Abstract
Antigenic polymorphism and HLA restriction may limit the immunogenicity of a subunit vaccine against liver-stage Plasmodium falciparum. We examined 59 clinical isolates and five laboratory clones of P. falciparum for polymorphism in the N- and C-terminal regions of LSA-1, evaluated binding of the corresponding peptides to selected HLA class I alleles, and measured IFN-γ responses in residents of a malaria-endemic area of Papua New Guinea where HLA-A*1101, -24, -B13, and -B40 are the most common class I alleles. LSA-1 polymorphism was limited to a single non-synonymous mutation encoding serine (S), proline (P), or threonine (T) at amino acid 85. Nine-mer 84–92 peptides with S, T, or P at the primary anchor position bound differentially to HLA-A11, -A2, and -B7. IFN-γ ELISPOT responses increased with age in malaria-exposed subjects: 14–16% and 30–36% of 2–5- and 6–54-year-olds, respectively, had ≥10 IFN-γ-secreting cells/106 peripheral blood mononuclear cells when stimulated with at least one peptide variant (P < 0·05). IFN-γ responses to all three peptides were also greater for older than younger individuals. No children <3 years old had lymphocytes that responded to all three 84–92 peptides, whereas 45% of adults (mean age 48 years) had aggregated IFN-γ responses. These data support the notion that age-related cumulative exposure to P. falciparum increases the frequency of IFN-γ responses to polymorphic epitopes of liver-stage antigens such as LSA-1.
Keywords: malaria, HLA, LSA-1, polymorphism, cytokine
INTRODUCTION
Falciparum malaria is a leading cause of morbidity and death in the tropics, especially among infants and other individuals who have not been repeatedly exposed to the parasite. Malaria infection is initiated when sporozoites are deposited in the bloodstream by anopheline mosquitoes. Within 30 min of inoculation, sporozoites invade hepatocytes where they subsequently undergo asexual reproduction. Liver-stage infection is followed in 1–2 weeks by the emergence of blood-stage parasites that may cause fever and other symptoms. Efforts to develop a vaccine against Plasmodium falciparum have been directed at enhancing resistance and immune responses to both pre-erythrocytic (sporozoite and liver-stage) and blood-stage parasites [1].
Study of the immune basis of resistance against liver-stage malaria in humans is difficult because there is no direct means of assessing parasite elimination and local immunity in this organ. Experimental work using murine malaria models indicates that vaccination with irradiated sporozoites, recombinant proteins, or plasmid DNA elicits cytotoxic T lymphocyte (CTL) and CD4 and/or CD8 T cell IFN-γ responses to peptide epitopes of pre-erythrocytic antigens. These antigen-specific responses are thought to contribute to resistance against liver-stage infection [2]. Analogous T cell responses develop in residents of endemic areas who are repeatedly infected with the P. falciparum[3,4] and immunologically naive volunteers in whom complete but transient resistance can be induced by prior exposure to radiation-attenuated sporozoites [5,6]. Several pre-erythrocytic antigens are currently under consideration for inclusion in a multistage vaccine against P. falciparum, e.g. circumsporozoite sporozoite protein (CSP), thrombospondin-related adhesive protein (TRAP, also known as sporozoite surface protein 2 or SSP2), and LSA-1. Immunodominant T cell epitopes of CSP are highly polymorphic, and T cell immunity to them is restricted by class I MHC molecules in mice [7] and humans [8]. The relationship between exposure to polymorphic epitopes of P. falciparum pre-erythrocytic antigens and human T cell immunity has been examined most thoroughly in the case of CSP. Udhayakumar and co-workers [9], for example, described natural polymorphism of CSP amino acid residues 368–390 and CTL reactions to the corresponding 23-mer peptides in four adult Kenyans. Evidence that variant epitopes of CSP may be selected by HLA-restricted CTL responses and that responses to such variants may be mutually antagonistic has been reported in a study conducted in the Gambia [10]. These and other data suggest that parasite antigenic polymorphism combined with HLA restriction of immunity to T cell epitopes may reduce the immunogenicity and efficacy of a subunit vaccine.
To date, LSA-1 is the only pre-erythrocytic antigen known to be expressed exclusively by liver-stage P. falciparum[11]. T cell and antibody responses to the N- and C-terminal non-repeat and central repeat regions of LSA-1 have been described in non-immune North Americans immunized with irradiated sporozoites [6] and Africans living in malaria-endemic areas [12]. Recent studies conducted in Kenya and Gabon indicate that cytokine responses to N- and C-terminal non-repeat regions of LSA-1 correlate with partial resistance to re-infection [13–15]. Using a consensus sequence based on the NF54 P. falciparum isolate, we previously characterized T cell cytokine responses to peptides encoded by LSA-1 amino acid residues 84–107, 1813–1835, and 1888–1909 in adults living in the Wosera area of East Sepik Province, Papua New Guinea [16]. Whereas only 18% of individuals had IL-4 or IL-5 responses, IFN-γ production stimulated by the N-terminal 84–107 peptide was observed in 33% of the subjects. The frequency of lymphocyte responses to the C-terminal peptides was even greater (87–88% of subjects). The reasons for the lower rate of IFN-γ responses to the N-terminal peptide are not known. In the current study, we determine if local isolates of P. falciparum have polymorphisms that were not represented in the peptide sequences used to stimulate lymphocyte cytokine production, assess whether 9-mer peptides corresponding to these polymorphic epitopes bind to HLA class I alleles common in this area of Papua New Guinea, and compare the ability of children and adults to make IFN-γ in response to the peptide variants.
SUBJECTS AND METHODS
Study site and human subjects
The study was conducted in the Wosera area of East Sepik Province, where P. falciparum, P. vivax, and P. malariae infection are holoendemic. Uncomplicated malaria morbidity peaks in 1–3-year-old children and decreases progressively with age so that the lowest frequency is in individuals ≥20 years old. Malaria is the major cause of death in 1–4-year-olds, accounting for 33% of the mortality in this age group [17,18].
Peripheral blood mononuclear cells (PBMC) for studies of T cell IFN-γ production and HLA typing were obtained from residents of the villages of Miko 1 and Miko 2. Healthy volunteers were recruited after explaining the purpose of the research. One hundred and fifty-one and 106 persons were typed at the HLA-A and -B loci, respectively. PBMC from 61 people were analysed for IFN-γ production in November 1997. Their age range was 14–67 years (mean ± 1 s.d. 28·3 ± 12 years). A second round of cytokine analysis was conducted in April 1999. At that time, PBMC from 43 children (age range 2–5 years; mean ± 1 s.d. 3·7 ± 1 years) and 74 older individuals (age range 6–54 years; mean ± 1 s.d. 19·2 ± 13 years) were obtained. None of the subjects had fever or other clinical manifestations of malaria. Twelve healthy North American adults (age range 30–45 years) who had never visited a malaria-endemic area also donated blood.
Malaria infection status was established by inspection of thick and thin blood smears according to standard protocols of the Papua New Guinea Institute of Medical Research. A thick smear was scored as negative if no parasites were observed microscopically in oil immersion fields that included a total of 200 leucocytes. Plasmodium falciparum, P. vivax, and P. malariae were detected in 38·7, 8·0 and 10·7% of the subjects in 1997, respectively. One individual was infected with all three species. Infection status was not determined in the subjects studied in 1999.
Ethical clearance and approval of the procedure for oral informed consent were obtained from the Medical Research Council of the Government of Papua New Guinea and the institutional Review Board for Human Studies of University Hospitals of Cleveland, Case Western Reserve University.
Genotyping of P. falciparum clones and field isolates
DNA samples isolated from the P. falciparum laboratory clones/isolates designated HB3 (origin Honduras), CAMP (Malaysia), D10 (Papua New Guinea), 3D7 (The Netherlands, of African origin), T2C6 (Thailand), and NF54 (Africa) were the gift of T. Wellems (National Institutes of Health, Bethesda, MD). Field isolates were obtained from a total of 59 residents of the Wosera in April 1996 and November 1997. Blood was collected by venepuncture or finger prick and blotted onto filter paper (Number 1 Whatman 3M, Minneapolis, MN). The dried samples were placed in 180 μl 5% w/v Chelex-100 (BioRad, Richmond, CA) preheated to 100°C. DNA was released into solution by vigorous shaking for 15 s and boiling for 10 min [19]. Following centrifugation, 5 μl of the supernatant were added to a polymerase chain reaction (PCR) mixture containing 25 mm MgCl2, 1× PCR buffer, 2·5 mm dNTPs, 1 U Taq polymerase (US Biochemical Corp., Cleveland, OH), and 10 μm oligonucleotide primer pair. Primer pairs were synthesized on the basis of the NF54 LSA-1 sequence (GenBank Accession no. X56203). The region of the LSA-1 gene encoding amino acids 1813–1909 was amplified using a primer set comprising the following oligonucleotides: 5′ GGAATTTATAA-AGAACTAGAAG 3′and 5′ AGTTTCATAAAATATTTAGTTAT 3′. The region encoding LSA-1 84–107 was amplified with the oligonucleotides 5′ TATGAGAAAACTAAAAATAATG 3′and 5′ CATCATCATT-TATTATGTGTTC 3′. PCR mixtures were initially denatured at 94°C for 30 s followed by 35 cycles of amplification (94°C × 10 s, 50°C × 20 s, 72°C × 160 s), and finally extended at 72°C × 7 min. The products were separated by electrophoresis in 1·5% agarose gel and fragments of appropriate size selected for sequencing. Sequencing was carried out across both strands using a PCR product sequencing kit (US Biochemical). Sequences were analysed using GeneWorks 2.1.
Restriction fragment length polymorphism (RFLP) analysis was performed on the 196 bp PCR product resulting from the second set of primers described above using the restriction enzymes ClaI and HpaI according to the manufacturer's protocol (US Biochemical). Digested DNA was separated by electrophoresis in a 4% agarose 3:1 high resolution blend gel (Amresco Corp., Solon, OH) in 0·5× TBE at 100 V for 1 h.
Experiments using either of the primer pairs with P. vivax genomic DNA as template did not produce PCR products detectable by staining with ethidium bromide.
HLA typing
HLA-A and -B alleles were determined serologically using Terasaki trays according to the manufacturer's instructions (One Lambda, Canoga Park, CA). Asian-specific reagents were used for typing the Papua New Guinea study subjects. The HLA-A*1101 subtype was confirmed by cloning and sequencing of the A-locus gene of five subjects [20].
LSA-1 variant peptides
Peptides were synthesized by fmoc chemistry [21], purified to ≥95% homogeneity by reverse phase high performance liquid chromatography (HPLC; Chiron Mimetopes, Raleigh, NC), and suspended in PBS pH 7 immediately before use. Assuming that position 2 of a 9-mer peptide corresponds to the primary anchor in the B pocket of the HLA class I dimer [22], peptides corresponding to LSA-1 84–92 with three different amino acids at position 2 were synthesized, i.e. L_MSNVKNV with T (threonine), P (proline), or S (serine).
Peptide binding to HLA class I molecules
Peptide binding was estimated using an HLA class I assembly assay based on binding of antibodies that recognize the conformation of stabilized class I dimers expressed on the surface of T2 lymphoma cells [23]. The.174/T2 cell line was obtained by fusion of the peptide transporter mutant.174 LCL with the T cell line CEM. These cells express HLA-A*0201 (A2/T2). T2 cells transfected with HLA-A*1101 (A11/T2) and HLA-B7 (B7/T2) were maintained as described [24]. Antibodies to HLA-A11 (A11.1M) and HLA-B7 (B27M1) were obtained from American Type Culture Collection (Rockville, MD). Stabilized HLA-A2 dimers were detected using antibody directed against HLA-A, -B and -C (Biodesign Int., Kennebunk, ME). FITC-conjugated secondary antibody was goat anti-mouse IgG (Life Technologies, Rockville, MD).
Differences in MHC class I expression between control and test MHC class I assembly assays were evaluated using the Mantel Haenzel χ2-test. P ≤ 0·0001 was considered significant.
T cell IFN-γ production
Blood was obtained by venepuncture and anti-coagulated with heparin. PBMC were separated from whole blood within 4 h of collection by Hypaque–Ficoll density gradient centrifugation and dispensed in duplicate in 96-well microtitre wells at a concentration of 1 × 106 cells per 300 μl RPMI 1640-containing 10% human AB serum, 80 μg/ml gentamycin, and 25 mm HEPES (cRPMI) [25]. To stimulate the cells for measurement of IFN-γ production, 10 μg/ml (9·7 μm) LSA-1 peptide were added and incubation continued for 48 h at 37°C in 5% CO2 in air. IFN-γ was measured by ELISPOT. Briefly, 24-well ELISPOT plates were coated by overnight incubation at 4°C with 5 μg/ml anti-IFN-γ MoAb (Endogen, Woburn, MA). Plates were washed three times with sterile PBS and blocked with 10% human serum in PBS at room temperature for 2 h. Pre-stimulated PBMC were washed and added to the wells at concentrations of 0·5 × 106 and 0·25 × 106 cells/300 μl complete medium. Incubation at 37°C was continued for 20 h, followed by washing with PBS−0·05% Tween and PBS alone. Biotinylated secondary antibody (200 μl; Endogen) was added at 4 μg/ml in PBS containing 0·05% Tween−1% bovine serum albumin (BSA) and incubated overnight at 4°C. The plates were then washed with PBS–Tween, 200 μl streptavidin-peroxidase (diluted 1:2000) (Dako, Carpinteria, CA) were added, and incubation carried out at room temperature for 2 h. After a final wash with PBS, spots were visualized by adding 200 μl substrate (800 μl of 1% 3-amino-9-ethyl-carbazole in 24 ml 0·1 m acetate buffer pH 5·0). Results are expressed as the net mean number of IFN-γ-secreting cells/106 PBMC (i.e. the value obtained after subtraction of the basal number). Only those preparations for which >100 IFN-γ-secreting cells/106 PBMC were observed following stimulation with 5 μg/ml phytohaemagglutinin (PHA; Sigma, St Louis, MO) were included in the analysis. Basal frequencies (i.e. culture medium alone) of IFN-γ-secreting cells from Papua New Guinea study subjects ranged from 0 to 5 per 106 PBMC.
PBMC from 12 North Americans who had never been exposed to malaria did not produce IFN-γ in response to any of the LSA-1 84–92 peptides on three separate occasions. The subjects included persons whose HLA class I alleles belonged to the A3-, A2-, and B7-like supertypes [26]. A positive response by a Papua New Guinean study subject was arbitrarily defined as a net value ≥10 IFN-γ-secreting cells/106 PBMC.
The expected frequency of response to all three variant peptides was calculated by multiplying the overall frequency of responses to individual peptides for all study subjects within a given age category. Differences in expected and observed frequencies were evaluated using the Mantel Haenzel χ2 test. P ≤ 0·05 was considered significant.
RESULTS
Polymorphism in the N- and C-terminal non-repeat regions of LSA-1
PCR products of 325 bp were generated with the primer pair encompassing codons for the C-terminal amino acid residues 1813–1909. The amplicons were cloned and sequenced. There were no synonymous or non-synonymous mutations in the T2C6, CAMP or 3D7 clones compared with the NF54 sequence (GenBank Accession no. X56203). DNA was prepared from the blood of five P. falciparum-infected individuals from the Wosera. The sequences were identical to the NF54 strain and did not include any synonymous or non-synonymous mutations.
PCR products of 196 bp were generated using the primer pair that includes nucleotides encoding the N-terminal amino acid residues 84–107. A single mutation predictive of an amino acid change at residue 85 was observed. The CAMP clone had T, which is identical to NF54. The 3D7 and HB3 clones had S, and the D10 clone had P (GenBank Accession no. U60974; Table 1). PCR products derived from the blood of 17 malaria-infected subjects studied in 1996 were sequenced. Samples from five subjects had non-synonymous mutations that encoded T, nine encoded S, and three encoded P at LSA-1 85. No other nucleotide changes relative to the NF54 sequence were observed.
Table 1.
Nucleotide and predicted amino acid sequences of Plasmodium falciparum LSA-1 84–92
| Field isolates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid and nucleotide sequence | Clones | 1996 | 1997 | ||||||||
| L | T | M | S | N | V | K | N | V | NF54, CAMP | 6/28 | 5/31 |
| tta | acg | atg | tct | aat | gta | aaa | aat | gtg* | (21%) | (16%) | |
| L | S | M | S | N | V | K | N | V | 3D7, HB3 | 17/28 | 25/31 |
| tta | tcg | atg | tct | aat | gta | aaa | aat | gtg | (61%) | (81%) | |
| L | P | M | S | N | V | K | N | V | D10 | 5/28 | 1/31 |
| tta | ccg | atg | tct | aat | gta | aaa | aat | gtg | (18%) | (3%) | |
LSA-1 nucleotides 325–354 (GenBank Accession no. X56203).
RFLP analysis of the 196-bp PCR product was possible since the nucleotide change that predicted S at LSA-1 85 produced a ClaI site. When a nucleotide substitution that corresponded to T occurred, a HpaI site was created. When P was encoded, no site was susceptible to digestion with either restriction enzyme. RFLP analysis of the 17 samples described above produced results predicted by sequencing. Eight, two, and one of 11 additional field isolates examined by RFLP analysis alone had codons that predicted S, P, or T, respectively. The data from all 28 samples obtained in 1996 are summarized in Table 1.
RFLP analysis was repeated using blood from a different group of 31 malaria-infected examined in 1997. There was no significant change in the frequency of the non-synonymous mutations at LSA-1 residue 85 compared with 1996 (P > 0·05, Table 1).
HLA-A and -B alleles
The most common alleles at the HLA-A locus were A11 (A*1101 subtype confirmed by nucleotide sequence), A24, and A34. All HLA-A alleles were within Hardy–Weinberg proportions. At the HLA-B locus, B13 and B40 were most frequent (Table 2). A greater frequency of HLA-B13/B40 was observed (60 of 106 individuals) than expected (38 of 106 individuals) from Hardy–Weinberg proportions (P < 0·05).
Table 2.
| HLA allele | No. of persons | Frequency |
|---|---|---|
| A11 | 102 | 0·675 |
| A24 | 108 | 0·715 |
| A31 | 10 | 0·066 |
| A34 | 40 | 0·265 |
| B13 | 69 | 0·651 |
| B27 | 8 | 0·075 |
| B40 | 93 | 0·877 |
| B56 | 18 | 0·170 |
The numbers of subjects typed at the HLA-A and -B loci were 151 and 106, respectively.
Other HLA-B alleles and their frequencies were: B15, 0·096; B54, 0·027; B70, 0·055; B37, B39, B45, B63, B75, B77 and B8101, all equal to 0·014.
Peptide binding to HLA-A11, -A2 and -B7 molecules
The T, S and P variants of LSA-1 84–92 led to variable levels of dimer stabilization for the three HLA class I molecules tested (Fig. 1). Expression of stable dimers of HLA-A*1101 increased significantly (P < 0·0001) in the presence of the S variant compared with cells to which no peptide was added. By contrast, when T and P were substituted at the second position, there was no detectable binding to HLA-A11. HLA-A2 expression increased in the presence of the T variant, while HLA-B7 expression increased in the presence of either the S or P variant (Fig. 1).
Fig. 1.
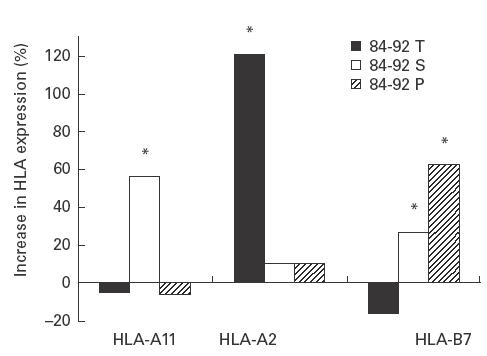
Binding of LSA-1 84–92 peptides to HLA-A*1101-, HLA-A2-, and HLA-B7-transfected T2 lymphoma cells. Assays were performed as described in SUBJECTS and METHODS. Results are the mean of three experiments. *Expression of HLA dimers was significantly greater (P < 0·0001) than in the presence of peptide compared with cells incubated without peptide.
LSA-1 peptide-stimulated IFN-γ production
The frequency of IFN-γ responses to at least one of the 84–92 peptides was lower in 2–5-year-olds (14–16%) compared with older individuals (30–36%). The age-related differences were statistically significant (P < 0·05) for the S and P but not T peptide (Table 3).
Table 3.
Frequency of IFN-γ responses to variants of LSA-1 84–92 by 2–5 and 6–54-year-old study subjects
| Number of subjects | T at position 2 | S at position 2 | P at position 2 | |
|---|---|---|---|---|
| 2–5 years old | 43 | 0·16 | 0·16 | 0·14 |
| 6–54 years old | 74 | 0·30 | 0·36 | 0·35 |
| P* | 0·104 | 0·020 | 0·013 |
Mantel Haenszel χ2 test.
Age-related changes in aggregated IFN-γ responses to all three LSA-1 peptide variants
No persons in the 2–3-year-old age group responded to all three peptides. PBMC from 8% of 4–5-year-olds produced IFN-γ in response to the T, S and P 84–92 peptides. Thereafter, the proportion of responders increased progressively from the age of 6–7 years into adulthood (Fig. 2, r2 = 0·8548). Among individuals between the ages of 6 and 54 years, responses to all peptide variants occurred more frequently together than expected if the responses to each variant were independent of each other. Assuming independence, the predicted value was 0·038 (0·30*0·36*0·35); the observed value was 0·216 (P = 0·001). By contrast, the observed and predicted values were similar for persons ≤5 years old (P = 0·155).
Fig. 2.
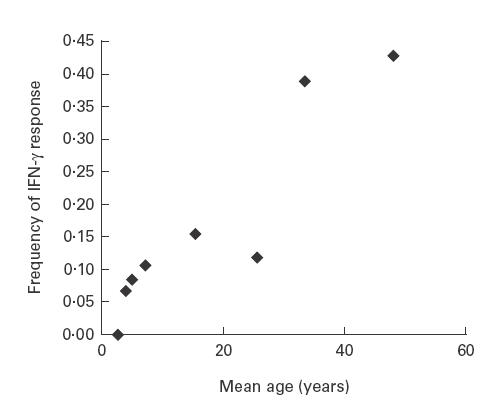
Age-related frequency of IFN-γ responses to all three variants of LSA-1 84–92. Peripheral blood mononuclear cells from a total of 117 individuals were incubated with individual peptides as described in SUBJECTS and METHODS. The number of persons in age group was the following: 2–3-year-olds, 16; 4-year-olds, 15; 5-year-olds, 12; mean 7-year-olds (range 6–10), 19; mean 15-year-olds (range 11–20), 13; mean 26-year-olds (range 21–30), 17; mean 33-year-olds (range 31–40), 18; mean 48-year-olds (range 41–54), 7. The trend line has r2 = 0.85.
Relationship between malaria infection status and IFN-γ responses to LSA-1 peptides
There was no significant positive or negative correlation between responses to any of the LSA-1 peptides and malaria infection status as determined by thick blood smear (examined only during the 1997 study). To examine the correlation between variant peptide-driven IFN-γ responses and clonality of infection, PCR genotyping of the N-terminal region of the LSA-1 gene was performed on samples obtained from adults who were immunologically characterized in 1997. Of 14 individuals infected with blood-stage P. falciparum encoding T at LSA-1 residue 85, five persons did (36%) and nine did not (64%) have IFN-γ responses to the 84–92 T peptide. These percentages did not differ from those of persons infected with P. falciparum of the S or P genotypes, or individuals whose blood did not generate a signal for the LSA-1 gene following PCR amplification (P > 0·05).
DISCUSSION
This study demonstrates that IFN-γ responses to three variants of an N-terminal polymorphic epitope of LSA-1 are common in persons living in a malaria-holoendemic area of Papua New Guinea where parasites of the corresponding genotypes circulate. The likelihood of responding increases with age, and by inference, cumulative exposure to clonal populations of parasites that express the variant epitopes. The overall frequency of IFN-γ responses reported here (30–36% in adults) is similar to that of polymorphic epitopes of other pre-erythrocytic antigens which are thought to be highly conserved, e.g. 32% of Gambians had IFN-γ-secreting cells when stimulated with a series of peptides that spanned TRAP/SSP2 [27].
The relatively greater frequency of non-synonymous than synonymous mutations in the N-terminal but not C-terminal non-repeat sequence of LSA-1 supports the notion that epitopes in the former region are subject to selection by the host immune response [28,29]. Previous descriptions of nucleotide sequences encoding the C-terminal region of LSA-1 have not identified mutations predictive of amino acid differences between the NF54 (African origin) and T9/96 (Thai origin) clones/strains of P. falciparum[12]. The data presented here confirm this in that the nucleotide sequences encoding amino acid residues 1813–1909 were identical among field isolates and several laboratory strains and clones of P. falciparum. By contrast, nucleotide changes that predicted three amino acid substitutions at the N-terminal residue 85 were identified. Yang and co-workers [30] have previously described nucleotide sequence variations in the non-repeat regions of the LSA-1 gene in field isolates from Kenya, Brazil, and Papua New Guinea. Five non-synonymous mutations between amino acid residues 1831 and 1869 were detected; 46·7% of isolates from the Madang area of Papua New Guinea (located approx. 300 km from the Wosera) had valine at residue 1867, and the remainder had aspartic acid at this position (identical to NF54). With respect to the N-terminus, only the threonine and serine (and not proline) alleles at amino acid residue 85 were identified. This earlier report also described nucleotide substitutions predictive of amino acid changes at residues 92, 95 and 104. These were not observed in the current study. Spatial and temporal differences of parasite populations in these geographically separate areas of Papua New Guinea may account for differences in the proportions of the various mutations. In addition, unlike Yang et al., we did not establish in vitro cultures of P. falciparum before genotyping. Thus, if low levels of circulating parasites with these genotypes were present, they may not have been detected by amplification of genomic DNA isolated directly from blood.
The results showing an age-related increase in the frequency of individual and aggregated IFN-γ responses to the variant peptides support the notion that cumulative exposure to multiple clones of parasites broadens the repertoire of T cell responses to polymorphic regions of pre-erythrocytic antigens such as LSA-1. Alternatively, the data are consistent with the possibility that T cell immunity to LSA-1 transcends various strains of P. falciparum or that recent malaria infections were clustered in the cohort of individuals studied. Antibody and lymphocyte proliferation responses to other pre-erythrocytic antigens have been reported to increase with age [31,32], but to our knowledge there are no published data describing the frequency of T cell IFN-γ or CTL responses to polymorphic epitopes of LSA-1. In the context of evaluating the potential antigenicity of candidate pre-erythrocytic vaccine molecules, it should be noted that the method used to study T cell immunity influences the frequency of responses detected in malaria-exposed populations. The IFN-γ ELISPOT was employed in the current study. This assay has recently been used to estimate frequencies of T cell immunity to TRAP peptide epitopes [27]. Since the current results are based on responses of lymphocytes incubated with peptides for 72 h, they probably reflect cytokine production by ‘re-stimulated’ T cells rather than ex vivo responses (i.e. short-term responses that occur before cell division occurs) [33]. It is not yet clear if these or other assays (e.g. CTL) are surrogates of protective immunity. Recent reports using LSA-1 peptides greater than 20-mer in length or recombinant LSA-1 proteins suggest that IL-10 responses to LSA-1 correlate with resistance to re-infection [13,15]. Nine-mer peptides were used here because we ultimately want to define the importance of HLA class I-restricted CD8 T cell responses in resistance to re-infection. Preliminary examination of lymphocyte responses to these and other 9-mer LSA-1 peptides in malaria-endemic areas of Kenya and Papua New Guinea indicate that <10% of individuals make IL-10 (unpublished observations).
Peptide binding experiments were performed to gain insight into whether HLA class I alleles may restrict responses to LSA-1 peptide variants. Binding of the three 84–92 variants to HLA-A*1101 was evaluated. This allele has a frequency of 67·5% in the population. Peptide with serine but not threonine or proline at the primary anchor position stabilized HLA-A11 dimers in vitro. Given the preference of HLA-A11 and other members of the HLA-A3-like supertype for hydroxyl-containing amino acids at position 2 [34], the results with serine peptide were anticipated. It is not clear however, why the peptide with threonine at position 2, another hyroxyl amino acid, did not bind. It is possible that more sensitive assays of peptide binding, such as those that rely on competition with known binders and allow determination of binding affinity, would have shown significant binding. More detailed studies of this problem will require examination of the responses of purified CD8 cells from persons exposed to malaria. Since this is not feasible in most endemic settings, especially when only limited amounts of blood can be obtained from children and infants, future studies will identify the surface phenotype and cytokine production profile of CD8+ T cells that bind HLA-A11 tetramers that include LSA-1 9-mer peptides [35].
Acknowledgments
This work was supported by a grant from the US Public Health Service, National Institutes of Health (AI-36478) and the Burrroughs-Wellcome Fund New Initiatives in Malaria Research program (J.W.K.).
REFERENCES
- 1.Nussenzweig RS, Long CA. Malaria vaccines: multiple targets. Science. 1994;265:1381–3. doi: 10.1126/science.8073276. [DOI] [PubMed] [Google Scholar]
- 2.Doolan DL, Sedegah M, Hedstrom RC, Hobart P, Charoenvit Y, Hoffman SL. Circumventing genetic restriction of protection against malaria with multigene DNA immunizations: CD8+ T cell-, IFN-γ-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–46. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aidoo M, Lalvani A, Allsopp CE, et al. Identification of conserved antigenic components for a cytotoxic T lymphocyte-inducing vaccine against malaria. Lancet. 1995;345:1003–6. doi: 10.1016/s0140-6736(95)90754-8. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman SL, Oster CN, Mason C, Bieir JC, Sherwood JA, Ballou WR, Mugambi M, Chulay JD. Human lymphocyte proliferative response to sporozoite T cell epitopes correlates with resistance to falciparum malaria. J Immunol. 1989;142:1299–303. [PubMed] [Google Scholar]
- 5.Malik A, Egan JE, Houghten RA, Sadoff JC, Hoffman SL. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci USA. 1991;88:3300–4. doi: 10.1073/pnas.88.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krzych U, Lyon JA, Jareed T, Schneider I, Hollingdale MR, Gordon DM, Ballou WR. Lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver- and blood-stage malaria antigens. J Immunol. 1995;155:4072–81. [PubMed] [Google Scholar]
- 7.Zevering Y, Khamboonruang C, Good MF. Human and murine T-cell responses to allelic forms of a malaria circumsporozoite protein epitope support a polyvalent vaccine strategy. Immunology. 1998;94:445–54. doi: 10.1046/j.1365-2567.1998.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Good MF, Pombo D, Quakyi IA, et al. Human T cell recognition of the circumsporozoite protein of Plasmodium falciparum-immunodominant T cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci USA. 1988;85:1199–203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udhayakumar V, Ongecha JM, Shi YP, et al. Cytotoxic T cell reactivity and HLA-B35 binding of the variant Plasmodium falciparum circumsporozoite protein CD8+ CTL epitope in naturally exposed Kenyan adults. Eur J Immunol. 1997;27:1952–7. doi: 10.1002/eji.1830270819. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert SC, Plebanski M, Gupta S, et al. Association of malaria parasite population structure, HLA, and immunological antagonism. Science. 1998;279:1173–7. doi: 10.1126/science.279.5354.1173. [DOI] [PubMed] [Google Scholar]
- 11.Guerin-Marchan C, Druihle P, Galey B, et al. A liver stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature. 1987;329:164–7. doi: 10.1038/329164a0. [DOI] [PubMed] [Google Scholar]
- 12.Fidock DA, Grans-Masse H, Lepers JP, et al. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol. 1994;153:190–204. [PubMed] [Google Scholar]
- 13.Luty AJ, Lell B, Schmidt-Ott R, et al. Parasite antigen-specific interleukin-10 and antibody responses predict accelerated parasite clearance in Plasmodium falciparum malaria. Eur Cytokine Netw. 1998;9:639–46. [PubMed] [Google Scholar]
- 14.Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis. 1999;179:980–8. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 15.Kurtis JD, Lanar DE, Opollo M, Duffy PE. Interleukin-10 responses to liver-stage antigen 1 predict human resistance to Plasmodium falciparum. Infect Immun. 1999;67:3424–9. doi: 10.1128/iai.67.7.3424-3429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connelly M, King CL, Bucci K, Walters S, Genton B, Alpers MP, Hollingdale MR, Kazura JW. T cell immunity to peptide epitopes of Liver Stage Antigen 1 in a malaria holoendemic area of Papua New Guinea. Infect Immun. 1997;65:5082–7. doi: 10.1128/iai.65.12.5082-5087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genton B, Al-Yaman F, Beck H-P, Hii J, Mellor S, Narara A, Smith T, Alpers MP. The epidemiology of malaria in the Wosera area, Papua New Guinea, in prearation for vaccine trials. I. Malariametric indices and immunity. Ann Trop Med Parasitol. 1995;89:359–76. doi: 10.1080/00034983.1995.11812965. [DOI] [PubMed] [Google Scholar]
- 18.Genton B, Al-Yaman F, Beck H-P, Mellor S, Rare L, Ginny M, Smith T, Alpers MP. The epidemiology of malaria in the Wosera area, Papua New Guinea, in preparation for vaccine trials. II. Mortality and morbidity. Ann Trop Med Parasitol. 1995;89:377–90. doi: 10.1080/00034983.1995.11812966. [DOI] [PubMed] [Google Scholar]
- 19.Kain KC, Wirtz RA, Fernandez I, Franke ED, Rodriguez MH, Lanar DE. Serologic and genetic characterization of Plasmodium vivax from whole blood-impregnated filter paper discs. Am J Trop Med Hyg. 1992;46:473–9. doi: 10.4269/ajtmh.1992.46.473. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, Tokunaga K, Ishikawa Y, et al. Sequence analysis of serological HLA-A11 split antigens, A11.1 and A11.2. Tissue Antigens. 1994;43:78–82. doi: 10.1111/j.1399-0039.1994.tb02304.x. [DOI] [PubMed] [Google Scholar]
- 21.Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen–antibody interactions at the level of individual amino acids. Proc Natl Acad Sci USA. 1985;82:5131–6. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelbard VH. Structure of peptides associated with class I and II MHC molecules. Ann Rev Immunol. 1994;12:181–207. doi: 10.1146/annurev.iy.12.040194.001145. [DOI] [PubMed] [Google Scholar]
- 23.Domenech N, Henderson RA, Finn OJ. Identification of an HLA-A11-restricted epitope from the tandem repeat domain of the epithelial tumor antigen mucin. J Immunol. 1995;155:4766–74. [PubMed] [Google Scholar]
- 24.Crumpacker DB, Alexander J, Cresswell P, Engelhard VH. Role of endogenous peptides in murine allogeneic cytotoxic T cell responses assessed using transfectants of the antigen-processing mutant.174xCEM.T2. J Immunol. 1992;148:3004–11. [PubMed] [Google Scholar]
- 25.Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW, King CL. Helminth- and Bacillus Calmette Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–8. [PubMed] [Google Scholar]
- 26.Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–12. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan KL, Plebanski M, Akinwunmi P, Lee EA, Reece WH, Robson KJ, Hill AV, Pinder M. Broadly distributed T cell reactivity, with no immunodominant loci, to the pre-erythrocytic antigen thrombospondin-related adhesive protein of Plasmodium falciparum in West Africans. Eur J Immunol. 1999;29:1943–54. doi: 10.1002/(SICI)1521-4141(199906)29:06<1943::AID-IMMU1943>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Hill AV, Jepson A, Plebanski M, Gilbert SC. Genetic analysis of host–parasite coevolution in human malaria. Philos Trans R Soc Lond B Biol Sci. 1997;352:1317–25. doi: 10.1098/rstb.1997.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escalante AA, Lal AA, Ayala FJ. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics. 1998;149:189–202. doi: 10.1093/genetics/149.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, Shi Y-P, Udhayakumar V, Alpers MP, Povoa MM, Hawley WA, Collins WE, Lal AA. Sequence variations in the non-repetitive regions of liver stage-specific antigen-1. Mol Biochem Parasitol. 1995;71:291–4. doi: 10.1016/0166-6851(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 31.Zevering Y, Khamboonruang C, Rungruengthanakit K, Tungviboonchai L, Ruengpipattanapan J, Pathurst I, Barr P, Good MF. Life-spans of human T-cell responses to determinants from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium vivax. Proc Natl Acad Sci USA. 1994;91:6118–22. doi: 10.1073/pnas.91.13.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Good MF, Doolan DL. Immune effector mechanisms in malaria. Curr Opin Immunol. 1999;11:412–9. doi: 10.1016/S0952-7915(99)80069-7. [DOI] [PubMed] [Google Scholar]
- 33.Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–65. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sidney J, Grey HM, Southwood S, et al. Definition of an HLA-A3-like supermotif demonstrates the overlapping peptide-binding repertoires of common HLA molecules. Hum Immunol. 1996;45:79–93. doi: 10.1016/0198-8859(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 35.Ogg GS, McMichael AJ. HLA-peptide tetrameric complexes. Curr Opin Immunol. 1998;10:393–6. doi: 10.1016/s0952-7915(98)80110-6. [DOI] [PubMed] [Google Scholar]


