Abstract
Eosinophil infiltration occurs in a variety of allergic and inflammatory diseases. The release of preformed mediators from eosinophils may contribute to inflammatory responses. We investigated the ability of eosinophil-derived major basic protein and eosinophil-derived neurotoxin to stimulate production of IL-8 from intestinal myofibroblasts. Intestinal myofibroblasts (18-Co cells) were incubated with major basic protein, eosinophil-derived neurotoxin, or a synthetic analogue of major basic protein, poly-l-arginine. Immunoreactive IL-8 was measured by ELISA and IL-8 mRNA levels were analysed by Northern blot or reverse transcription-polymerase chain assay. Major basic protein induced IL-8 mRNA production and release of significant levels of IL-8 immunoreactive protein. By contrast, eosinophil-derived neurotoxin stimulated little IL-8 release. The induction of IL-8 mRNA by poly-l-arginine was significantly inhibited by actinomycin D. These findings demonstrate a novel interaction between eosinophils and intestinal fibroblasts that may be involved in the pathogenesis of diseases associated with tissue eosinophilia.
Keywords: eosinophil, IL-8, major basic protein, gastrointestinal, fibroblast
INTRODUCTION
Eosinophils normally reside in tissues with mucosal surfaces such as the gastrointestinal tract. A variety of inflammatory and allergic diseases, including inflammatory bowel disease (IBD), parasitic infections, eosinophilic gastroenteritis, asthma, atopic dermatitis, and allergic rhinitis are associated with increases in the number of eosinophils within affected tissues [1,2]. While the histopathological patterns of eosinophil infiltration in these conditions have been well characterized, the precise contribution of the eosinophil in these diseases is uncertain.
Eosinophils contain a wide variety of biologically active mediators including the granule-associated preformed mediators, major basic protein (MBP) and eosinophil-derived neurotoxin (EDN). Despite the fact that MBP and EDN are both located within the eosinophil secondary granule, these proteins have important structural and functional differences. MBP is a 14-kD polypeptide that is localized within the central core of the secondary granule. It possesses an isoelectric point of 10·9. MBP exhibits cytotoxic properties, such as the ability to kill Schistosomes. MBP also has multiple non-cytolytic actions, including the selective ability to induce histamine release from human basophils and mast cells; stimulate tumour necrosis factor-alpha (TNF-α) release from murine mast cells; stimulate the release of platelet-derived serotonin; induce neutrophil chemiluminescence; stimulate IL-8 release from neutrophils and eosinophils; and generate neutrophil superoxide anion production [3–7]. By contrast, EDN is an 18-kD protein that is found in the granule matrix and nuclear membrane. It has an isoelectric point of 8·9. EDN is a weak helminthotoxin, but possesses other potent biologic properties including neurotoxicity and RNase activity [8].
Eosinophils lie juxtaposed to immune, endothelial, and epithelial cells in the gastrointestinal (GI) tract. As well, eosinophils are in close anatomic proximity to the supportive matrix of the GI tract, including interstitial fibroblasts. A growing literature supports the concept that fibroblasts not only function as passive structural cells, but may also actively participate in immune and inflammatory responses. For example, fibroblasts can produce a variety of immunomodulatory and proinflammatory cytokines such as IL-8 and granulocyte-macrophage colony-stimulating factor (GM-CSF) [9,10]. Furthermore, eosinophils and mediators produced by eosinophils influence fibroblast cytokine production and function. Respiratory tract fibroblasts exposed to eosinophil granule MBP produce IL-6 and IL-11, and co-culture of eosinophils and fibroblasts increase fibroblast proliferation and stimulate glycosaminogylcan degradation [11–15]. These studies suggest that interactions between eosinophils and fibroblasts may have important functional consequences that contribute to the pathogenesis of inflammatory reactions. In this regard, the proinflammatory chemokine IL-8 may be particularly important in intestinal inflammation. IL-8 is a member of the C-X-C chemokine family that acts primarily on the neutrophil as an activator and chemoattractant. Neutrophil infiltration is a pathological hallmark of IBD and increased IL-8 is seen in this condition [16].
We initiated these studies to determine whether eosinophil-derived granule proteins influence IL-8 production from fibroblasts of intestinal origin. We report that MBP, but not EDN, can stimulate the release of IL-8 from the intestinal myofibroblasts cell line, 18-Co.
MATERIALS AND METHODS
Cells
18-Co intestinal myofibroblasts were purchased and studied between passages 5 and 12 (American Type Cell Culture CRL-1459, Manassas, VA) [17]. Primary cell fibroblast cultures were established from neonatal foreskin by a previously described explant technique and studied between passages 4 and 8 [18]. Fibroblasts were grown to near confluence, washed twice with serum-free medium, and incubated with either TNF-α, poly-l-arginine (PA), eosinophil granule protein (MBP or EDN), serum-free medium with vehicle buffer (control) or serum-free media. Supernatants were harvested at different intervals (30 min to 24 h) and immunoreactive IL-8 protein was measured in duplicate or triplicate by ELISA analysis (Endogen, Woburn, MA).
Isolation of human eosinophils
Human eosinophils were collected from whole blood from healthy and hypereosinophilic donors in the Clinical Research Center at the Beth Israel Deaconess Medical Center as described previously [3]. After centrifugation, the buffy coat was collected and separated by density centrifugation on Ficoll–Paque (Pharmacia, Piscataway, NJ). Erythrocytes in the cell pellet were lysed in NH4Cl erythrocyte lysis buffer and the remaining cells washed twice in PBS with 2% fetal bovine serum (FBS). Granulocytes were resuspended in PBS with 2% FBS and an aliquot stained with Randolph's stain and counted. Eosinophils were then isolated by negative selection using the MACS system [19]. The granulocytes were incubated with CD16 antibodies conjugated to magnetic particles (50 μl of particles per 5 × 107 cells) for 30 min at 6°C with gentle mixing every 5 min. CD16 is expressed on neutrophils but not eosinophils. After incubation, the cells were passed through a Miltenyi MACS column in a magnetic field and unlabelled eosinophils were collected below. An aliquot of the collected cells was stained with Randolph's stain. Using this approach, eosinophil preparations of >98% purity were routinely obtained.
Purification of eosinophil granule proteins
Eosinophil-derived granule proteins were isolated as previously described [3]. Briefly, eosinophils were washed with ice-cold 0·25 m sucrose and resuspended in the same medium. Cell membranes were disrupted by vigorous pipetting through a narrow bore pipette. Cell lysates were centrifuged at 600 g to remove cell debris and unbroken cells and at 13 000 g for 20 min to pellet the granules. Eosinophil granules were lysed in 10 mm HCl and sonicated. Granule lysates were centrifuged at 40 000 g and the resulting supernatant was fractionated on a Sephadex G-50 12 × 100 cm column equilibrated with 0·025 m acetate buffer pH 4·3, containing 0·15 m NaCl. Acetate buffer was harvested for vehicle control conditions. Eosinophil granule proteins were identified by their distinctive chromatographic elution profile from the G-50 column. The third protein peak yields fractions containing pure MBP. The purity of protein isolation was determined by SDS–PAGE gel and distinctive bands for the recently described MBP homologue were not identified [20]. The concentration of MBP was determined by spectrophotometric absorbance at 277 nm with an extinction coefficient of 26·3 and EDN at 280 nm with a extinction coefficient of 15·5. The identity of each protein was confirmed by radioimmunoassay or Western blot analysis.
Lactate dehydrogenase assay
The time point for protein harvest was 24 h and we therefore utilized lactate dehydrogenase (LDH) assays as an assessment of cell viability [21,22]. Supernatants from each cell condition, stimulated and unstimulated, were assayed for their LDH content. Cell supernatant (50 μl) was mixed with 100 μl of 2 mm NADH2 and 750 μl of 100 mm phosphate buffer, followed by 100 μl of sodium pyruvate. The decrement of absorbance measured at 334 nm was measured for 3 min and the percentage of LDH release from unstimulated cells was compared with stimulated and Triton X-100-treated cells.
Northern blot analysis of IL-8 mRNA expression
18-Co myofibroblasts were stimulated by PA as defined above, in the presence and absence of the transcriptional inhibitor actinomycin D (10 ng/ml). RNA was isolated and Northern blot analysis performed. RNA from 18-Co myofibroblasts stimulated by PA or MBP for varying times or unstimulated cells was isolated by the guanidine method (Ultraspec, Houston, TX). Total RNA (10 μg) was electrophoresed in a 1% agarose gel and was transferred to nitrocellulose membranes. Blots were hybridized with a 32P-labelled cDNA probe for human IL-8. The IL-8 probe was constructed as previously described [23]. A cDNA probe for chicken glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was used to determine RNA loading equivalence.
Reverse transcription-polymerase chain reaction determination of IL-8 mRNA expression
Total RNA (1 μg) was reverse transcribed to cDNA using Moloney murine leukaemia virus reverse transcriptase. The mixture (10 μl) was amplified with 0·4 mm of IL-8 primers (Clontech, Palo Alto, CA), 1× PCR reaction buffer, 0·8 mm dNTPs, and 2 U of Thermus aquaticus DNA polymerase (Perkin Elmer Cetus) using a DNA thermal cycler (Perkin Elmer, Forest City, CA). The cycle parameters for polymerase chain reaction (PCR) were as follows: 95°C for 30 s, 55°C for 1 min, 72°C for 2 min for 35 cycles and 72°C for 8 min. Products were visualized in a 1·5% TAE agarose gel. Specificity for primer pairs was confirmed by Southern blot analysis using an oligonucleotide probe internal to the primer sequences for IL-8 [23].
RESULTS
Recombinant human TNF-α stimulates IL-8 protein release from human intestinal myofibroblasts
We first established whether intestinal 18-Co myofibroblasts have the capacity to produce IL-8. TNF-α is a potent agonist for the production of IL-8 in multiple cell types. We therefore determined the response of 18-Co intestinal myofibroblasts to recombinant human (rh)TNF-α. rhTNF-α at concentrations of 10 ng/ml and 100 ng/ml induced little or no IL-8 production from 18-Co myofibroblasts after 30 or 90 min (data not shown). However, significant levels of IL-8 protein were seen after 24 h in rhTNF-α-stimulated compared with medium stimulated cells (Fig. 1). Thus, 18-Co intestinal myofibroblasts have the capacity to produce IL-8.
Fig. 1.
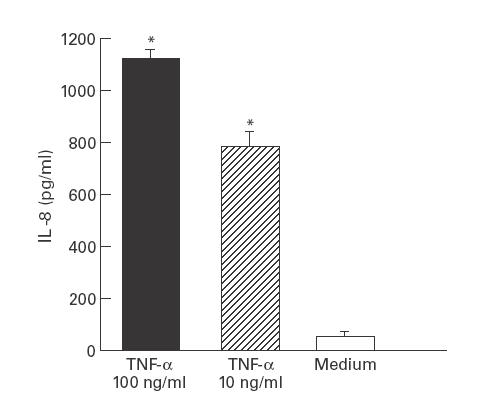
Recombinant human tumour necrosis factor-alpha (rhTNF-α) stimulates IL-8 release from intestinal myofibroblasts. 18-Co human intestinal myofibroblasts were incubated with different concentrations of rhTNF-α or medium alone for 24 h. Supernatants were collected and analysed in triplicate for immunoreactive IL-8 protein by ELISA. *P < 0·05 compared with medium alone by Student's t-test. Similar results were obtained in a repeat experiment.
Eosinophil-derived granule MBP, but not eosinophil-derived neurotoxin, stimulates IL-8 protein production
We next determined the effect of the eosinophil-derived granule cationic proteins MBP and EDN on the production of IL-8 mRNA and protein from 18-Co intestinal myofibroblasts. MBP (10−6 m) stimulated significant release of IL-8 immunoreactive protein at 24 h compared with buffer-stimulated (control) cells (Fig. 2). By contrast, a similar concentration of EDN had no significant impact on IL-8 production after 24 h incubation (Fig. 2). Neither MBP nor EDN at the concentrations examined had a significant influence on cell viability compared with unstimulated cells as determined by measurements of LDH release (data not shown). The concentration of MBP examined has been previously shown to stimulate cytokine production without altering cell viability [3]. We also determined the effect of MBP on cutaneous fibroblasts; MBP induced a four-fold increase in the level of IL-8 protein compared with buffer-stimulated cells (Fig. 3).
Fig. 2.
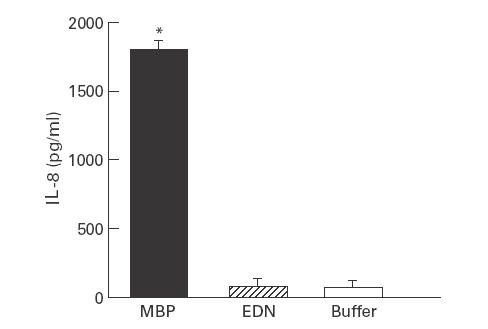
Major basic protein (MBP), but not eosinophil-derived neurotoxin (EDN), stimulates IL-8 release from human intestinal myofibroblasts. 18-Co human intestinal fibroblasts were incubated with MBP 10−6 m, EDN 10−6 m, or buffer (control) for 24 h. Supernatants were collected and analysed in triplicate for immunoreactive IL-8 protein by ELISA. *P < 0·01 compared with buffer-stimulated cells by Student's t-test. Similar experiments were obtained in a repeat experiment.
Fig. 3.
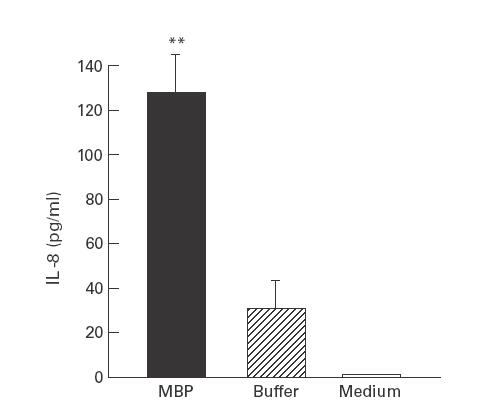
Major basic protein (MBP) stimulates IL-8 protein release from foreskin fibroblasts. Primary cultures of human foreskin fibroblasts were grown to confluence and incubated with MBP (10−6 m), buffer + medium, or medium alone for 24 h. Supernatants were collected and analysed in duplicate for immunoreactive IL-8 by ELISA. **P < 0·05 compared with buffer-stimulated cells by Student's t-test.
We next examined whether MBP induced an increase in IL-8 mRNA levels in intestinal myofibroblasts. We first determined IL-8 mRNA levels by reverse transcription (RT)-PCR and Southern blot analysis with normalization of IL-8 mRNA levels to housekeeping gene GAPDH. Using this approach, IL-8 mRNA levels were virtually undetectable in buffer-stimulated 18-Co cells. By contrast, myofibroblasts stimulated with MBP exhibited an increase in IL-8 mRNA levels after 3 h (Fig. 4).
Fig. 4.
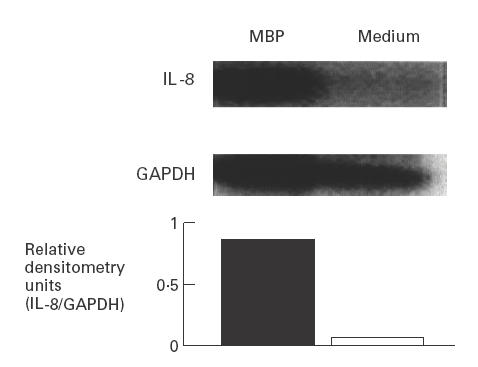
Major basic protein (MBP) induces IL-8 mRNA levels in human intestinal myofibroblasts. 18-Co human intestinal fibroblasts were incubated for 3 h with MBP (10−6 m) or medium alone. Total RNA was isolated and reverse transcription-polymerase chain reaction performed using primers specific for human IL-8. Southern blot analysis was performed with a 32P-labelled IL-8 cDNA probe. Blots were stripped and re-probed with a chicken glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA probe to assess RNA loading.
Poly-l-arginine induces IL-8 production in intestinal myofibroblasts
MBP is a highly charged cationic protein of which no commercial source is available. Several studies suggest that the mechanism of action of MBP on target cells is related to its high net positive charge [15,24]. We therefore examined the effect of PA, a synthetic ‘analogue’ of MBP that is similarly charged, on IL-8 production from intestinal 18-Co cells. PA (10−6 m) induced a significant increase in IL-8 immunoreactive protein (Fig. 5) and IL-8 mRNA by Northern blot analysis (Fig. 6). The stimulation of 18-Co cells with PA resulted in IL-8 mRNA accumulation at 3, 6, 12 and 24 h and maximal IL-8 protein levels were seen at 12 h. PA had no significant effect on cell viability compared with unstimulated controls (data not shown).
Fig. 5.
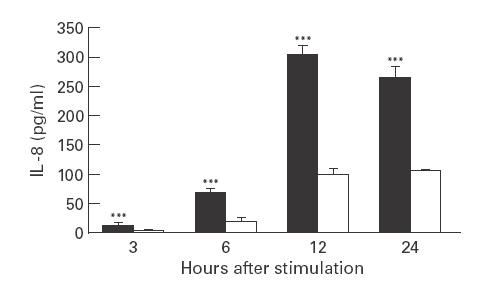
Poly-l-arginine (PA) stimulates IL-8 release from intestinal myofibroblasts. 18-Co human intestinal fibroblasts were incubated with PA at 10−6 m (▪) or medium alone (□) for 24 h. Supernatants were collected at different times and analysed in triplicate for immunoreactive IL-8 protein by ELISA. ***P < 0·05 compared with cells in medium alone by Student's t-test. Similar results were obtained in two repeat experiments.
Fig. 6.
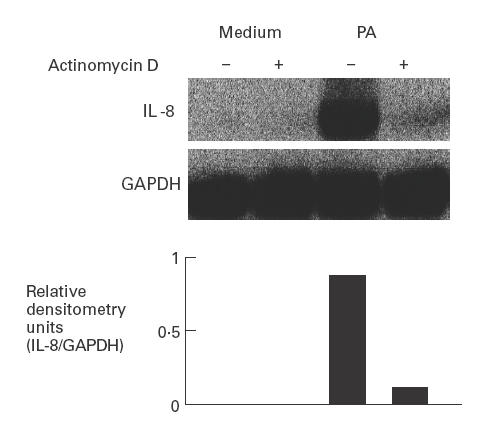
Poly-l-arginine (PA) induces IL-8 gene transcription in intestinal myofibroblasts. 18-Co human intestinal fibroblasts were incubated for 3 h with PA (10−6 m) or medium alone. Some cells were treated with actinomycin D (10 ng/ml) for 30 min prior to exposure to PA. Total RNA was isolated and Northern blot analysis performed for IL-8 mRNA expression. Blots were subsequently stripped and re-probed with a chicken glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA probe to assess RNA loading equivalence. Similar results were obtained in repeat experiments.
We next determined whether PA-induced IL-8 production was transcriptionally regulated using actinomycin D, a specific inhibitor of RNAP II. Actinomycin D treatment significantly diminished PA-induced IL-8 mRNA accumulation in 18-Co myofibroblasts (Fig. 6), suggesting that PA initiates new IL-8 gene transcription.
DISCUSSION
Eosinophils are normally present in the GI tract [25] and increase during certain allergic and inflammatory conditions, suggesting roles in both health and disease. Increased eosinophils have been observed in association with IBD, oesophagitis, eosinophilic gastroenteritis, food hypersensitivity reactions, asthma, and parasitic disease [26–31]. Others have demonstrated extracellular deposition of MBP in atopic dermatitis, ulcerative colitis, eosinophilic gastroenteritis and coeliac disease [26,28,29,32,33]. Increased quantities of MBP have been found in the stool effluents from patients affected with Crohn's colitis and in bronchial lavages of patients with asthma [34–36]. Taken together, these studies suggest that eosinophils and components of the cytoplasmic granules of eosinophils, such as MBP, may be involved in inflammatory responses.
The current study demonstrates that eosinophil-derived granule protein MBP can induce IL-8 protein production from human intestinal myofibroblasts and, albeit to a lesser degree, from primary cultures of dermal fibroblasts. The over 10-fold difference in IL-8 protein seen between the two stimulated fibroblasts probably represents heterogeneity between fibroblasts, i.e. intestinal fibroblasts may be more susceptible to the effects of MBP compared with skin fibroblasts. Whether this is due to differences in local microenvironments or differences in yet unidentified MBP receptors on the surface of the fibroblast is not known. We found that poly-l-arginine, a synthetic analogue of MBP, also induced IL-8 gene transcription and protein release from 18-Co myofibroblasts, suggesting that at least part of the action of MBP may be related to its cationic charge. We also show that, in these experimental conditions, EDN did not stimulate significant IL-8 protein release. These findings suggest that MBP, but not EDN, induces synthesis of a potent chemokine associated with neutrophil recruitment and activation.
Eosinophils reside in the lamina propria of the intestinal mucosa in close proximity to fibroblasts. Previous evidence suggests that this anatomic relationship could facilitate interactions between the two cell types. For example, the co-culture of eosinophils with fibroblasts in the presence of IL-5 induced a phenotypic change in eosinophils from normodense to hypodense and increased cytotoxicity of eosinophils toward Schistosomae [14]. As well, eosinophils can modulate fibroblast function. Studies have demonstrated that eosinophils stimulate fibroblast proliferation and induce production of IL-6 and transforming growth factor-beta (TGF-β) [15,37,38]. These studies and ours demonstrate that communication between eosinophils and fibroblasts occurs in vitro, but the exact nature of the interactions between these cells in vivo is still not clear.
The precise concentration of extracellular MBP in affected tissues during inflammation is not known, but the intimate juxtaposition of eosinophils and fibroblasts and the increased numbers of eosinophils in inflammatory conditions make it likely that MBP reaches increased concentrations in local microenvironments. The concentration examined here corresponds to that used in other in vitro studies examining MBP functions and correlates to those found in other inflamed tissue sites [39,40].
Further studies will be necessary to elucidate fully the mechanism(s) involved in MBP-induced IL-8 production by intestinal fibroblasts, but our findings suggest that the cationic charge of MBP may contribute to this effect. Numerous studies have used poly-l-arginine as a synthetic ‘analogue’ of MBP, because 14% of MBP's amino acid sequence comprises arginine residues [41,42]. The ability of PA to stimulate myofibroblast release of IL-8 protein and mRNA accumulation suggests a role for surface charge in mediating IL-8 production by intestinal myofibroblasts. In other systems, the effects of PA and MBP can be blocked by anionic molecules, such as heparin and polyacidic amino acids, suggesting that the cationic nature of eosinophil-derived granule proteins, at least in part, confers biological activity, probably through interactions with negatively charged lipid membrane [15,43,44]. However, the quantity of IL-8 protein released after PA stimulation was less than native MBP, and the kinetics differed, suggesting that charge alone is not the only factor conferring MBP's activity. The specificity of the response raises the question of whether a specific MBP receptor-mediated signalling pathway is involved. Although a receptor has not yet been identified, a recent study by Thomas et al. shows that the action of MBP involves activation of an NADPH oxidase complex through a tyrosine kinase-, and calmodulin-dependent pathway [45].
Our results show that eosinophil granule MBP stimulates human intestinal myofibroblast and dermal fibroblast IL-8 production. This action of MBP suggests a novel mechanism that may participate in the recruitment of neutrophils during intestinal and dermal inflammation. We speculate that this interaction between eosinophils and fibroblasts participates in the initiation and/or perpetuation of the inflammatory process through the recruitment of neutrophils.
Acknowledgments
Supported by NIH grants DK 33506 and Core B (Morphology), DK46819 and DK40561 (Clinical Nutrition Research Center at Harvard) from the National Institutes of Health (B.K.W.); a Career Development Award from the Crohn's and Colitis Foundation of America, NIH grant DK02564 and Charles Janeway Scholarship from Children's Hospital of Boston (G.T.F.); grant number AR42309 from the National Institutes of Health (J.V.); Falk Medical Scholar Grant from the Greater Chicago Chapter of the Arthritis Foundation (A.M.S.); and grant AI25230 from the National Institutes of Health (S.J.A.). The authors thank Tim Turner for his expert technical advice, Sean P. Colgan for his critical review of the manuscript, and Naofumi Mukaida for the kind gift of the IL-8 probe.
REFERENCES
- 1.Spry J. Natural history of eosinophils. In: Smith H, Cook R, editors. Immunopharmacology of eosinophils. London: Academic Press; 1993. pp. 1–9. [Google Scholar]
- 2.Leiferman KM, Butterfield JH. Eosinophil-associated diseases. In: Smith H, Cook RM, editors. Immunopharmacology of eosinophils. London: Academic Press; 1993. pp. 151–80. [Google Scholar]
- 3.Furuta GT, Ackerman SJ, Lu L, Williams RE, Wershil BK. Stem cell factor influences mast cell mediator release in response to eosinophil derived granule major basic protein. Blood. 1998;92:1055–61. [PubMed] [Google Scholar]
- 4.Kita H, Abu-Ghazaleh RI, Sur S, Gleich GJ. Eosinophil major basic protein induces degranulation and IL-8 production by human eosinophils. J Immunol. 1995;154:4749–58. [PubMed] [Google Scholar]
- 5.Moy J, Gleich G, Thomas L. Noncytotoxic activation of neutrophils by eosinophil granule major basic protein: effect on superoxide anion generation and lysosomal enzyme release. J Immunol. 1989;145:2626–32. [PubMed] [Google Scholar]
- 6.O'donnel MC, Ackerman SJ, Gleich GJ, Thomas LL. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J Exp Med. 1983;157:1981–91. doi: 10.1084/jem.157.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohrbach MS, Wheatley CL, Slifman NR, Gleich GJ. Activation of platelets by eosinophil granule proteins. J Exp Med. 1990;172:1271–4. doi: 10.1084/jem.172.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackerman SJ, Loegering DA, Venge P, Olsson I, Harley JB, Fauci AS, Gleich GJ. Distinctive cationic proteins of the human eosinophil granule: major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin. J Immunol. 1983;131:2977. [PubMed] [Google Scholar]
- 9.Rolfe MW, Kunkel SL, Standiford TJ, Chensue SW, Allen RM, Evanhoff HL, Pham SH, Streiter RM. Pulmonary fibroblast expression of interleukin-8: a model for alveolar macrophage-derived cytokine networking. Am J Respir Cell Mol Biol. 1991;5:493–501. doi: 10.1165/ajrcmb/5.5.493. [DOI] [PubMed] [Google Scholar]
- 10.Vancheri C, Gauldie J, Bienenstock J, Cox G, Scicchitano R, Stanisz A, Jordana M. Human lung fibroblast-derived granulocyte-macrophage colony stimulating factor mediates eosinophil survival in vitro. Am J Respir Cell Mol Biol. 1989;1:289–95. doi: 10.1165/ajrcmb/1.4.289. [DOI] [PubMed] [Google Scholar]
- 11.Birkland T, Cheavens M, Pincus S. Human eosinophils stimulate DNA synthesis and matrix production in dermal fibroblasts. Arch Dermatol Res. 1994;286:312–8. doi: 10.1007/BF00402221. [DOI] [PubMed] [Google Scholar]
- 12.Halttunen T, Marttinen A, Rantala I, Kainulainen H, Maki M. Fibroblasts and transforming growth factor B induce organization and differentiation of T84 human epithelial cells. Gastroenterology. 1996;111:1252–62. doi: 10.1053/gast.1996.v111.pm8898639. [DOI] [PubMed] [Google Scholar]
- 13.Hernnas J, Sarnstrand B, Lindroth P, Peterson CG, Venge P, Malmstrom A. Eosinophil cationic protein alters proteoglycan metabolism in human lung fibroblasts. Eur J Cell Biol. 1992;59:352–63. [PubMed] [Google Scholar]
- 14.Owen WF, Petersen J, Austen KF. Eosinophils altered phenotypically and primed by culture with granulocyte leukotriene C4 response to FMLP. J Clin Invest. 1991;87:1958–63. doi: 10.1172/JCI115222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochester CL, Ackerman SJ, Zheng T, Elias JA. Eosinophil–fibroblast interactions: granule major basic protein interacts with IL-1 and transforming growth factor-β in the stimulation of lung fibroblasts IL-6-type cytokine production. J Immunol. 1996;156:4449–56. [PubMed] [Google Scholar]
- 16.Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA, Mueller C. Expression of interleukin 8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 17.Hinterleitner TA, Saada JI, Berschneider HM, Powell DW, Valentich JD. IL-1 stimulates intestinal myofibroblast COX gene expression and augments activation of CL-secretion in T84 cells. Am J Physiol. 1996;271:C1262–8. doi: 10.1152/ajpcell.1996.271.4.C1262. [DOI] [PubMed] [Google Scholar]
- 18.Varga JA, Olsen Herhal J, Constantine G, Rosenbloom J, Jimenez S. Interferon-gamma reverses the stimulation of collagen but not fibronectin gene expression by transforming growth factor-beta in normal human fibroblasts. Eur J Clin Invest. 1990;20:487–93. doi: 10.1111/j.1365-2362.1990.tb01890.x. [DOI] [PubMed] [Google Scholar]
- 19.Miltenyi S, Muller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–8. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 20.Plager D, Loegering D, Weiler D, et al. A novel and highly divergent homology of human eosinophil granule major basic protein. J Biol Chem. 1999;274:14464–73. doi: 10.1074/jbc.274.20.14464. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Ghazaleh RI, Fujisawa T, Mestecky J, Kyle RA, Gleich GJ. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–400. [PubMed] [Google Scholar]
- 22.Kubo H, Loegering D, Tohda Y, et al. Discordant and anomalous results among cytotoxicity assays: the confounding properties of eosinophil granule major basic protein on cell viability assays. J Immunol Methods. 1999;227:1–15. doi: 10.1016/s0022-1759(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 23.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366. [PubMed] [Google Scholar]
- 24.Coyle AJ, Ackerman SJ, Burch R, Proud D, Irvin CG. Human eosinophil-granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. J Clin Invest. 1995;95:1735–40. doi: 10.1172/JCI117850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowichik A, Weinberg A. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Modern Pathol. 1996;9:110–4. [PubMed] [Google Scholar]
- 26.Colombel JF, Torpier G, Janin A, Klein O, Cortot A, Capron M. Activated eosinophils in adult coeliac disease; evidence for a local release of major basic protein. Gut. 1992;33:1190–4. doi: 10.1136/gut.33.9.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvorak AM, Osage JE, Monahan RA, Dickersin GR. Crohn's disease: transmission electron microscopic studies. III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum Pathol. 1980;11:620–34. doi: 10.1016/s0046-8177(80)80073-6. [DOI] [PubMed] [Google Scholar]
- 28.Rabb Y, Fredens K, Gerdin B, Hallgren R. Eosinophil activation in ulcerative colitis: studies on mucosal release and localization of eosinophil granule constituents. Dig Dis Sci. 1998;43:1061–70. doi: 10.1023/a:1018843104511. [DOI] [PubMed] [Google Scholar]
- 29.Talley NJ, Kephart GM, McGovern TW, Carpenter HA, Gleich GJ. Deposition of eosinophil granule major basic protein in eosinophilic gastroenteritis and celiac disease. Gastroenterol. 1992;102:137–45. doi: 10.1016/0016-5085(92)91106-e. [DOI] [PubMed] [Google Scholar]
- 30.Walsh S, Antonioli D, Goldman H, Fox V, Bousvaros A, Leichtner A, Furuta G. Allergic esophagitis in children: a clinicopathologic entity with allergic features. Am J Surg Pathol. 1999;23:390–6. doi: 10.1097/00000478-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Wardlaw AJ, Kay AB. The role of the eosinophil in the pathogenesis of asthma. Allergy. 1987;42:321–35. doi: 10.1111/j.1398-9995.1987.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 32.Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis—comparison with onchocerciasis. N Engl J Med. 1985;313:282–5. doi: 10.1056/NEJM198508013130502. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak AM, McLeod RS, Monahan-Earley RA, et al. Ultrastructural evidence for piecemeal and anaphylactic degranulation of human gut mucosal mast cells in vivo. Int Arch Allergy Immunol. 1992;99:74–83. doi: 10.1159/000236338. [DOI] [PubMed] [Google Scholar]
- 34.Bischoff S, Grabowsky J, Manns M. Quantification of inflammatory mediators in stool samples of patients with inflammatory bowel disorders and controls. Dig Dis Sci. 1997;42:394–403. doi: 10.1023/a:1018886423475. [DOI] [PubMed] [Google Scholar]
- 35.Frigas E, Loegering DA, Solley GO, Farrow GM, Gleich GJ. Increased levels of eosinophil granular major basic protein in the sputum of patients with bronchial asthma. Mayo Clin Proc. 1981;56:345–53. [PubMed] [Google Scholar]
- 36.Levy AM, Gleich GJ, Sandborn WJ, Tremaine WJ, Steiner BL, Phillips SF. Increased eosinophil granule proteins in gut lavage fluid from patients with inflammatory bowel disease. Mayo Clin Proc. 1997;72:117–23. doi: 10.4065/72.2.117. [DOI] [PubMed] [Google Scholar]
- 37.Pincus SH, Ramesh KS, Wyler DJ. Eosinophils stimulate fibroblast DNA synthesis. Blood. 1987;70:572–4. [PubMed] [Google Scholar]
- 38.Shock A, Rabe KF, Dent G, Chambers RC, Gray AJ, Chung KF, Barnes PJ, Laurent GJ. Eosinophils adhere to and stimulate replication of lung fibroblasts ‘in vitro’. Clin Exp Immunol. 1991;86:185–90. doi: 10.1111/j.1365-2249.1991.tb05793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzichini E, Pizzichini MMM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154:308–17. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 40.Butterfeld J, Weiler D, Peterson E, Gleich G, Leiferman K. Sequestration of eosinophil granule major basic protein in human mast cells. Lab Invest. 1990;62:77–86. [PubMed] [Google Scholar]
- 41.Coyle AJ, Perretti F, Manzini S, Irvin CG. Cationic protein-induced sensory nerve activation: role of substance P in airway hyperresponsiveness and plasma extravasation. J Clin Invest. 1994;94:2301–6. doi: 10.1172/JCI117594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hulsmann AR, Raatgeep HR, Hollander JCD, Bakker WH, Saxena PR, Jongste JCD. Permeability of human isolated airways increases after hydrogen peroxide and poly-l-arginine. Am J Respir Crit Care Med. 1996;153:841–6. doi: 10.1164/ajrccm.153.2.8564141. [DOI] [PubMed] [Google Scholar]
- 43.Abu-Ghazaleh R, Gleich G, Prendergast F. Interaction of eosinophil granule major basic protein with synthetic lipid bilayers: a mechanism for toxicity. J Membrane Biol. 1992;128:153–64. doi: 10.1007/BF00231888. [DOI] [PubMed] [Google Scholar]
- 44.Barker R, Gundel R, Gleich G, Checkel J, Loegering D, Pease L, Hamann K. Acidic polyamino acids inhibit human eosinophil granule major basic protein toxicity. J Clin Invest. 1991;88:798–805. doi: 10.1172/JCI115379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haskell MD, Moy JN, Gleich GJ, Thomas LL. Analysis of signaling events associated with activation of neutrophil superoxide anion production by eosinophil granule major basic protein. Blood. 1995;86:4627–37. [PubMed] [Google Scholar]


