Abstract
The expression of CR2 (CD21) by synovial B and T lymphocytes of patients suffering from various forms of arthritis was analysed with cytofluorometry and with reverse transcriptase-polymerase chain reaction. CR2 (CD21) cell surface protein was detected in normal quantities on peripheral B cells, but was almost absent on synovial B lymphocytes of the same patients. This reduction was most severe in patients with rheumatoid arthritis, but also observed in all other cases. CR2 (CD21) did not reappear after in vitro culture. CR2 (CD21) mRNA was also strongly reduced in synovial B and T lymphocytes. Synovial fluid B lymphocytes were larger than peripheral blood B lymphocytes, while T cells from the same patients showed no size differences. We conclude that synovial fluid B lymphocytes have undergone an irreversible step towards terminal differentiation. The presence or absence of CR2 (CD21) mRNA in peripheral versus synovial T cells indicates that CR2 (CD21) is also differentially expressed by T lymphocytes.
Keywords: rheumatoid arthritis, CR2 (CD21), synovial lymphocytes, complement receptor type 2
Introduction
Rheumatoid arthritis (RA) has a prevalence of about 1% in the human population and is accompanied by an increased mortality [1,2]. The disease is characterized by signs of local inflammation, chronic cartilage and joint destruction and immunological abnormalities such as the occurrence of rheumatoid factors and other autoantibodies. Heavy joint destruction is observed together with changes in the synovial lining layer, which increases in size. This pannus tissue consists of a variety of cell types including fibroblasts, macrophages and lymphocytes which together sustain the chronic cartilage and bone erosion [3]. Most of the cells recruited into the rheumatoid joint are of haematopoietic origin. The increase in cellularity is accompanied by angiogenesis in the synovial tissue, thus increasing delivery of cells and molecules to areas of inflammation [4].
B cells can proliferate and differentiate in the rheumatoid joint. The synovial tissue B lymphocytes undergo selection by antigen in local structures similar to germinal centres [5,6] and display mainly IgG or IgA as antigen-specific receptors [7,8]. Compared with peripheral blood cells the synovial B cell population contains relatively more cells specific for local autoantigens, including collagen type II and rheumatoid factors (RF) and fewer cells specific for foreign antigens [8]. Using a potential polyclonal activation system we showed before that only a small proportion of synovial B cells could be activated to proliferation and differentiation. We speculated that part of the population would not be expanded in vitro, because they were already further differentiated in vivo [8]. Now we analysed the differentiation state of synovial B cells with antibodies specific for B cell surface proteins and detected a strong reduction in the expression of the complement receptor type 2 (CR2 (CD21)), which is known to disappear when B cells differentiate into plasma cells.
PATIENTS and METHODS
Patients and healthy blood donors
Synovial fluid (SF) and peripheral blood (PB) were obtained from patients with various rheumatic diseases (Table 1) treated in the department of Rheumatology and Clinical Immunology at the University Hospital Freiburg. In all instances synovial tapping was therapeutically indicated and patients gave their informed consent. Altogether, we examined lymphocytes from SF and PB from 49 patients with inflammatory joint diseases. Twenty-one patients fulfilled the ACR criteria [2] for RA, 18 were classified as reactive arthritis (ReA), five patients suffered from psoriasis arthritis (PA), one patient had adult onset Still's disease, one ankylosing spondylitis (AS) and three were unclassified. SFL and PBL of all patients were examined for CR2 (CD21) surface expression, but due to the limited number of SFL, not all patients were included in other studies. In addition, PBL were obtained from healthy blood donors (HD) (laboratory personal).
Table 1.
Study subjects
| Disease | n* | F/M | Age (years) |
|---|---|---|---|
| Rheumatoid arthritis | 21 | 12/9 | 53 ± 16 |
| Reactive arthritis | 18 | 4/14 | 40 ± 16 |
| Psoriasis arthritis | 5 | 2/3 | 26 ± 8 |
| Still's disease | 1 | 1/0 | 32 |
| Ankylosing spondylitis | 1 | 0/1 | 40 |
| Unclassified | 3 | 2/1 | 29, 69, 80 |
The total numbers of patients (n), numbers of female and male patients (F/M) and their age (mean ±s.d.) are listed.
Preparation of mononuclear cells
PB and SF samples from patients and HD were diluted 1:2 in PBS and mononuclear cells (MNC) purified by Ficoll–Hypaque density gradient centrifugation (Pharmacia, Uppsala, Sweden) according to standard protocols.
Cytofluorometry
Anti-CR2 (CD21) MoAb BL13–FITC was purchased from Immunotech (Hamburg, Germany), anti-CR2 (CD21) MoAb BU33-FITC from Harlan Sera-lab (Belton, UK) and CD19–PE from Dakopatts (Glostrup, Denmark). Anti CD4–PE and anti-CD8–FITC antibodies were purchased from Dakopatts and Medac (Hamburg, Germany), respectively. Surface expression of CR2 (CD21) and CD19 was measured by cytofluorometry using a FACScan (Becton Dickinson, Mountain View, CA). Scatter profiles were used to gate for lymphocytes. For quantitative comparisons of CR2 (CD21) expression we gated on CD19+ cells. All data were analyzed using Cell Quest software (Becton Dickinson).
Cell cultures
MNC from PB or SF were cultured in vitro for up to 48 h in standard medium with 5% fetal calf serum (FCS).
Preparation of RNA and cDNA synthesis
Single-cell suspensions were washed in ice-cold PBS and taken up in denaturing solution at 107 cells/ml. Denaturing solution contained 4 m guanidinium isothiocyanate, 25 mm sodium citrate pH 7, 0·1 m 2-mercaptoethanol (2-ME), 0·5% sodium lauroyl sarcosinate. The cells were passed ten times through a 20 × 11/2 gauge needle and kept on ice for 15 min to allow complete solubilization and denaturation of proteins. The RNA was prepared by phenol extraction and isopropanol precipitation. To synthesize cDNA 10 μg of RNA were incubated with Superscript II (Gibco BRL, Eggenstein, Germany) for 1 h at 42°C. Each sample was checked with GAPDH-specific oligonucleotides for successful cDNA synthesis.
Reverse transcriptase-polymerase chain reaction
To analyse the expression of CR2 (CD21) mRNA we performed reverse transcriptase-polymerase chain reaction (RT-PCR) as described elsewhere [9]. In brief, a region surrounding the transmembrane domain was amplified under the following optimized PCR conditions: 95°C 20 min, 57°C 60 min, 72°C 60 min, 30 cycles, 0·1 U Taq polymerase/reaction. The primers were 5′ to 3′: GGA ACC TGG AGC CAA CCT GCC (21S2761) and CTG GGC TCC CAT CTT TAC CAT (21R3360).
Results
B and T lymphocytes in SF and blood of rheumatic patients
SF samples of all patients examined contained 0·25–0·5% CD19+ B cells within the lymphocyte gate, while CD4+ or CD8+ T cells amounted to >90%. The percentages of B lymphocytes in the PB of these patients were comparable to those of HD (1–11%).
Reduced surface expression of CR2 (CD21) on B lymphocytes from SF
To study the differentiation stage of SF B lymphocytes in RA patients, we analysed several cell surface markers, using specific MoAbs and flow cytometry analysis. For most, no difference between PB and SF B lymphocytes was detected (not shown). In contrast, the expression of CR2 (CD21) was clearly diminished on SF B lymphocytes compared with PB B lymphocytes taken from the same patient at the same time (Fig. 1). Following these early observations, a total of 49 patients entering the clinics with various diagnoses (Table 1) and requiring synovial tapping were examined for the cell surface expression of CR2 (CD21) on their SF and PB B lymphocytes. In all cases the surface expression of CR2 (CD21) was reduced or even absent on SF B lymphocytes, irrespective of the type of arthritis, the age or gender of the patient, and the type of treatment (Fig. 1a,b). T lymphocytes from both compartments studied at the same time did not express detectable amounts of CR2 (CD21) on their surface (not shown).
Fig. 1.
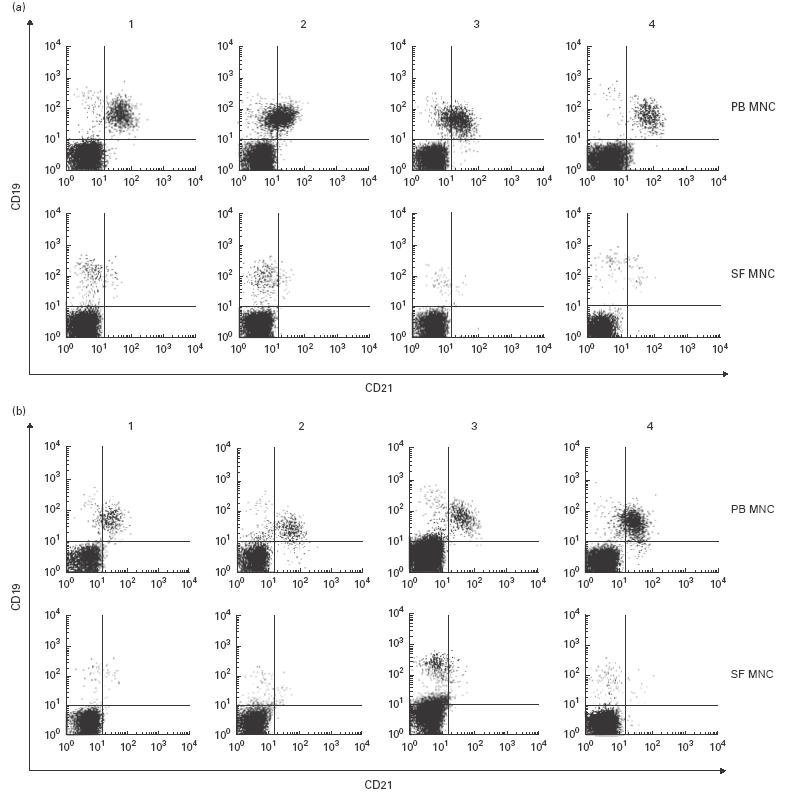
Expression of CR2 (CD21) on the surface of peripheral blood (PB) and synovial fluid (SF) B lymphocytes from patients with rheumatoid arthritis (RA) or reactive arthritis (ReA). PB mononuclear cells (MNC) and SF MNC were stained with anti-CD19–PE and anti-CR2 (CD21)–FITC MoAbs. Depicted are eight representative examples of lymphocytes from RA patients (a) and patients with ReA (b).
Quantitative comparison of the reduction in CR2 (CD21) surface expression
The degree of CR2 (CD21) reduction differed from patient to patient (Fig. 1). For a quantitative comparison we therefore measured the means of the fluorescence intensities (MFI) of the CR2 (CD21) expression in PB and SF B lymphocytes, and calculated the ratios between these values (MFIPB/MFISF). These ratios allowed the comparison of data from different patients as well as from different dates. On average, MFI ratios were slightly higher in RA patients (3·5 ± 0·8 (mean ±s.d.); n = 15) than in non-RA patients (3·0 ± 0·7; 17 cases of ReA), without being statistically significant (Fig. 2).
Fig. 2.
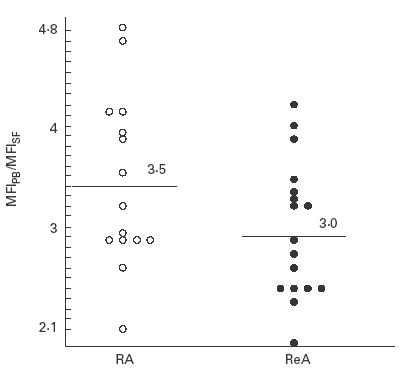
Ratios of the mean fluorescence intensities (MFI) of CR2 (CD21) on CD19+ peripheral blood (PB) and synovial fluid (SF) B lymphocytes. Individual values are given for patients with rheumatoid arthritis (RA; •) or reactive arthritis (ReA; ○). MFI averages of CR2 (CD21) expression (black bars) on PB B lymphocytes were 3·5 ± 0·8 (RA) and 3·0 ± 0·7 (ReA).
Stability of low CR2 (CD21) expression by synovial B lymphocytes
SF, especially when derived from the inflamed joint, differs in its composition from PB, both in quality and quantity of various soluble factors, including enzymes and cytokines. The reduction of CR2 (CD21) on synovial B lymphocytes therefore could be due to a site-specific effect brought about by the microenvironment of the inflamed synovium, e.g. digestion of parts of the molecule by a protease. In this case, removal of SF could be expected to result in re-appearance of CR2 (CD21) on the B cell surface. However, culturing synovial and peripheral B lymphocytes for 2 days under standard tissue culture conditions, i.e. in medium containing no SF, did not change their expression of CR2 (CD21) (Fig. 3). Also, the addition of IL-2 or IL-4 did not change their phenotype (data not shown).
Fig. 3.
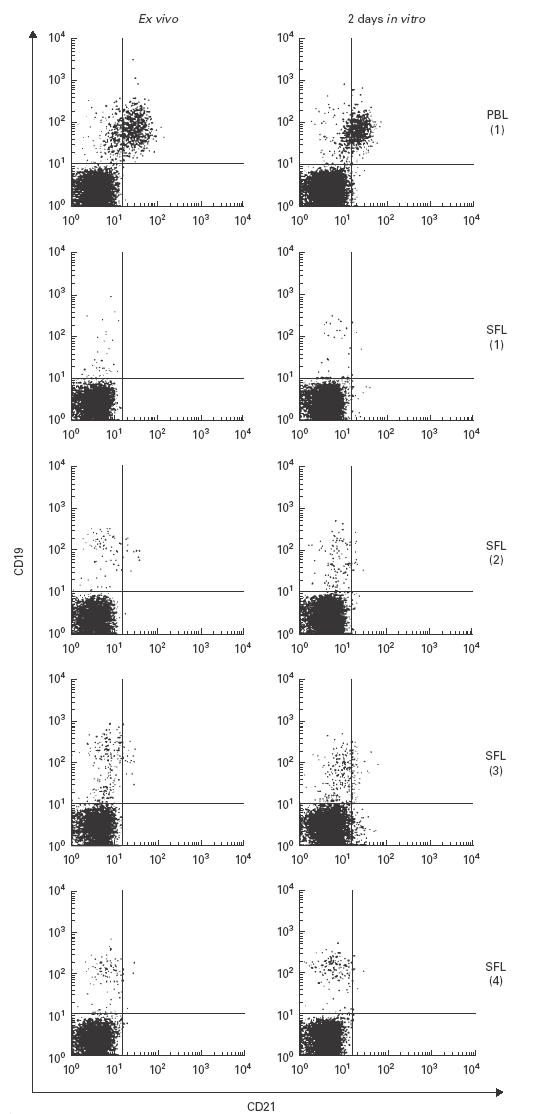
(See previous page.) Stability of CR2 (CD21) expression by synovial and peripheral B lymphocytes in vitro. Peripheral blood (PB) mononuclear cells (MNC) and synovial fluid (SF) MNC of rheumatoid arthritis (RA) patients were analysed directly after isolation, or after in vitro culture for 48 h in medium containing 5% fetal calf serum.
Analysis of CR2 (CD21) mRNA
Recently we published that peripheral blood CD4+ and CD8+ T cells express amounts of CR2 (CD21) mRNA similar to CD19+ B lymphocytes [10]. In contrast, CR2 (CD21) protein could only be detected on the surface of B lymphocytes, but not on the surface of T lymphocytes [10]. To compare the expression of CR2 (CD21) mRNA in SF- and PB-MNC, these cells were isolated from five patients with different forms of arthritis, mRNA and cDNA prepared and subjected to RT-PCR using CR2 (CD21)-specific primers. Interestingly, CR2 (CD21) mRNA was strongly reduced in the mRNA derived from SF MNC compared with the mRNA derived from PB MNC from the same patient (Fig. 4). As most SF MNC are T lymphocytes and only few B lymphocytes, the effect seen is probably due to a reduction in the expression of T lymphocyte-derived CR2 (CD21) mRNA. In addition, B lymphocytes may also be affected. At this point we did not however attempt to separate these populations in order to prove the involvement of both types of lymphocytes.
Fig. 4.
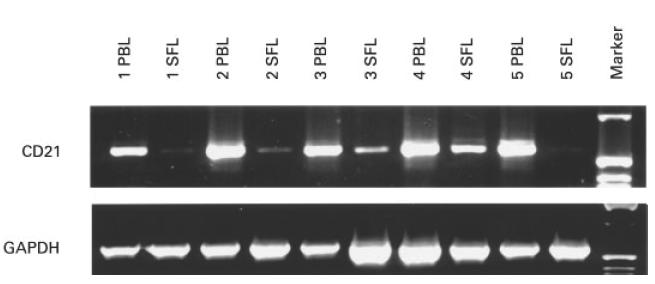
Reduction of CR2 (CD21) mRNA in synovial but not peripheral blood lymphocytes of the same patients. Equal amounts of RNA were reverse transcribed into cDNA and used to amplify the transmembrane region of CR2 (CD21). GAPDH primers were used to show that equal amounts of cDNA were used for each polymerase chain reaction. The patients suffered from rheumatoid arthritis (lanes 1 and 5), reactive arthritis (lane 2), psoriasis arthritis (lane 3) or unclassified polyarthritis (lane 4).
Advanced differentiation state of SF B lymphocytes
Is the loss of CR2 (CD21) due to plasma cell differentiation? CR2 (CD21) is differentially expressed during B cell development, it appears, when pre-B cells develop into mature B cells, which stay CR2 (CD21)+. When mature B cells encounter antigen they differentiate into B cell blasts and further into antibody-secreting plasma cells which no longer express CR2 (CD21) [11]. Plasma cells can easily be discriminated from other types of B cells by their distinct morphology and size. Therefore, we analysed these parameters for SF and PB B lymphocytes (gated as CD19+ cells) by comparison of their forward scatter profiles. This analysis comprised 19 patients of our panel. In all cases we found a strong difference between the CD19+ cells from both compartments. Patients' SF B lymphocytes were significantly enlarged, while PB B lymphocytes were smaller (Fig. 5a) and indistinguishable in their forward scatter profile from PB B lymphocytes derived from HD. Forward scatter analysis of CD4+ and CD8+ T cells did not display such enlargement (Fig. 5b). Thus CD19+ B cells in the synovium represent a phenotype which could be best described as B cell blasts.
Fig. 5.
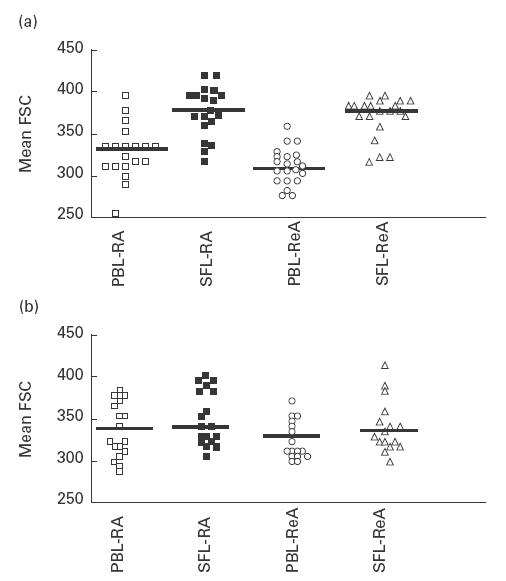
Size of synovial fluid B and T lymphocytes in comparison with peripheral blood (PB) cells. Mean forward scatters of lymphocytes expressing either CD19 (a) or CD4 or CD8 (b) were calculated. (a) Peripheral blood and synovial B cells from rheumatoid arthritis (RA) patients (□ and ▪, respectively) and reactive arthritis (ReA) patients (○ and Δ, respectively) are depicted. (b) Mean forward scatter from RA patients' CD4+ and CD8+ T cells from PB (□ and ○) and synovial fluid (▪ and Δ, respectively) display the same average.
Discussion
We have shown that the CR2 (CD21) glycoprotein is strongly reduced on synovial B cells of arthritis patients while still being present on the PB B cells of the same patients. Moreover, this reduction in surface CR2 (CD21) glycoprotein is accompanied by the reduction of CR2 (CD21) mRNA as revealed by RT-PCR analysis. Recently we have shown that normal PB CD19+ B cells, CD4+ and CD8+ T cells express similar amounts of CR2 (CD21) mRNA, although T cells are lacking CR2 (CD21) surface expression [11]. Because B cells are a minor fraction of the synovial lymphocytes (< 0·5%), the reduction of the CR2 (CD21) mRNA can only be explained by the lack of these transcripts in synovial T cells as well. The small B cell numbers did not allow to use quantitative PCR or Northern blotting to discriminate between the CR2 (CD21) mRNA content in B and T cells in our samples. Therefore we can not formally conclude that CR2 (CD21) mRNA is also reduced in the synovial B cells.
Expression of CR2 (CD21) on B cells is found only on mature B cells, but not on immature or on plasma B cells [10]. CR2 (CD21) plays a pivotal role in the activation and proliferation of B cells and is a prerequisite for T-dependent immune responses. Engagement of CR2 (CD21) with the B cell receptor lowers the threshold required for activation of B cells by antigen and is involved in T-dependent immunity [12–18]. According to these results one can suppose that SF B cells would need high-affinity antigen to be further activated via the B cell receptor, because the CR2 (CD21) co-receptor is lacking. Alternatively, synovial B cells might have undergone antigen-dependent selection and are prone to terminal differentiation.
Recent studies in mice have demonstrated that CR2/CR1 (CD21/CD35) is involved in the induction of anergy and peripheral tolerance. In anti-hen-egg lysozyme (HEL) immunoglobulin/sHEL double transgenic mice crossed to CR2 (CD21)-deficient mice the induction of anergy to HEL-binding self-reactive B cells required expression of CR2/CR1 (CD21/CD35). These mice showed a strong reduction in tolerance as the HEL-specific B cells matured and accumulated in the peripheral lymphoid compartment. In further experiments CR2 (CD21)/35-deficient mice were crossed into Fas-deficient lpr/lpr mice developing spontaneously a mild form of lupus-like disease. The deficiency of CR2/CR1 (CD21/CD35) in lpr/lpr mice resulted in increased lymphadenopathy, antinuclear and anti-dsDNA autoantibodies, increased renal immunoglobulin deposits and glomerulonephritis [19,20].
To account for the CR2 (CD21) deficiency on B cells from the SF of patients with arthritis, two mechanisms can be considered. First, CR2 (CD21) can be down-regulated upon contact with immune complexes, autoantibodies or by proteases. Second, the synovial B cells might represent a specific step in B cell differentiation towards the generation of plasma cells and/or memory B cells. With respect to the first possibility, immune complexes bearing C3 fragments are present in the SF [21] of patients suffering from RA. These immune complexes may interact via C3d with CR2 (CD21) and cause the removal of CR2 (CD21) from the cell surface. Autoantibodies directed against CR2 (CD21) have been observed in the SF [22]. Alternatively CR2 (CD21) may be shed from the surface by means of a cell-bound or soluble protease. Proteolytic activities which shed CR2 (CD21) and lead to a soluble form of the molecule have been demonstrated [23,24]. When we cultured synovial B cells devoid of CR2 (CD21) for 2 days in the absence of SF and growth factors, the CR2 (CD21)− phenotype remained stable. This suggests that the reduction of CR2 (CD21) on SF B cells is not a transient phenotype mediated by immune complexes, autoantibodies or soluble proteases.
Studies of the ontogeny of B cells showed that terminal differentiation is accompanied by the loss of CR2 (CD21) expression [10]. The possibility that synovial B cells are more differentiated is supported by our findings that synovial B cells are enlarged compared with peripheral blood B cells from the same patients. Furthermore synovial B cells have been shown to express several different immunoglobulin isotypes [8,25,26]. Immunoglobulin class switch recombination characterizes B cells, which have encountered antigen and have been selected in germinal centres [27,28]. B cells enter the synovium through vessels in the pannus tissue. In order to reach the articular lumen they have to cross this tissue. Analysis of the V gene repertoire of SF B cells has suggested that they are selected by antigen because of the restricted repertoire of the V elements and common length of CDR3 regions they use [29], and more recently a germinal centre type of reaction has been demonstrated for B cells of the pannus tissue [5]. The differentiation of mature B cells towards plasma cells might have as many steps as the differentiation of precursor B cells into mature cells [30,31]. It should be possible to define these steps of plasma cell differentiation by differential gene expression. The lack of CR2 (CD21) and the enlargement of the B cells, while CD19 is still present, might represent such a step towards terminal differentiation.
The expression of CR2 (CD21) glycoprotein in T cells might be similarly regulated as during B cell differentiation. CR2 (CD21) has been reported to be expressed on the surface of a subfraction of PB T lymphocytes and on thymocytes. In addition, Epstein–Barr virus (EBV) can infect thymocytes and T cells [32–38]. CR2 (CD21) mRNA was shown to be expressed in CD4+ and CD8+ PB T cells to a similar extent as in B cells [11]. Although our staining protocol failed to detect CR2 (CD21) on the surface of T cells, others have described low levels of CR2 (CD21) on a fraction of PB T cells [32]. Recently Prodinger et al. [38] described a new complex of CR2 (CD21) emerging on T cells after CR2 (CD21) ligation. There are currently no other data supporting a functional role of CR2 (CD21) expression in T cells. Our data are the first to describe the reduction of CR2 (CD21) mRNA in T cells and may enable us to investigate a possible function in relation to activation and differentiation processes.
In conclusion, we show that patients suffering from arthritic diseases have reduced CR2 (CD21) expression in synovial but not peripheral blood lymphocytes. We speculate that the reduction of CR2 (CD21) might be involved in the autoimmune-related progression of the arthritic diseases.
Acknowledgments
We like to thank Dr H. Eibel for critical reading of the manuscript and helpful discussion during the experimental work. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Me604/4) to I.M. and a fellowship of the Hans-Hench-Stiftung to M.B.
References
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.Arnett FC, Edworthy SM, Block DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 3.Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37:783–9. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 5.Schröder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93:221–5. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randen I, Mellbye OJ, Førre O, Natvig JB. The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand J Immunol. 1995;41:481–6. doi: 10.1111/j.1365-3083.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]
- 7.Munthe E, Natvig JB. Immunoglobulin classes, subclasses and complexes of IgG rheumatoid factor in rheumatoid plasma cells. Clin Exp Immunol. 1972;12:55–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Rudolphi U, Rzepka R, Batsford S, et al. The B cell repertoire of patients with rheumatoid arthritis. II. Increased frequencies of IgG+ and IgA+ B cells specific for mycobacterial heat-shock protein 60 or human type II collagen in synovial fluid and tissue. Arthritis Rheum. 1997;40:1409–19. doi: 10.1002/art.1780400808. [DOI] [PubMed] [Google Scholar]
- 9.Illges H, Braun M, Peter HH, Melchers I. A plasmid for the quantification of the CD21 human interferon-alpha receptor expression by PCR. Eur Cyt Net. 1996;7:375–6. [PubMed] [Google Scholar]
- 10.Braun M, Peter HH, Melchers I, Illges H. Human B and T lymphocytes have similar amounts of CD21-mRNA, but differ in surface expression of the CD21-glycoprotein. Int Immunol. 1998;10:1197–202. doi: 10.1093/intimm/10.8.1197. [DOI] [PubMed] [Google Scholar]
- 11.Tedder TF, Clement LT, Cooper MD. Expression of C3d receptors during human B cell differentiation: immunofluorescence analysis with the HB-5 monoclonal antibody. J Immunol. 1984;133:678–83. [PubMed] [Google Scholar]
- 12.Tedder TF, Inaoki M, Sato S. The CD19–CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–18. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 13.Carter RH, Spycher MO, Ng YC, Hoffman R, Fearon DT. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J Immunol. 1988;141:457–63. [PubMed] [Google Scholar]
- 14.Ahearn JM, Fischer MB, Croix D, et al. Disruption of the CR2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–62. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 15.Croix DA, Ahearn JM, Rosengard AM, et al. Antibody response to a T-dependent antigen requires B cell expression of complement receptors. J Exp Med. 1996;183:1857–64. doi: 10.1084/jem.183.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina H, Holers VM, Li B, et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–61. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–49. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 18.Mongini PK, Vilensky MA, Highet PF, Inman JK. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon co-ligation of the antigen receptor with CD21 (CR2) J Immunol. 1997;159:3782–91. [PubMed] [Google Scholar]
- 19.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–68. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 20.Prodeus AP, Goerg S, Shen LM, et al. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–31. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 21.McDuffie FC. Immune complexes in the rheumatic diseases. J Allergy Clin Immunol. 1978;62:37–43. doi: 10.1016/0091-6749(78)90071-4. [DOI] [PubMed] [Google Scholar]
- 22.Barel M, Kahan A, Charriaut-Marlangue C, Frade R. Autoantibodies against gp140, the Epstein–Barr virus and C3d receptor in sera from rheumatoid arthritis patients. Eur J Immunol. 1986;16:1357–61. doi: 10.1002/eji.1830161108. [DOI] [PubMed] [Google Scholar]
- 23.Ling N, Hansel T, Richardson P, Brown B. Cellular origins of serum complement receptor type 2 in normal individuals and in hypogammaglobulinaemia. Clin Exp Immunol. 1991;84:6–22. doi: 10.1111/j.1365-2249.1991.tb08117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fremeaux-Bacci V, Fischer E, Kazatchkine MD. Membrane and soluble forms of CD21 (the C3dg/EBV receptor) Immunol Letters. 1996;54:201–4. doi: 10.1016/s0165-2478(96)02673-9. [DOI] [PubMed] [Google Scholar]
- 25.Outschoorn I, Rowley MJ, Cook AD, Mackay IR. Subclasses of immunoglobulins and autoantibodies in autoimmune diseases. Clin Immunol Immunopathol. 1993;66:59–66. doi: 10.1006/clin.1993.1008. [DOI] [PubMed] [Google Scholar]
- 26.Berek C, Kim HJ. B cell activation and development within chronically inflamed synovium in rheumatoid and reactive arthritis. Semin Immunol. 1997;9:261–8. doi: 10.1006/smim.1997.0076. [DOI] [PubMed] [Google Scholar]
- 27.Lindhout E, Koopman G, Pals ST, de Groot C. Triple check for antigen specificity of B cells during germinal centre reactions. Immunol Today. 1997;18:573–7. doi: 10.1016/s0167-5699(97)01160-2. [DOI] [PubMed] [Google Scholar]
- 28.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–26. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 29.Bridges SLJ, Lavelle JC, Lee SK, Byer S, Schroeder HWJ. CDR3 fingerprinting of immunoglobulin kappa light chains expressed in rheumatoid arthritis. Evidence of antigenic selection or dysregulation of gene rearrangement in B cells. Ann NY Acad Sci. 1997;815:423–6. doi: 10.1111/j.1749-6632.1997.tb52093.x. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa K, Li YS, Wasserman R, Sauder S, Shinton S, Hardy RR. B lymphocyte developmental lineages. Ann NY Acad Sci. 1997;815:15–29. doi: 10.1111/j.1749-6632.1997.tb52041.x. [DOI] [PubMed] [Google Scholar]
- 31.Osmond DG, Rolink A, Melchers F. Murine B lymphopoiesis: towards a unified model. Immunol Today. 1998;19:65–68. doi: 10.1016/s0167-5699(97)01203-6. [DOI] [PubMed] [Google Scholar]
- 32.Fingeroth JD, Clabby ML, Strominger JD. Characterization of a T lymphocyte Epstein–Barr virus/C3d receptor (CD21) J Virol. 1988;62:1442–7. doi: 10.1128/jvi.62.4.1442-1447.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsoukas CD, Lambris JD. Expression of CR2/EBV receptors on human thymocytes detected by monoclonal antibodies. Eur J Immunol. 1988;8:299–302. doi: 10.1002/eji.1830180823. [DOI] [PubMed] [Google Scholar]
- 34.Delibrias CC, Mouhoub A, Fischer E, Kazatchkine MD. CR1 (CD35) and CR2 (CD21) complement C3 receptors are expressed on normal human thymocytes and mediate infection of thymocytes with opsonized human immunodeficiency virus. Eur J Immunol. 1994;24:2784–8. doi: 10.1002/eji.1830241131. [DOI] [PubMed] [Google Scholar]
- 35.Watry D, Hedrick JA, Siervo S, et al. Infection of human thymocytes by Epstein–Barr virus. J Exp Med. 1991;173:971–80. doi: 10.1084/jem.173.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy E, Ambrus J, Kahl L, Molina H, Tung K, Holers VM. T lymphocyte expression of complement receptor 2 (CR2/CD21): a role in adhesive cell–cell interactions and dysregulation in a patient with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1992;90:235–44. doi: 10.1111/j.1365-2249.1992.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikuta H, Taguchi Y, Tomizawa K, et al. Epstein–Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature. 1988;333:455–7. doi: 10.1038/333455a0. [DOI] [PubMed] [Google Scholar]
- 38.Prodinger WM, Larcher C, Schwendinger M, Dierich MP. Ligation of the functional domain of complement receptor type 2 (CR2, CD21) is relevant for complex formation in T cell lines. J Immunol. 1996;156:2580–4. [PubMed] [Google Scholar]


