 ± 12·4 |
| IL-2 | 28·1 ± 12·4 | 32·7 ± 15·8 |
| IL-4 | 41·2 ± 8·4 | 53·8 ± 24·6 |
| IL-5 | 20·0 ± 7·5 | 22·0 ± 7·1 |
Values expressed as mean ±s.d.
P < 0·01 compared with control.
Effect of anti-TNF-α antibody
Treatment with anti-TNF-α antibody resulted in a significant reduction in the number of pulmonary lymphocytes, but no change was noted in the number of macrophages (Fig. 1). We also examined the effect of anti-TNF-α antibody on IL-10 concentration in aqueous lung extracts by ELISA. The mean concentration of IL-1β significantly decreased following such treatment. However, TNF-α antibody did not influence the number of bacteria isolated from the lungs (Fig. 2).
Fig. 1.
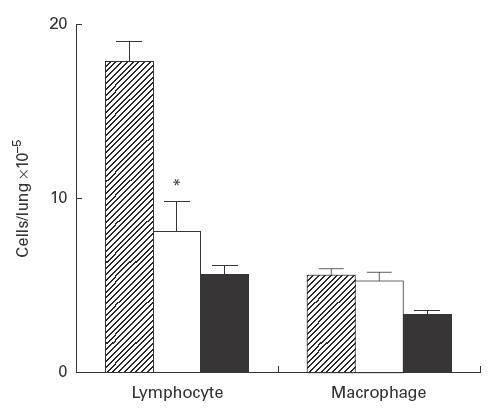
Effects of anti-tumour necrosis factor-alpha (TNF-α) antibody on lymphocyte and macrophage counts in the lung. Pulmonary intraparenchymal lymphocytes and macrophages were examined. The total number of cells was counted, and each population was identified based on morphological analysis of at least 300 cells stained by May–Giemsa staining. Cell numbers in the lung obtained from control (hatched), mice treated with anti-TNF-α MoAb (□) and uninfected (intubated with sterile tubes) mice (▪). Each bar represents the mean ±s.d. of five animals. *P < 0·01 compared with control.
Fig. 2.
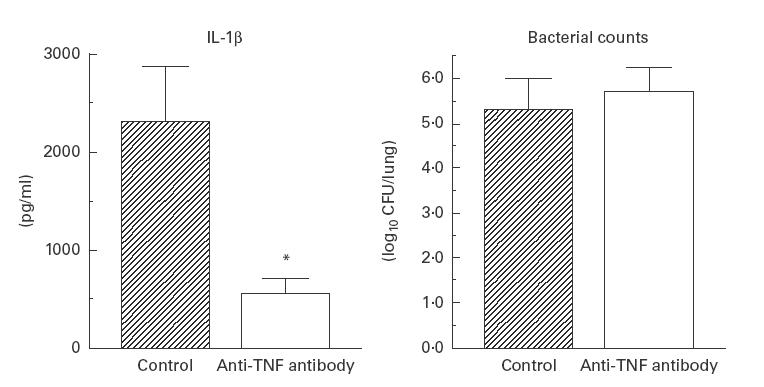
Effects of anti-tumour necrosis factor-alpha (TNF-α) antibody on IL-1β and the number of viable bacteria in the lung. Data are mean ±s.d. of five animals. *P < 0·01 compared with control.
DISCUSSION
In this study, the concentrations of proinflammatory cytokines were measured up to 60 days after induction of respiratory infection in mice to evaluate their effects on the accumulation of lymphocytes in chronic respiratory infection. Our data showed a significant increase in the concentration of all cytokines measured in the present study (Table 1). The significant rise in TNF-α and IL-1β concentrations was in agreement with the clinical efficacy of macrolide antibiotics in patients with DPB reported by Sakito and co-workers [6]. These two cytokines play an important role in chronic infection. The first subset of T helper lymphocytes (Th1 cells) produce various cytokines such as IFN-γ, IL-2, TNF-α and IL-1β, while Th2 cells produce IL-4 and IL- 5. The Th1 response is known to exert a self-limiting, curative response characterized by production of IFN-γ, IL-2 and IL-12, which enhance cell-mediated immunity. In contrast, the Th2 immune response induces a chronic, fatal disease characterized by production of IL-3, IL-4, IL-5, IL-6 and IL-10 cytokines that promote humoral immunity [11]. Previous studies have shown the predominance of the Th2 immune response in patients with cystic fibrosis (CF) [12,13]. Moser et al. reported that the cytokine pattern observed in genetically different strains of mice during P. aeruginosa infection indicated that the Th1 response might be beneficial in CF patients with chronic P. aeruginosa infection [14]. Although there are no reports that suggest the predominance of the Th1 or Th2 immune response in DPB, the present animal studies demonstrated Th1 predominance during the late stage of the disease at day 60. These results suggest differences in the pathoimmune response between DPB and CF.
To examine the effect of clarithromycin, we measured the concentrations of cytokines in aqueous lung extracts. Treatment for 10 days significantly reduced the levels of TNF-α and IL-1β. These effects were in agreement with the clinical efficacy of macrolides in patients with DPB [6]. We have previously reported that clarithromycin treatment can reduce lymphocyte counts to baseline levels. These findings suggested that the macrolide antibiotic may down-regulate cytokine production or secretion in the lung, and ultimately reduce the accumulation of the lymphocytes in the site of the inflammation. Clarithromycin also reduced the mean concentrations of IFN-γ, IL-2, IL-4 and IL-5, albeit insignificantly. Thus, macrolide antibiotics seem to preferentially affect TNF-α and IL-1β relative to IFN-γ, IL-2, IL-4 and IL-5.
TNF-α plays a key role in resistance to several pulmonary infections [15,16], but when produced in excess it can lead to shock, lung damage, fibrosis, and tissue dysfunction [17–20]. Thus, it was of interest to determine the exact role of TNF-α in the response of mice with chronic respiratory P. aeruginosa infection. To examine the role of TNF-α, mice were treated with anti-TNF-α antibody 7 days after inoculation. The antibody resulted in a significant reduction of lymphocytes in the lung but did not affect the number of viable bacteria recovered from the lungs. These results suggest that TNF-α enhances lymphocyte accumulation/function in the lung in mice with chronic respiratory infection and that neutralizing anti-TNF-α antibody improved inflammation of the lung. Because the effects of TNF-α and anti-TNF-α antibody on lymphocytes have not been well characterized, we hypothesized that anti-TNF-α antibody suppresses lymphocyte accumulation by inhibiting the production of IL-1β. The level of IL-1β was significantly decreased after administration of anti-TNF-α antibody, which did not affect bacterial counts in the lung. In this regard, Fong and co-workers [21] reported that anti-TNF-α antibody reduced IL-1β levels during lethal bacteraemia. Thus, it seems that the suppressive effect of anti-TNF-α antibody on lymphocytes may be related to inhibition of IL-1β production in chronic respiratory infection.
Several investigators have recently suggested that anti-inflammatory cytokines may play a role in suppressing P. aeruginosa-induced tissue damage during chronic infection [22,23]. Our results also showed that anti-TNF-α antibody is useful for treatment of chronic respiratory P. aeruginosa infection. However, many investigators reported that TNF-α is required in the protective immune response against several infectious diseases. Thus, the use of anti-TNF treatment for chronic P. aeruginosa infection might be dangerous due to the possibility of infection by other pathogens.
In conclusion, our data show that chronic lung infection is accompanied by significant increases in the local concentrations of several cytokines, including TNF-α, IL-1β, IFN-γ, IL-2, IL-4 and IL-5. We also demonstrate that treatment with clarithromycin significantly reduced the levels of TNF-α and IL-1β in the lung. Treatment with anti-TNF-α antibody significantly attenuated both lymphocyte numbers and the level of IL-1β in the lung. These data suggest that TNF-α is essential for the accumulation of lymphocytes in the lung and that anti-TNF-α antibody may be a useful new strategy for the treatment of chronic pulmonary infections.
Acknowledgments
The authors thank Dr F. G. Issa (Department of Medicine, University of Sydney, Sydney, Australia) for his assistance in editing the manuscript.
REFERENCES
- 1.Homma H, Yamanaka A, Tanimoto S, et al. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest. 1983;83:63–69. doi: 10.1378/chest.83.1.63. [DOI] [PubMed] [Google Scholar]
- 2.Sato A, Chida K, Iwata M, et al. Study of bronchus-associated lymphoid tissue in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1992;146:473–8. doi: 10.1164/ajrccm/146.2.473. [DOI] [PubMed] [Google Scholar]
- 3.Kudoh S, Uetake T, Hagiwara K, et al. Clinical effect of low-dose and long-term erythromycin chemotherapy on diffuse panbronchiolitis. Jpn J Thoracic Dis. 1987;25:632–42. [PubMed] [Google Scholar]
- 4.Kadota J, Sakito O, Kohno S, et al. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1993;147:153–9. doi: 10.1164/ajrccm/147.1.153. [DOI] [PubMed] [Google Scholar]
- 5.Mukae H, Kadota J, Kohno S, et al. Increase of activated CD8+ cells in bronchoalveolar lavage fluid in patients with diffuse panbronchiolitis. Am J Respir Crit Care Med. 1995;152:613–8. doi: 10.1164/ajrccm.152.2.7633715. [DOI] [PubMed] [Google Scholar]
- 6.Sakito O, Kadota J, Kohno S, et al. Interleukin-1 beta, tumor necrosis factor-α and interleukin-8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: potential mechanism of macrolide therapy. Respiration. 1996;63:42–48. doi: 10.1159/000196514. [DOI] [PubMed] [Google Scholar]
- 7.Fujii T, Kadota J, Morikawa T, et al. Inhibitory effect of erythromycin on interleukin-8 production by 1alpha, 25-dihydroxyvitamin D3-stimulated THP-1 cells. Antimicrob Agents Chemother. 1996;40:1548–51. doi: 10.1128/aac.40.6.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagihara K, Tomono K, Sawal T, et al. Effect of clarithromycin on lymphocytes in chronic respiratory Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 1997;155:337–42. doi: 10.1164/ajrccm.155.1.9001333. [DOI] [PubMed] [Google Scholar]
- 9.Abraham E, Fretas AA, Coutino AA. Purification and characterization of intraparenchymal lung lymphocytes. J Immunol. 1990;144:2117–22. [PubMed] [Google Scholar]
- 10.Konno S, Asano K, Kurokawa M, et al. Antiasthmatic activity of a macrolide antibiotic, Roxythromycin: analysis of possible mechanisms in vitro and in vivo. Respiration. 1994;105:308–16. doi: 10.1159/000236773. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 12.Koller DY, Gotz M, Elcher I, et al. Eosinophilic activation in cystic fibrosis. Thorax. 1994;49:496–9. doi: 10.1136/thx.49.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen M, Hougen HP, Cryz SJ, et al. Vaccination promotes Th1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am J Respir Crit Care Med. 1995;152:1337–46. doi: 10.1164/ajrccm.152.4.7551392. [DOI] [PubMed] [Google Scholar]
- 14.Moser C, Hougen HP, Song Z, et al. Early immune response in susceptible and resistant mice strains with chronic Pseudomonas aeruginosa lung infection determines the type of T-helper cell response. APMIS. 1999;107:1093–100. doi: 10.1111/j.1699-0463.1999.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 15.Kolls JK, Beck JM, Nelson S, et al. Alveolar macrophage release of tumor necrosis factor during murine Pneumocystis carinii pneumonia. Am J Respir Cell Mol Biol. 1993;8:370–6. doi: 10.1165/ajrcmb/8.4.370. [DOI] [PubMed] [Google Scholar]
- 16.Gosselin D, Desanctis J, Boule M, et al., editors. Role of tumor necrosis factor α in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995:3272–8. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolls JK, Nelson S, Summer WR. Recombinant cytokines and pulmonary host defense. Am J Med Sci. 1993;306:330–50. doi: 10.1097/00000441-199311000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Krongorg G, Hansen B, Svenson M, et al. Cytokines in sputum and serum from patients with cystic fibrosis and chronic Pseudomonas aeruginosa infection as markers of destructive inflammation in the lungs. Pediatr Pulmonol. 1993;15:292–7. doi: 10.1002/ppul.1950150506. [DOI] [PubMed] [Google Scholar]
- 19.Piguet PF, Collart MA, Grau GE, et al. Requirement of tumor necrosis factor-α for development of silica-induced pulmonary fibrosis. Nature (London) 1990;344:245–7. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- 20.Tracey KJ, Cerami A. Tumor necrosis factor-α: a pleiotropic cytokine and therapeutic target. Ann Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 21.Fong Y, Tracey KJ, Moldawer LL, et al. Antibodies to cachectin/tumor necrosis factor-α reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1990;170:1627–33. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonfield TL, Panuska JR, Konstan MW, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Hanes M, Chrisp CE, et al. Microbial pathogenesis in cystic fibrosis: pulmonary clearance of mucoid Pseudomonas aeruginosa and inflammation in a mouse model of repeated respiratory challenge. Infect Immun. 1998;66:280–8. doi: 10.1128/iai.66.1.280-288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


