Abstract
Apoptosis followed by macrophage phagocytosis is the principal mechanism by which neutrophil granulocytes (PMN) are removed from the site of inflammation. To investigate whether Streptococcus pneumoniae causes apoptosis of PMN, we exposed PMN to viable and heat-killed pneumococci and purified pneumococcal cell walls (PCW). The occurrence of PMN cell death was quantified by flow cytometry using annexin V/propidium iodide labelling of the cells. Intracellular histone-associated DNA fragments were quantified by ELISA. The presence of apoptosis was confirmed by in situ tailing. Exposure of PMN to viable pneumococci caused necrosis of the cells. The pneumococcal cytotoxin pneumolysin, the bacterial production of hydrogen peroxide, and PCW contributed to necrosis. Heat-killed pneumococci accelerated the process of apoptosis observed in cultivated non-stimulated PMN in vitro. These results demonstrated that pneumococci induce PMN cell death. Depending on the intensity of the stimulus, PMN necrosis and apoptosis were observed.
Keywords: neutrophil cytology, PMN, cell death, pneumococcal cell walls, pneumolysin, hydrogen peroxide
INTRODUCTION
Neutrophils (PMN) play a principal role in host defence against bacterial infection. During the onset of inflammation, mononuclear cells and PMN invade the tissue by transmigration through the layer of activated endothelial cells. One major function of PMN consists of phagocytosis of bacteria. After a short life span PMN are removed from the site of inflammation by macrophage engulfment after undergoing apoptosis [1–3]. The programme of apoptosis is constitutive and regulated by microenvironmental signals [4,5]. The process of transmigration itself prolongs the survival time of the granulocytes. In this process cross-linking of β2-integrins on the surface of PMN seems to play a major role [5]. Once PMN have reached the site of inflammation, the cells are exposed to a variety of cytokines. Granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), adenosine polyphosphates, IL-2, IL-4, IL-6, IL-8, IL-15, platelet-activating factor (PAF), and the complement component C5a lead to prolonged survival of PMN [6–13]. The interaction of PMN with monocytes and platelets delays apoptotic cell death [14,15]. Glucocorticoids prolong PMN survival [16,17]. Tumour necrosis factor-alpha (TNF-α) and IL-10 are host mediators inducing PMN apoptosis [18–20]. During anti-bacterial host defence, bacteria and bacterial products act on PMN function and life span. In vitro stimulation of PMN with the Gram-negative organisms Escherichia coli and Haemophilus somnus [21–23] supported the hypothesis that PMN were cleared from the site of inflammation by apoptosis in bacterial infection. PMN underwent apoptosis after contact with these Gram-negative bacteria. However, although stimulation with low-dose E. coli resulted in apoptosis, high doses induced necrosis of the PMN [24].
Streptococcus pneumoniae is the most frequent bacterial pathogen causing otitis media, pneumonia and meningitis in humans. Three distinct pneumococcal products are released during bacterial growth which have been shown to damage host cells: the cytotoxin pneumolysin [25], hydrogen peroxide [26,27], and pneumococcal cell wall components [28]. In this study we evaluated the influence of viable and heat-killed pneumococci, and of cytotoxic pneumococcal products on the regulation of PMN survival.
MATERIALS AND METHODS
Preparation of PMN
PMN were isolated from buffy coats derived from healthy human donors using plasma Percoll gradients (Pharmacia Biotech, Freiburg, Germany) and were cultured in Dulbecco's minimal essential medium (DMEM) with 10% fetal calf serum (FCS). Stimulation was performed at 37°C immediately after isolation of the cells. At least three distinct preparations were used for the experiments. Representative results were shown in the Figures.
Pneumococcal strain
An encapsulated virulent pneumococcal strain D39 (NCTC 7466) and its unencapsulated avirulent variant (R6x [29]) were used in this study. For purification of pneumococcal cell walls (PCW) the unencapsulated strain was used. A pneumolysin-deficient knock-out mutant of the strain D39 was generated by insertion duplication mutagenesis of the pneumolysin gene using the plasmid pJDC9 (D39ply::pJDC9) [30]. Mutants were screened on antibiotic selection media (LB-agar, 5% sheep blood, 1 μg/ml erythromycin). Correct insertion was confirmed by polymerase chain reaction (PCR) and sequencing. The deficiency of pneumolysin production was checked by haemolysis assay as described previously [31].
The bacteria were grown in Todd–Hewitt broth to mid-logarithmic phase, harvested by centrifugation, washed in PBS, and heat-killed by incubation at 60°C for 20 min. Bacteria were resuspended in cell culture medium to a final concentration equalling 107 colony-forming units (CFU)/ml.
Preparation of PCW
The bacteria were washed in 50 mm Tris buffer pH 7·0 and added to preheated 5% SDS solution and boiled for 15 min. Bacterial cell walls were obtained from the supernatant by centrifugation at 17 000 g, 10 min. The pellets were washed twice in 1 m NaCl and three times in distilled water to remove remaining SDS. The pellet containing larger bacterial cell fragments were broken mechanically by a glass bead stirrer. The supernatant was spun down at 700 g and the cell-free supernatant was spun down again at 38 000 g for 15 min to obtain bacterial fragments. The pellet was resuspended in 100 mm Tris buffer pH 7·5 containing NaN3 0·05%, MgSO4 20 mm, DNase 10 μg/ml, RNase 50 μg/ml, and incubated at 37°C for 2 h. Then, trypsin was added to a final concentration of 50 μg/ml and CaCl2 to a concentration of 10 mm. The solution was digested overnight at 37°C. SDS was added to a final concentration of 1% and incubated at 60°C for 15 min. Pneumococcal cell walls were pelleted at 38 000 g and washed four times with distilled water until SDS had been completely removed. The pellet consisting of PCW was lyophilized and resuspended to a final concentration of 50 mg/ml in distilled water. PCW were used at a final concentration of 50–200 μg/ml for stimulation experiments.
Analysis of PMN by flow cytometry for apoptosis and necrosis
For identification of apoptosis and necrosis of the PMN the binding of annexin V and the uptake of propidium iodide were measured by flow cytometry [32] (FACSCalibur; Becton Dickinson, Heidelberg, Germany) using the apoptosis detection kit TACS annexin V–FITC (R&D Systems, Wiesbaden, Germany) according to the manufacturer's instructions. Flow cytometric analysis was performed using the Cell-Quest software program (Becton Dickinson). Logarithmic fluorescence intensity of annexin V–FITC was plotted versus the fluorescence intensity of propidium iodide (PI) in a dot plot. Data from 10 000 PMN were analysed for each plot. The experiments were performed three times, and results of a representative experiment are shown.
ELISA detection of histone-associated DNA fragments
For measuring intracellular histone-associated DNA fragments of PMN an ELISA was used according to the manufacturer's instructions (Cell Death Detection ELISAPLUS; Roche Molecular Biochemicals, Mannheim, Germany). PMN (10 000) were obtained from the culture and pelleted. The cells were lysed and the supernatant containing the nuclear derived histone-associated DNA fragments was used for ELISA. The optical density (OD)405nm gave the degree of DNA fragmentation.
In situ tailing
Smears of PMN after 24 h in vitro culture were used for staining apoptotic nuclei by in situ tailing. Cells were fixed by ethanol, rehydrated, and treated for 15 min at 37°C with 50 μg/ml proteinase K (Sigma, Deisenhofen, Germany). The preparations were incubated for 1 h at 37°C in a reaction mixture containing 10 μl of 5× tailing buffer, 1 μl digoxigenin (DIG) DNA labelling mix, 2 μl cobalt chloride, 12 U terminal transferase and the necessary amount of distilled water to give a volume of 50 μl. After washing, the preparations were incubated with 10% FCS for 15 min at room temperature and then washed again. A solution of alkaline phosphatase-labelled anti-DIG antibody in 10% FCS was placed on the sections for 60 min at 37°C. The colour reaction was developed with 4-nitroblue-tetrazolium-chloride/5-bromine-4-chloride-3-indolyl-phosphate (NBT/BCIP). The preparations were counterstained with nuclear fast red–aluminium hydroxide. All reagents were purchased from Roche Molecular Biochemicals.
RESULTS
After preparation of PMN from buffy coats, the cells were cultured in vitro. Before adding the bacterial stimuli, fresh isolated PMN were used for annexin V/propidium iodide staining and subsequent FACS analysis to check the viability of the cells. Ninety percent of PMN were viable, only a small population showed signs of necrosis, and few cells were labelled with annexin V (Fig. 1a). After 19 h of in vitro culture the PMN were stained for cell viability. In unstimulated control cultures the quantity of annexin V+ cells increased (14% versus 1% at the time of preparation), indicating that apoptosis had been induced. Only a small population became necrotic, as revealed by propidium iodide staining (12%) (Fig. 1b).
Fig. 1.
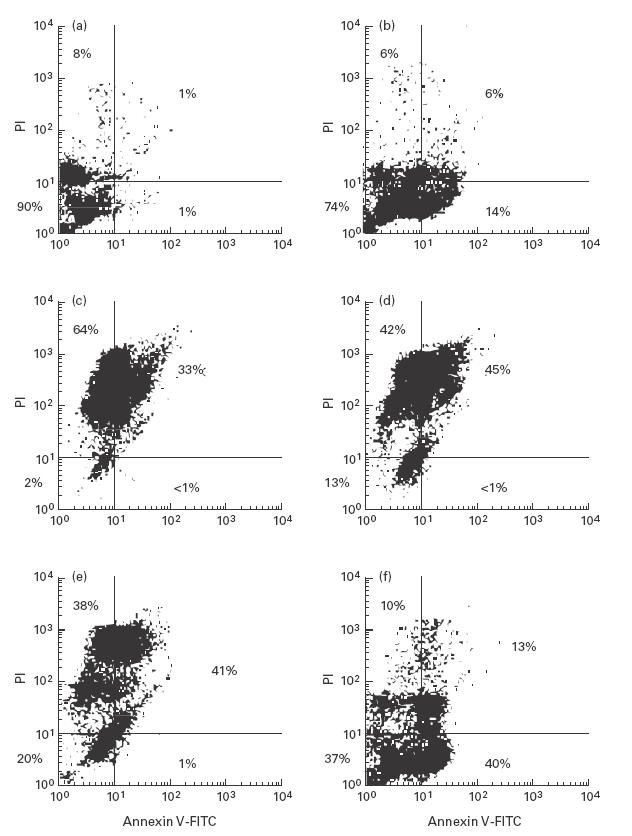
FACS analysis of PMN after labelling the cells with annexin V–FITC and propidium iodide (PI). Viable cells were negative for annexin V–FITC and PI (lower left quadrant). Annexin V labelling indicated apoptosis (lower right quadrant). Cells which were stained with PI were necrotic (upper quadrants) [32]. Unstimulated freshly isolated PMN (a) and PMN after 19 h in vitro culture: unstimulated controls (b), after addition of 107 viable pneumococci (D39)/ml (c), 107 viable pneumolysin-deficient pneumococci (D39ply::pJDC9)/ml (d), 107 viable pneumolysin-deficient pneumococci (D39ply::pJDC9)/ml + catalase (2000 EU/ml) (e), 107 heat-killed pneumococci (D39)/ml (f).
Experiments with encapsulated strain D39 revealed comparable results to those with the unencapsulated strain R6x. Purified cell walls were prepared from the strain R6x. Therefore, the results obtained from stimulation experiments using the virulent strain D39 are shown. Viable pneumococci at 107 CFU/ml predominantly induced PMN necrosis, as revealed by propidium iodide uptake (Fig. 1c). To investigate the influence of the major cytotoxic products of the pneumococcus—pneumolysin, hydrogen peroxide, and PCW—the experiments were performed with a pneumolysin-negative pneumococcal mutant (D39ply::pJDC9) in the presence and absence of catalase and with PCW at concentrations ranging from 50 to 200 μg/ml. Viable bacteria of the pneumolysin-negative mutant induced necrosis of PMN (Fig. 1d). However, a slight reduction in cytotoxicity was observed (13% viable cells versus 2% in cultures stimulated with the viable D39). The experiment with the pneumolysin-negative mutant was performed in the presence of catalase (2000 EU/ml) to investigate whether the production of hydrogen peroxide by the pneumococcus was the mechanism by which the mutant caused necrosis of PMN. In this experiment, the rate of viable PMN increased up to 20% (Fig. 1e). These experiments demonstrated that pneumolysin as well as hydrogen peroxide contributed to the necrosis of PMN in the experiments with viable pneumococci. Pneumococci undergo autolysis during growth. During autolysis PCW are released into the surrounding bacteria. Therefore, in another experiment, purified PCW were added to the PMN culture at concentrations up to 200 μg/ml. The experiments resulted in poor reproducibility of the results. Signs of enhanced necrosis were observed, and the rate of apoptotic PMN was comparable to that of unstimulated controls. In some experiments, especially in those with a background of necrotic PMN in the primary preparation, the results did not differ from those of unstimulated controls.
For a second set of experiments, heat-killed pneumococci were used at a concentration equalling 107 CFU/ml. Nineteen hours after adding the inactivated bacteria to the PMN culture, 40% of the PMN were labelled with annexin V–FITC and showed no staining with propidium iodide. Therefore, an early phase of apoptosis was observed (Fig. 1f).
The induction of PMN apoptosis by heat-killed pneumococci was confirmed by two additional methods. After 4 h, the concentration of histone-associated DNA fragments (HA-DNA) in the cellular fraction was measured. The HA-DNA in the cellular fraction should be elevated if apoptosis has induced. Heat-inactivated pneumococci used at a concentration equalling 107 CFU/ml led to a significant increase of HA-DNA compared with controls. PCW (50 μg/ml) decelerated the increase, indicating a lower quantity of apoptotic cells (Fig. 2). The induction of PMN apoptosis by heat-killed pneumococci was visualized by in situ tailing. Twenty-four hours after pneumococci had been added to the culture medium almost all PMN were stained positively by in situ tailing, whereas only few PMN of control or PCW-stimulated cultures showed nuclei marked by this method (Fig. 3).
Fig. 2.
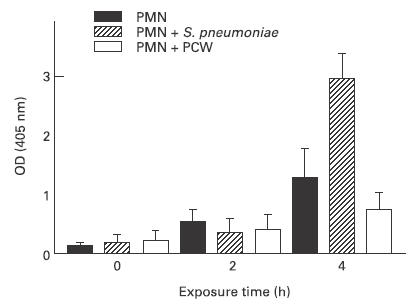
Apoptosis was determined by ELISA measuring the concentration of cellular histone-associated DNA fragments. The concentrations were determined 0 h, 2 h, and 4 h after the addition of heat-killed pneumococci (equalling 107 colony-forming units (CFU)/ml) or pneumococcal cell walls (PCW; 50 μg/ml) to the in vitro culture of PMN. OD, Optical density.
Fig. 3.
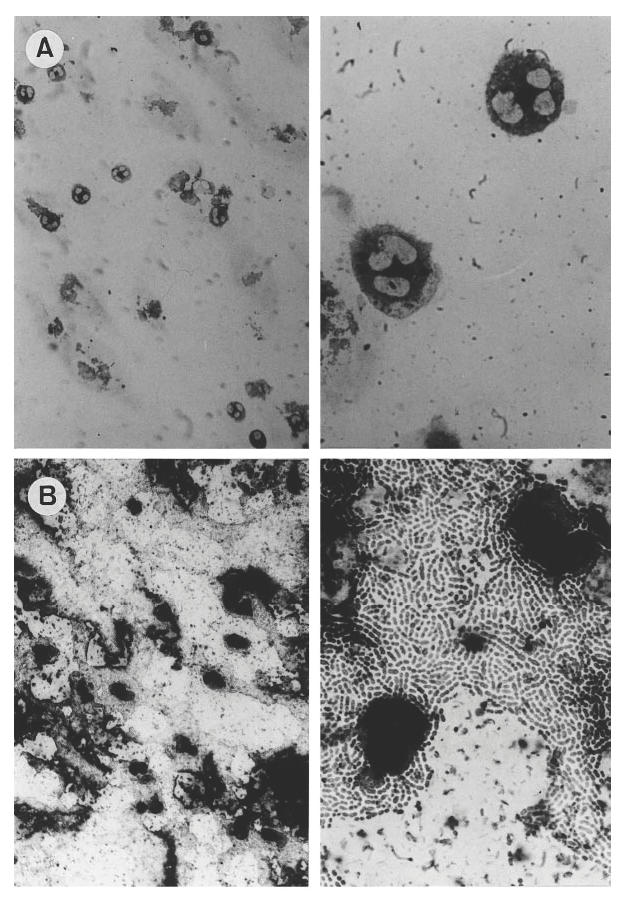
Detection of apoptotic nuclei (dark staining) of PMN by in situ tailing. The tailing reaction was performed 24 h after PMN had been isolated. Controls rarely showed apoptosis (A), whereas in cultures stimulated with heat-killed pneumococci a high rate of PMN apoptosis was observed (B).
DISCUSSION
This study demonstrates that S. pneumoniae is capable of inducing cell death in PMN. Viable pneumococci undergo autolysis during growth and treatment with anti-bacterial agents which primarily affect cell wall synthesis [33,34]. During bacterial lysis not only cell wall components are released into the surrounding but also the cytotoxin pneumolysin [35]. Hydrogen peroxide has been identified as a product of pneumococcal metabolism. Hydrogen peroxide and pneumolysin are toxic to eukaryotic cells [26,27]. After heat inactivation pneumococci do not produce hydrogen peroxide and the bacteria do not undergo further autolysis. Therefore, neither pneumolysin nor cell wall components are released. The bacteria act on eukaryotic cells by the components present at the cell surface.
In this study, PMN were exposed to metabolically active pneumococci, pneumolysin-negative pneumococcal mutants, the latter in the presence of hydrogen peroxide-neutralizing activity of catalase, heat-killed pneumococci, and PCW. The results of the study demonstrate differences in the cytotoxic capacity of the stimuli. Whereas viable wild-type pneumococci cause necrosis of most of the PMN, knocking out the pneumolysin gene and neutralization of hydrogen peroxide attenuate the cytotoxic potential of pneumococci. Heat-killed pneumococci caused PMN apoptosis instead of necrosis. In the experiments with heat-killed pneumococci, neither pneumolysin nor hydrogen peroxide, nor the release of PCW could play a significant role in the interaction with PMN. Therefore, heat-killed pneumococci are a less potent stimulus with reduced cytotoxic potential. Probably, the switch between apoptosis and necrosis primarily depends on the intensity of the stimulus. Similarly, in stimulation experiments of PMN with E. coli low concentrations of viable bacteria caused apoptosis, whereas high concentrations induced necrosis [24].
In vivo, PMN are exposed to both toxic products of the bacterial metabolism and bacterial components. Which of the tested bacterial stimuli displayed best the situation in vivo remains speculative. In vivo, apoptosis has been identified as the major form of death by which leucocytes terminate inflammation. Up to 40% of apoptotic PMN have been reported in the cerebrospinal fluid of patients suffering from bacterial meningitis [2]. In an animal model of pneumococcal meningitis the rate of apoptotic PMN peaked 8 h after the initiation of antibiotic treatment with β-lactam antibiotics [2]. These experiments suggest that killed pneumococci and the release of pneumococcal products trigger the induction of PMN apoptosis in vivo.
In this study, we could not clarify the role of PCW in necrosis and apoptosis induction in vitro. According to present knowledge about the pneumococcus, the remaining cytotoxic potential of the pneumolysin-negative pneumococcus in the presence of catalase should be due to PCW released during autolysis of the cells. Therefore, we performed stimulation experiments with purified PCW. However, these experiments did not give clear results. By flow cytometry, necrosis and apoptosis were observed. The rate of apoptosis was comparable to the rate of apoptosis induced during in vitro culture without any further stimulus, a well-described phenomenon [36]. The additional necrosis could be interpreted as being induced by PCW. In stimulation experiments with PCW, reduced concentrations of cellular histone-associated DNA fragments were measured, revealing either reduced spontaneously occurring apoptosis or induction of necrosis. The latter in conjunction with the results described above appears the most likely interpretation.
In conclusion, pneumococcal components including pneumolysin, hydrogen peroxide, and cell wall components initiate inflammation and oppress host defence. The induction of necrosis of PMN by viable pneumococci supports the spreading of bacterial infection. The induction of PMN apoptosis later in the course of disease, induced by metabolically inactive bacteria and probably followed by ingestion of total bacteria, is teleologically beneficial for resolving inflammation.
Acknowledgments
The authors thank Bernd Nietzgen, Gabriele Zysk and Adriana Soto for excellent technical assistance.
REFERENCES
- 1.Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–7. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- 2.Nau R, Zettl U, Gerber J, et al. Granulocytes in the subarachnoid space of humans and rabbits with bacterial meningitis undergo apoptosis and are eliminated by macrophages. Acta Neuropathol. 1998;96:472–80. doi: 10.1007/s004010050921. [DOI] [PubMed] [Google Scholar]
- 3.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–75. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sendo F, Tsuchida H, Takeda Y, Gon S, Takei H, Kato T, Hachiya O, Watanabe H. Regulation of neutrophil apoptosis—its biological significance in inflammation and the immune response. Hum Cell. 1996;9:215–22. [PubMed] [Google Scholar]
- 5.Watson RW, Rotstein OD, Nathens AB, Parodo J, Marshall JC. Neutrophil apoptosis is modulated by endothelial transmigration and adhesion molecule engagement. J Immunol. 1997;158:945–53. [PubMed] [Google Scholar]
- 6.Biffl WL, Moore EE, Moore FA, Barnett CCJ. Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J Trauma. 1996;40:575–8. doi: 10.1097/00005373-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Cox G, Gauldie J, Jordana M. Bronchial epithelial cell-derived cytokines (G-CSF and GM-CSF) promote the survival of peripheral blood neutrophils in vitro. Am J Respir Cell Mol Biol. 1992;7:507–13. doi: 10.1165/ajrcmb/7.5.507. [DOI] [PubMed] [Google Scholar]
- 8.Cox G. IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am J Physiol. 1996;271:L566–71. doi: 10.1152/ajplung.1996.271.4.L566. [DOI] [PubMed] [Google Scholar]
- 9.Gasmi L, McLennan AG, Edwards SW. The diadenosine polyphosphates Ap3A and Ap4A and adenosine triphosphate interact with granulocyte-macrophage colony-stimulating factor to delay neutrophil apoptosis: implications for neutrophil platelet interactions during inflammation. Blood. 1996;87:3442–9. [PubMed] [Google Scholar]
- 10.Girard D, Paquet ME, Paquin R, Beaulieu AD. Differential effects of interleukin-15 (IL-15) and IL-2 on human neutrophils: modulation of phagocytosis, cytoskeleton rearrangement, gene expression, and apoptosis by IL-15. Blood. 1996;88:3176–84. [PubMed] [Google Scholar]
- 11.Girard D, Paquin R, Beaulieu AD. Responsiveness of human neutrophils to interleukin-4: induction of cytoskeletal rearrangements, de novo protein synthesis and delay of apoptosis. Biochem J. 1997;325:147–53. doi: 10.1042/bj3250147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kettritz R, Gaido ML, Haller H, Luft FC, Jennette CJ, Falk RJ. Interleukin-8 delays spontaneous and tumor necrosis factor-alpha-mediated apoptosis of human neutrophils. Kidney Int. 1998;53:84–91. doi: 10.1046/j.1523-1755.1998.00741.x. [DOI] [PubMed] [Google Scholar]
- 13.Leuenroth S, Lee C, Grutkoski P, Keeping H, Simms HH. Interleukin-8-induced suppression of polymorphonuclear leukocyte apoptosis is mediated by suppressing CD95 (Fas/Apo-1) Fas-1 interactions. Surgery. 1998;124:409–17. [PubMed] [Google Scholar]
- 14.Andonegui G, Trevani AS, López DH, Raiden S, Giordano M, Geffner JR. Inhibition of human neutrophil apoptosis by platelets. J Immunol. 1997;158:3372–7. [PubMed] [Google Scholar]
- 15.Lee A, Haslett C. Human monocyte-conditioned medium inhibits neutrophil apoptosis in vitro. Biochem Soc Trans. 1994;22:254S. doi: 10.1042/bst022254s. [DOI] [PubMed] [Google Scholar]
- 16.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154:4719–25. [PubMed] [Google Scholar]
- 17.Kato T, Takeda Y, Nakada T, Sendo F. Inhibition by dexamethasone of human neutrophil apoptosis in vitro. Nat Immun. 1995;14:198–208. [PubMed] [Google Scholar]
- 18.Gon S, Gatanaga T, Sendo F. Involvement of two types of TNF receptor in TNF-alpha induced neutrophil apoptosis. Microbiol Immunol. 1996;40:463–5. doi: 10.1111/j.1348-0421.1996.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 19.Takeda Y, Watanabe H, Yonehara S, Yamashita T, Saito S, Sendo F. Rapid acceleration of neutrophil apoptosis by tumor necrosis factor-alpha. Int Immunol. 1993;5:691–4. doi: 10.1093/intimm/5.6.691. [DOI] [PubMed] [Google Scholar]
- 20.Cox G. IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am J Physiol. 1996;271:L566–71. doi: 10.1152/ajplung.1996.271.4.L566. [DOI] [PubMed] [Google Scholar]
- 21.William R, Watson G, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–92. [PubMed] [Google Scholar]
- 22.Watson RW, Redmond HP, Wang JH, Bouchier-Hayes D. Bacterial ingestion, tumor necrosis factor-alpha, and heat induce programmed cell death in activated neutrophils. Shock. 1996;5:47–51. doi: 10.1097/00024382-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Yang YF, Sylte MJ, Czuprynski MJ. Apoptosis: a possible tactic of Haemophilus somnus for evasion of killing by bovine neutrophils? Microb Pathog. 1998;24:351–9. doi: 10.1006/mpat.1998.0205. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda T, Saito H, Inoue T, et al. Ratio of bacteria to polymorphonuclear neutrophils (PMNs) determines PMN fate. Shock. 1999;12:365–72. [PubMed] [Google Scholar]
- 25.Rubins JB, Janoff EN. Pneumolysin: a multifunctional pneumococcal virulence factor. J Lab Clin Med. 1998;131:21–27. doi: 10.1016/s0022-2143(98)90073-7. [DOI] [PubMed] [Google Scholar]
- 26.Duane PG, Rubins JB, Weisel HR, Janoff EN. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect Immun. 1993;61:4392–7. doi: 10.1128/iai.61.10.4392-4397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirst RA, Sikand KS, Rutman A, Mitchell TJ, Andrew PW, O'callaghan C. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect Immun. 2000;68:1557–62. doi: 10.1128/iai.68.3.1557-1562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geelen S, Bhattacharyya C, Tuomanen E. The cell wall mediates pneumococcal attachment to and cytopathology in human endothelial cells. Infect Immun. 1993;61:1538–43. doi: 10.1128/iai.61.4.1538-1543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiraby JG, Fox MS. Marker discrimination in transformation and mutation of pneumococcus. Proc Natl Acad Sci USA. 1973;70:3541–5. doi: 10.1073/pnas.70.12.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JD, Morrison DA. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene. 1988;64:155–64. doi: 10.1016/0378-1119(88)90489-1. [DOI] [PubMed] [Google Scholar]
- 31.Benton A, Paton JC, Briles DE. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect Immun. 1997;65:1237–44. doi: 10.1128/iai.65.4.1237-1244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermes I, Haanen C, Steffens-Nakken H, Reutlingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 33.Fischer H, Tomasz A. Production and release of peptidoglycan and wall teichoic acid polymers in pneumococci treated with beta-lactam antibiotics. J Bacteriol. 1984;157:507–13. doi: 10.1128/jb.157.2.507-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuertz K, Schmidt H, Eiffert H, Schwartz P, Mäder M, Nau R. Differential release of lipoteichoic and teichoic acids from Streptococcus pneumoniae as a result of exposure to beta-lactam antibiotics, rifamycins, trovafloxacin, and quinupristin-dalfopristin. Antimicrob Agents Chemother. 1998;42:277–81. doi: 10.1128/aac.42.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinfort C, Wilson R, Mitchell T, et al. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun. 1989;57:2006–13. doi: 10.1128/iai.57.7.2006-2013.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leblebicioglu B, Walters J. Alkaline conditions accelerate polymorphonuclear leukocyte apoptosis in vitro. Infect Immun. 1999;67:2019–21. doi: 10.1128/iai.67.4.2019-2021.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


