Abstract
This study provides evidence that cardiolipin (CL) molecules are expressed on the surface of apoptotic cells and are recognized by antiphospholipid antibodies, purified from patients with the antiphospholipid antibody syndrome (APS). CL expression on cell surface was demonstrated by high performance thin layer chromatography analysis of phospholipids from plasma membrane purified fractions and by the positive staining with the CL-specific dye nonyl-acridine orange. This finding was complemented with the observation that aCL IgG purified from patients with APS bind to the surface of apoptotic cells. This staining shows a clustered distribution mostly localized on surface blebs. Interestingly, CL exposure on the cell surface preceded the DNA fragmentation, as shown by cytofluorimetric analysis. These findings demonstrate that exposure of CL molecules on the cell plasma membrane is an early event of the apoptotic cellular program that may represent an in vivo trigger for the generation of aCL.
Keywords: cardiolipin, apoptosis, anticardiolipin antibodies, antiphospholipid antibody syndrome
Introduction
Programmed cell death (PCD) or apoptosis, a widespread phenomenon that concerns any nucleated cell type, is a cardinal element of immune system homeostasis [1,2]. Apoptosis may be defined morphologically and biochemically [3]. In most cases, PCD is accompanied by characteristic ultrastructural alterations (cell shrinkage, cytoplasmic compaction, membrane blebbing, nuclear chromatin condensation) [4] and internucleosomal ‘ladder-type’ DNA fragmentation [5,6]. Most cells undergoing apoptosis in vivo are removed by phagocytosis before the death process culminates in low-molecular-weight DNA fragmentation and apoptotic modification of nucleus morphology [7]. It has recently been suggested that phosphatidylserine (PS), which is normally almost totally confined to the inner leaflet of the plasma membrane, represents a membrane ‘flag’ on apoptotic cells [8,9] and thereby acts as recognition signal for phagocytosis [10]. Changes in the asymmetric distribution of PS precedes the loss of membrane integrity by several hours. The investigation of lipid topology in eukaryotic cells demonstrates a physiological asymmetrical distribution of phospholipids in cell membranes [11,12]. Phosphatidylcholine (PC) and sphingomyelin (SM) are almost exclusively located in the outer leaflet of the lipid bilayer, while phosphatidylserine (PS) and 70% of phosphatidylethanolamine (PE) are located in the inner leaflet of the plasma membrane. Cardiolipin (CL) is mostly confined to the mitochondrial membrane. This asymmetrical distribution, which results from transbilayer movements, can be perturbed, transiently or permanently, by a variety of events [13–15], including apoptosis [8,9].
Increased levels of apoptosis of MRL-lpr/lpr T lymphocytes [16], as well as of peripheral blood lymphocytes (PBL) from patients with systemic lupus erythematosus (SLE), have recently been reported [17]. Because of accelerated apoptosis, increased amounts of nucleosomes are released into the extracellular space. However, the role of apoptosis in SLE pathogenesis is still controversial. In apparent contrast with the observation of an increased rate of apoptosis in PBL, overexpression of bcl-2 has been described in T lymphocytes and related to disease activity [18]. In HIV infection, a correlation between serum antiphospholipid antibodies and the level of apoptosis has been suggested [19]. We recently developed a highly selective method for detection of antiphospholipid reactivity [20]. This method allows the selection of highly specific anticardiolipin antibodies (aCL) from sera of patients with antiphospholipid antibody syndrome (APS) [20]. Using this method, we show that affinity-purified highly specific aCL, isolated from patients with APS, target CL molecules exposed on the cell surface of apoptotic cells. Exposure of CL on plasma membrane of apoptotic cells may be a relevant contributing factor in vivo to the generation of aCL in the course of APS or HIV infection.
Materials and methods
Cells and reagents
Human promonocytic U937 cells were cultured in RPMI 1640 (Gibco-BRL, Life technologies Italia srl, Milano, Italy) containing foetal calf serum (FCS) 10% at 37°C in a humified 5% CO2 athmosphere. Apoptosis was induced by incubating the cells at a concentration of 5 × 105 cells/ml in serum-free medium supplemented with insulin (5 mg/l) and transferrin (5 mg/l), and by adding either Tumor Necrosis Factor α (TNFα, Genzyme Diagnostics, Cambridge MA, USA), 1000 IU/ml for 0 min, 30 min, 1 h, 2 h and 3 h), or anti-Fas (CD95) IgM mAb (Immunotech, Marseille, France), 100 ng/ml for 0 min, 30 min, 1 h, 2 h and 4 h.
Phospholipid analysis of isolated plasma membrane fractions
Plasma membrane fractions from U937 cells were isolated as previously reported [21]. Briefly, after homogenization, untreated and anti-FAS treated cells (100 ng/ml for 4 h) were sedimented at 200 000 g for 20 min and the pellet was resuspended in 65% sucrose. This resuspended pellet was throughly homogenized. It was then brought to a refractive index of 1·430 (68% w/v) with cold saturated sucrose. The sample (1·5 ml) was placed on the bottom of 15 × 90 mm cellulose nitrate tubes. Continuous 25–65% (w/v) density gradients of sucrose were formed above the particulate homogenate. The gradients were then centrifuged at 90 000 g for 16 h in a SW41 rotor. The membranes and organelles in the homogenate were separated into bands of differing density. The bands corresponding to the plasma membranes were removed and the material from these layers was diluted with 0·16 m NaCl and sedimented at 200 000 g for 40 min. The purity of plasma membrane preparations was evaluated by testing specific enzymatic activities [21]. Nucleotidase and alkaline phosphodiesterase I activities were considered as markers for the plasma membrane fraction and showed an approximately 45-fold enrichment compared to the whole homogenate. On the contrary, NADPH-cytochrome c reductase and glucose-6-phosphatase (lysosomal markers) showed a significant decrease of their specific activities in the plasma membrane compared to those in the initial homogenate.
The purity of plasma membrane preparation was inferred from the absence of marker activities for mitochondrial organelles. Cytochrome c oxidase (mitochondrial marker) activity was measured by following ferrocytochrome c oxidation at 550 nm in 1 ml of a medium containing plasma membrane fraction, 10 mm phosphate buffer (pH 7·4) and 50 µm ferrocytochrome c. The concentration of cytochrome c was determined using a molar extinction coefficient at 550 nm of 19 600 m−1 × cm−1 for the reduced form.
On the basis of these enzymatic activities analyses, the lysosomal and mitochondrial contaminations of the plasma membrane preparations were 4 ± 0·3% and 1·1 ± 0·1%, respectively. The virtual absence of mitochondrial contaminants in the plasma membrane preparation was confirmed by immunoblotting analysis, using an anticytochrome c monoclonal antibody (clone 7H8·2 CL2., Pharmingen, San Diego, CA, USA).
The pellets were normalized for protein content by Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA) and analysed for their phospholipid composition.
Phospholipids were extracted according to the technique described by Folch [22] and separated by thin layer chromatography, using high performance thin layer chromatography (HPTLC) silica gel 60 (10 × 10) plates (Merck, Darmstadt, Germany). Chromatography was performed in chloroform: methanol:acetic acid:water [100:75:7:4] (v:v:v:v). Phospholipids were stained by exposure to iodide vapours.
Scanning confocal microscopic analysis of 10-N-nonyl-acridine orange (NAO) fluorescence
Untreated and Fas-treated (100 ng/ml for 4 h) U937 cells were incubated with 45 µm 10-N-nonyl-acridine orange (Molecular Probes, Inc., USA) [23] for 15 min at room temperature and then analysed by scanning confocal microscopy. Images were acquired through confocal laser scanning microscope (Sarastro 2000, Molecular Dynamics, Sunnyvale, CA) adapted to a NIKON OPTIPHOT microscope (objective PLAN-APO 60/1·4 oil) and equipped with argon ion laser (25 mW). FITC was excited at 488 nm and laser power was set at 1 mW. Images were collected at 512 × 512 pixels with voxel dimensions 0·08 mm (lateral), 0·49 mm (axial). After having been processed with routines for noise filtering, serial optical sections were assembled in Depth-Coding mode. Acquisition and processing were carried out using Image Space software (Molecular Dynamics).
Antibodies
Human aCL (IgG), purified by affinity chromatography from five patients with the antiphospholipid antibody syndrome, according to the technique described elsewhere [24], were selected on the basis of their highly specific binding to CL, as demonstrated by TLC immunostaining, using graded concentrations of PS (1, 2, 5, 10 µg) and ELISA. No reactivity against PL other than CL such as PS, PC, PE and PI, or β2-glycoprotein I was found.
Adsorption tests with cardiolipin were performed according to the method described by Harris [25]. Briefly, the aCL (700 µg/ml, 280 µg/ml, 140 µg/ml or 70 µg/ml) were incubated (2:1, v:v) with CL or PS micelles (3 mg/ml) for 1 h at 37°C, in order to give a final concentration of phospholipid micelles of 1 mg/ml, and overnight at 4°C. The mixture was centrifuged for 15 min at 27 000 g at 4°C; the supernatants were kept as adsorbed antibody and used in immunofluorescence studies. The percent of inhibition is calculated with the following:
[MFI (mean fluorescence intensity) of adsorbed sample
− MFI of control secondary antibody
× 100]/[ MFI of unadsorbed sample
− MFI of control secondary antibody]
Affinity purified normal human IgG (from blood donors) were used as control.
Immunostaining on TLC plates
Immunostaining was performed as previously described [20], using 2 µg of five different phospholipid antigens: CL, PC, PE, PS and PI (Sigma Chemical Company, St Louis, MO, USA). Affinity purified IgG fraction (7 mg/ml) was diluted 1:100 in 0·5% (w/v) gelatin/PBS. Parallel blots were processed without first antibody or without antigen as control for nonspecific reactivity.
Evaluation of apoptosis
Apoptosis was measured both by morphologic analysis and by flow cytometry. PS inversion on apoptotic cell plasma membranes was evaluated by FITC-conjugated annexin V binding (Bender MedSystem Diagnostic, Wien, Austria), according to manufacturer's instructions. DNA fragmentation was studied by propidium iodide staining followed by flow cytometric analysis (EPICS Profile, Coulter Electronics, Hialeah, FL, USA) [26]. Cells were fixed with cold 70% ethanol in PBS for 1 h at 4°C. After centrifugation at 200 g for 10 min at 4°C, cells were washed once in PBS. The pellet was resuspended in 0·5 ml PBS, 50 µl of RNAse (Type I-A, Sigma, 10 mg/ml in PBS) was added, followed by 1 ml propidium iodide (Sigma, 100 µg/ml in PBS) solution. The cells were incubated in the dark at room temperature for 15 min and kept at 4°C until measured. A Trypan blue exclusion test was performed to evaluate the viability of the cultures.
Immunofluorescence and flow cytometric analysis
Indirect immunofluorescence was performed to analyse CL expression on the cell plasma membrane of U937 cells. One × 106 cells (untreated, Fas-treated and TNFα-treated, as reported above) were fixed in 4% formaldehyde/PBS for 1 h at 4°C. After washing three times with PBS, cells were incubated with human purified IgG aCL or, in parallel experiments with human purified IgG aCL previously adsorbed with CL or PS, in PBS/1%BSA, for 1 h at 4°C. Fluorescein isothiocyanate (FITC)-conjugated antihuman IgG (γ-chain specific, Sigma) were then added and incubated at 4°C for 30 min. After washing with PBS, fluorescence intensity was analysed with a Becton Dickinson cytometer (Becton Dickinson, Mountain View, CA, USA). Cells were gated on the basis of forward angle light scatter and 90° light scatter parameters.
Formaldehyde fixed untreated and Fas-treated (100 ng/ml for 4 h) cells, labelled with human purified IgG aCL and then with FITC-conjugated goat antihuman IgG were also analysed by scanning confocal microscopy, as reported above.
In separate parallel experiments, cells were directly stained with anti-CL Ab before fixing the cells with 4% formaldehyde in PBS. Alternatively, cells were processed for a second formaldehyde fixation immediately after the incubation with anti-CL Ab and before the addition of the secondary antibody. Both fixation procedures did not affect CL staining on the cell surface.
Results
Evidence for the appearance of CL molecules on isolated plasma membranes of U937 cells undergoing apoptosis
Plasma membranes of untreated and anti-FAS treated (100 ng/ml for 4 h) U937 cells were isolated and purified. Phospholipids were extracted and analysed by HPTLC (Fig. 1a). This method revealed the presence of PC, PE, PS, SM and, at to a lesser extent, PI comigrating bands in both untreated and anti-FAS treated cells. A CL comigrating band was clearly evident only in anti-FAS treated cell preparations. The identity of this band was confirmed by TLC immunostaining using the highly specific human aCL IgG (not shown). The purity of plasma membrane preparations was checked by testing specific enzymatic activities. As a control, the virtual absence of mitochondrial contaminants in the plasma membrane preparation was confirmed by immunoblotting analysis, using an anticytochrome c monoclonal antibody (Fig. 1b).
Fig. 1.
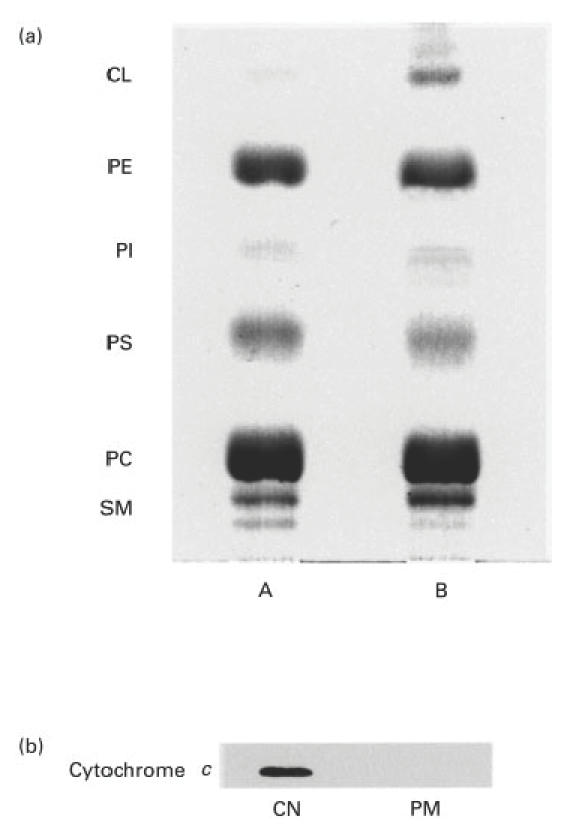
(a) HPTLC analysis of plasma membrane phospholipids in anti-Fas-treated U937 cells. Apoptosis was induced by incubating the cells (5 × 105 cells/ml), with anti-Fas (CD95) IgM mAb, 100 ng/ml for 4 h. Plasma membrane fractions from U937 cells were isolated by continuous 25–65% (w/v) density gradients of sucrose. Phospholipids were extracted according to the technique described by Folch [22] and separated by thin-layer chromatography, in chloroform: methanol: acetic acid: water (100:75:7:4) (v:v:v:v). Phospholipids were stained with iodide vapours. Lane A, phospholipids obtained from untreated U937 cells; lane B, phospholipids obtained from anti-Fas treated U937 cells. (b) Immunoblotting analysis of the cytochrome c (mitochondrial marker) contamination in plasma membrane preparation. Whole cell extract and the plasma membrane preparation were subjected to SDS-PAGE (12%), Western blot and immunodetection of cytocrome c using a specific monoclonal antibody. CN, whole cell extract; PM, plasma membrane preparation.
Distribution of CL on the plasma membrane of apoptotic U937 cells
Experiments with NAO, a dye which interacts stechiometrically with CL, were performed in order to confirm the appearance of CL molecules on the cell surface of apoptotic cells and to analyse CL distribution during apoptosis. Scanning confocal microscopic analysis of anti-Fas treated cells (100 ng/ml for 4 h) showed an uneven surface distribution of the staining mostly located in plasma membrane areas with apoptotic blebs. Cytoplasm was also extensively stained (Fig. 2b). As expected, untreated U937 cells showed a staining confined only to the cytoplasm without any cell surface staining (Fig. 2a).
Fig. 2.
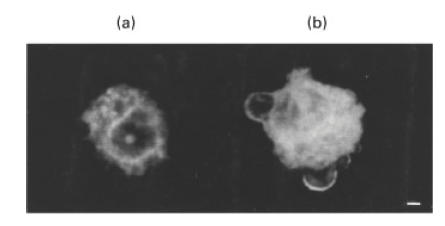
Scanning confocal analysis of NAO staining on the surface blebs of U937 apoptotic cells. Apoptosis was induced by incubating the cells (5 × 105 cells/ml), with anti-Fas (CD95) IgM mAb, 100 ng/ml for 4 h. Cells were stained with 45 mm 10-N-nonyl acridine orange for 15 min at room temperature. Images were acquired through confocal laser scanning microscope equipped with argon ion laser (25 mW). FITC was excited at 488 nm and laser power was set at 1 mW. Images were collected at 512 × 512 pixels with voxel dimensions 0·08 mm (lateral), 0·49 mm (axial). Acquisition and processing were carried out using Image Space software. (a) Untreated cell, stained with NAO; (b) anti-Fas treated cell, stained with NAO. Magnification ×2250; bar = 1 µm.
ACL binding to the surface of apoptotic cells
Cytofluorimetric analysis was performed using the affinity-purified human aCL IgG, which had been shown to have highly specific reactivity by TLC immunostaining (not shown). This analysis demonstrated that aCL IgG purified from patients with APS bind specifically to the surface of apoptotic cells. This binding was almost completely inhibited by previous adsorption with CL micelles (1 mg/ml) (82·2 ± 6% of inhibition), but not with the same concentration of PS (5·0 ± 1% of inhibition). The same aCl fraction, incubated in the absence of phospholipid micelles, gave virtually the same reactivity to CL on the cell surface showed in Fig. 3. As a negative control antibody, we used affinity purified normal human IgG.
Fig. 3.
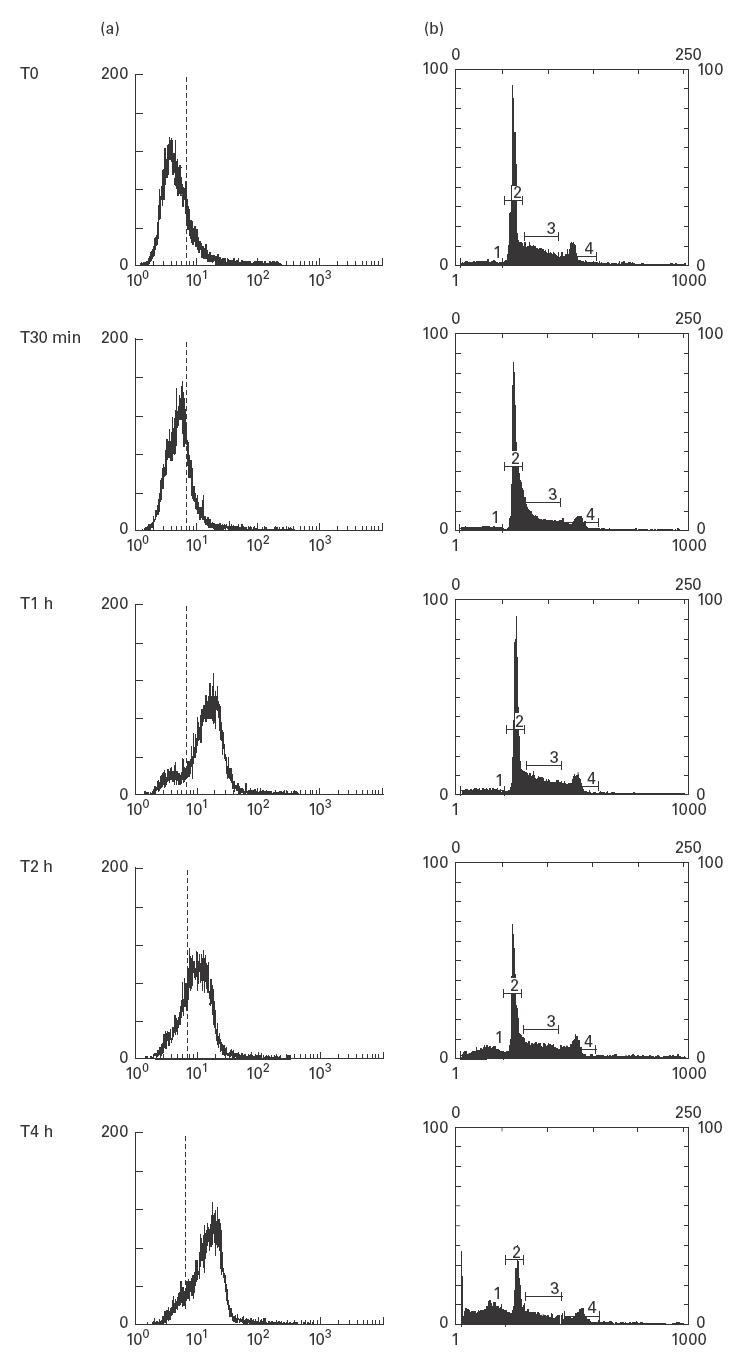
Cytofluorimetric analysis of CL expression and cell cycle in anti-Fas-treated U937 cells. Apoptosis was induced by incubating the cells (5 × 105 cells/ml) with anti-Fas (CD95) IgM mAb, 100 ng/ml for 30 min, 1 h, 2 h, 4 h. (a) Time-course of CL expression on cell plasma membrane after treatment with anti-Fas. The cells were stained with affinity purified aCL and subsequently incubated with FITC-conjugated goat anti‐human IgG. Histograms represent log fluorescence versus cell number, gated on cell population of a side scatter/forward scatter (SS/FS) histogram. Cell number is indicated on the y-axis and fluorescence intensity is represented in three logarithmic units on the x-axis. The dotted line indicates specific reactivity. (b) Time-course of the cell cycle analysis after treatment with anti-Fas. Cells were fixed with 70% ethanol and, after adding RNAse, were stained with propidium iodide. A subdiploid peak in flow cytometry histograms (cursor 1) identifies DNA fragmentation as the typical nuclear change that defines apoptosis. Cursor 2 reveals the diploid peak, cursor 3 the hyperdiploid peak and cursor 4 the tetraploid peak. Cell number is indicated on the y-axis and fluorescence intensity is represented at the x-axis. The cytofluorimetric analysis of DNA staining with propidium iodide showed a subdiploid peak of fluorescence of: 7·4% (T0), 7·6% (T30 min), 9·4% (T1 h), 18·6% (T2 h), 33·5% (T4 h) (an example of five different experiments).
The cytofluorimetric analysis showed a time-dependent binding of aCL IgG to cell surface (Fig. 3a). The increase in cell membrane binding was highly significant after 1 h of treatment with anti-Fas Ab (85 ± 7·5%) and was more evident after longer incubations (2, 4 h). No reactivity was shown on the plasma membrane of untreated cells.
DNA staining with propidium iodide of anti-Fas treated U937 cells followed by cytofluorimetric analysis showed a subdiploid peak of fluorescence at the different time-points: T0 4·9 ± 1%, T30 min 7·5 ± 0·9%, T1 h 10·1 ± 1·2%, T2 h 18·6 ± 1·3%, T4 h 35 ± 1·4% (Fig. 3b).
The percentage of necrotic cells was less than 2%, as a result of Trypan blue exclusion test.
A parallel treatment of U937 cells with TNFα showed an aCL binding of 63·5 ± 6·3% as early as after 30 min of incubation. The percentage of bound cells increased within 1 h (Fig. 4). As shown in Fig. 4, aCL binding to TNFα-treated cells clearly precedes DNA degradation. As a control of PS inversion on cell plasma membrane, annexin V binding is also shown.
Fig. 4.
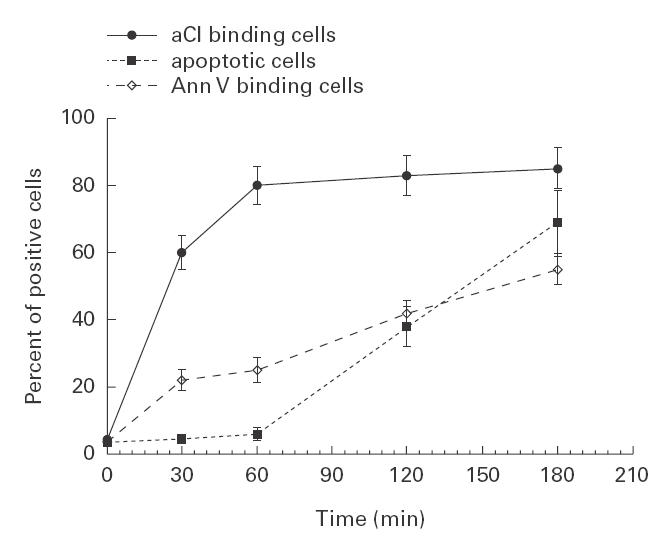
Cytofluorimetric analysis of CL expression, annexin V binding and hypodiploid cells after TNFα treatment. Apoptosis was induced by incubating U937 cells at a concentration of 5 × 105 cells/ml, in the presence of TNFα, 1000 IU/ml for 0 min, 30 min, 1 h, 2 h and 3 h. The cells were stained with affinity purified anti-CL Ab, followed by incubation with FITC-conjugated goat antihuman IgG or with FITC-conjugated annexin V. For DNA fragmentation analysis, cells were fixed with 70% ethanol and, after adding of RNAse, were stained with propidium iodide solution. The dotted line with solid squares indicates the cells with typical DNA fragmentation, identified by a subdiploid peak in the flow cytometry histograms. Mean of five experiments.
The human aCL IgG preparation was used to analyse the distribution pattern of CL molecules on the cell surface by scanning confocal microscopy. This analysis showed that the antibodies bound to U937 cells undergoing apoptosis, after treatment with anti-Fas (100 ng/ml for 4 h) or TNFα (1000 IU/ml for 3 h), but not to viable cells. The signal appeared uneven and punctate over the plasma membrane. This distribution indicated that CL molecules mostly localized on the surfaces of both small blebs and apoptotic bodies (Fig. 5b). A virtual absence of immunolabelling across internal membranes was observed. These findings suggest that, in cells undergoing apoptosis, surface expression of CL precedes the loss of plasma membrane integrity. Sequential single sections are showed in Fig. 5(d). Untreated U937 cells showed a fairly low immunolabelling (Fig. 5a). Virtually no reactivity was detected using control human IgG (Fig. 5c).
Fig. 5.
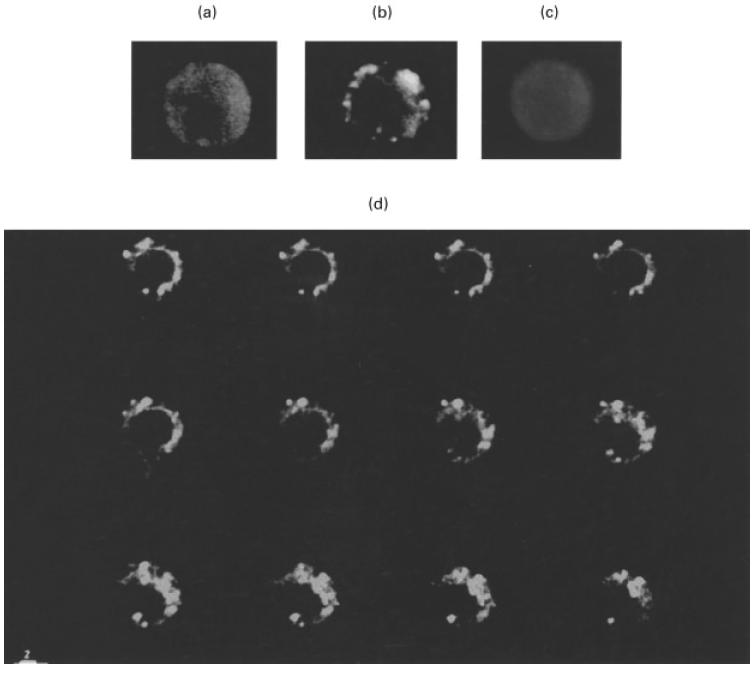
Scanning confocal analysis of CL expression and distribution on the surface of U937 apoptotic cells. Apoptosis was induced by incubating the cells at a concentration of 5 × 105 cells/ml, with anti-Fas (CD95) IgM mAb, 100 ng/ml for 4 h. Cells were stained with affinity purified anti-CL Ab, followed by incubation with FITC-conjugated goat anti‐human IgG (a,b) or with control human IgG (c). Images were acquired through confocal laser scanning microscope equipped with argon ion laser (25 mW). FITC was excited at 488 nm and laser power was set at 1 mW. Images were collected at 512 × 512 pixels with voxel dimensions 0·08 mm (lateral), 0·49 mm (axial). Acquisition and processing were carried out using Image Space software. (a) Untreated cell stained with affinity purified aCL; (b) anti-Fas treated cell stained with affinity purified aCL; (c) anti-Fas treated cells stained with control human IgG; (d) anti-Fas treated cell stained with affinity purified aCL. A complete z-series of 12 sections processed with routine for noise filtering is shown (Gaussian filter). Magnification ×1500; bar = 2 µm.
Discussion
In this investigation, we analysed CL expression on the plasma membrane of cells undergoing apoptosis in vitro and the reactivity of human antiphospholipid antibodies with CL on the surface of apoptotic cells.
Our results obtained by HPTLC analysis and staining with nonyl-acridine orange, a dye which interacts stoichiometrically with CL [27], clearly indicated that cells undergoing apoptosis exposed on their surface CL molecules, which are normally localized in mitochondria [28]. Additionally, scanning confocal microscopy observation of U937 cells highlighted that most of NAO-stained CL molecules were localized not only in the cytoplasm, but also on the cell surface with an uneven and clustered distribution. Interestingly, CL appeared to be concentrated in apoptotic blebs. This finding suggests that these areas of plasma membrane may represent CL-enriched domains.
Studies on transbilayer lipid movements during apoptosis [10] indicated that PS translocation was due to a downregulation of the adenosine triphosphate-dependent aminophospholipid translocase and an activation of a nonspecific lipid scramblase [29,30]. Water-soluble carrier proteins, called ‘phospholipid exchange proteins’, have been shown to have the ability to transfer individual phospholipid molecules between membranes [13,30]. Relatively little is known about mechanisms and intracellular pathways regulating CL membrane translocation. It is well known that apoptosis is accompanied by mitochondrial perturbations, such as the reduction in mitochondrial transmembrane potential and the increase in mitochondrial generation of superoxide anion. Both events precede nuclear DNA fragmentation [31,32]. A structural defect of the inner mitochondrial membrane which incorporates CL has been previously reported [33]. After the apoptotic signal, cells sustain progressive lipid peroxidation, resulting from the generation of lipid-diffusible reactive oxygen species [34]. The major sites of free radical generation include mitochondria, endoplasmic reticulum (ER) and nuclear membranes [34–37]. Two additional mechanisms have been proposed to account for phospholipid movement to mitochondria [13,30]. These include the involvement of a collision-based mechanism involving the ER and the mitochondria or specialized domains of the mitochondrial membranes and the transient fusion between ER and mitochondrial membranes. Further studies are needed to clarify the molecular mechanism(s) which regulate this transport.
Moreover, cytofluorimetric analysis revealed that aCL IgG purified from the serum of patients with APS bind to CL on the surface of apoptotic cells. This analysis showed that CL molecules are exposed on the cell plasma membrane time-dependently and that their appearance precedes DNA degradation and cell lysis by several hours. This result suggests that CL molecules may function as self-antigen molecules. A common fate of self-antigens at these sites might be their vulnerability to oxidative damage [38]. CL may form stable complexes with specific mitochondrial (glyco)proteins which are translocated to the plasma membrane during apoptosis.
It is of interest to observe that autoantibodies directed to mitochondrial components have been found in patients with lupus and related diseases [39], a finding with might suggest an exposure of such molecule(s) on the cell surface. The observation that aCL bind to surface blebs of apoptotic cells, a site where most of the autoantigens found in SLE are clustered [38], is therefore of great importance. Since binding of autoantibody to one component of a multicomponent complex can influence the subsequent processing and presentation of the other antigens in the complex [40], it is possible that coating of apoptotic blebs by aCL enhances the immunogenicity of these autoantigens [41]. When apoptosis occurs in a microenvironment in direct contact with the plasma, the procoagulant role of the apoptotic surface may be expressed additionally [41]. Opsonization of apoptotic cells by antiphospholipid antibodies has recently been shown to enhance recognition and phagocytosis by macrophages, with massive TNFα secretion [42,43]. The release of TNFα may amplify this process by inducing further apoptosis and promoting the maturation of APC towards a more efficient antigen processing and presentation capability.
The clearance of disrupted cell membranes from physiologically dead cells, such as PBL, may account for the exposure of CL and the subsequent production of autoantibodies. Indeed, we observed a correlation between the increased expression of CL on lymphocytes from patients with APS and the rate of apoptosis in PBL (unpublished observations). Moreover, it has recently been reported that antiphospholipid antibodies bind to apoptotic, but not viable thymocytes [44]. Thus, apoptotic cells may provide an abundant source of antigen and be immunogen for aPL generation. This hypothesis has been confirmed and extended by the recent study of Mevorach et al. [45], who demonstrated that systemic exposure to apoptotic cells can induce an anti-CL response in normal mice. These findings suggest that aCL may be generated as an immune response in diseases characterized by increased rates of apoptosis such as AIDS and, possibly, SLE or APS and may help to explain the autoantigen selection and initiation of the immune response occurring in these diseases. In conclusion, CL on the cell surface of apoptotic cells may represent an in vivo trigger for the production of antiphospholipid antibodies.
Acknowledgments
We thank Professor Maria Grazia Cifone, Università di L'Aquila, Italy, for helpful discussion and Dr Maria Giammatteo, Centro Interdipartimentale di Microscopia Elettronica Università di L'Aquila, for precious help on image aquisition with laser confocal microscope. G.M. Pontieri, A. Pavan and G. Valesini were supported by grants from Ministero dell'Università e della Ricerca Scientifica, Italy.
References
- 1.Williams GT, Smith CA, McCarthy NJ, Grimes EA. Apoptosis: final control point in cell biology. Trends Cell Biol. 1992;2:263–7. doi: 10.1016/0962-8924(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 2.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fesus L. Biochemical events in naturally occurring forms of cell death. FEBS Lett. 1993;328:1–5. doi: 10.1016/0014-5793(93)80952-q. [DOI] [PubMed] [Google Scholar]
- 4.Duvall E, Wyllie AH. Death and the cell. Immunol Today. 1986;7:115–9. doi: 10.1016/0167-5699(86)90152-0. [DOI] [PubMed] [Google Scholar]
- 5.Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of endonuclease. Am J Pathol. 1990;136:593–608. [PMC free article] [PubMed] [Google Scholar]
- 6.Peitsch MC, Mannhers HG, Tschopp J. The apoptosis endonucleases: cleaning up after cell death? Trends Cell Biol. 1994;4:37–41. doi: 10.1016/0962-8924(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 7.Savil J, Fadok VA, Henson PM, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–6. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 8.Martin SJ, Reutelinsperger CPM, McGahon AJ, Rader JA, van Schie RCAA, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mower Da, Jr, Peckam, Illera VA, Fishbaugh JK, Stunz LL, Asham RF. Decreased membrane phospholipid packing and decreased cell size precede DNA cleavage in mature mouse B cell apoptosis. J Immunol. 1994;152:4832–42. [PubMed] [Google Scholar]
- 10.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bretsche MS. Asymmetrical lipid bilayer structure for biological membranes. Nature. 1972;236:11–2. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- 12.Op den Kamp JAF. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 13.Zachowski A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem J. 1993;294:1–14. doi: 10.1042/bj2940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaux PF, Zachowski A. Maintenance and consequences of membrane phospholipid asymmetry. Chem Phys Lipids. 1994;73:107–20. [Google Scholar]
- 15.Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernest JD, Lubin BH. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87:1179–86. [PubMed] [Google Scholar]
- 16.VanHouten N, Budd RC. Accelerated programmed cell death of MRL-lpr/lpr T lymphocytes. J Immunol. 1992;149:2513–7. [PubMed] [Google Scholar]
- 17.Emlen W, Niebur JA, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152:3685–92. [PubMed] [Google Scholar]
- 18.Aringer M, Wintersberger W, Steiner CW, Kiener H, Presterl E, Jaeger U, Smolen JS, Graninger WB. High levels of bcl-2 protein in circulating T lymphocytes, but not B lymphocytes of patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37:1423–30. doi: 10.1002/art.1780371004. [DOI] [PubMed] [Google Scholar]
- 19.Silvestris F, Frassanito MA, Cafforio P, et al. Antiphosphatidylserine antibodies in human immunodeficiency virus 1+ patients correlate with evidence of T cell apoptosis and mediate antibody dependent cellular cytotoxicity. Blood. 1996;87:5185–95. [PubMed] [Google Scholar]
- 20.Sorice M, Griggi T, Circella A, et al. Detection of antiphospholipid antibodies by immunostaining on thin layer chromatography plates. J Immunol Meth. 1994;173:49–54. doi: 10.1016/0022-1759(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 21.Perdue JF. The isolation and characterization of plasma membrane from cultured chicken embryo fibroblasts. Meth Enzymol. 1974;31:162–8. doi: 10.1016/0076-6879(74)31017-8. [DOI] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 23.Leprat P, Ratinaud MH, Maftah A, Petit JM, Julien R. Use nonyl acridine orange and rhodamine 123 to follow biosynthesis and functional assembly of mitochondrial membrane during. L1210 cell cycle. Exp Cell Res. 1990;186:130–7. doi: 10.1016/0014-4827(90)90219-z. [DOI] [PubMed] [Google Scholar]
- 24.McNeil HP, Krillis SA, Chesterman CN. Purification of antiphospholipid antibodies using a new affinity method. Thromb Res. 1988;52:641–8. doi: 10.1016/0049-3848(88)90136-3. [DOI] [PubMed] [Google Scholar]
- 25.Harris EN, Gharavi AE, Loizou S. Crossreactivity of antiphospholipid antibodies. J Clin Lab Immunol. 1985;16:1–6. [PubMed] [Google Scholar]
- 26.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Meth. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 27.Gallet PF, Mafta A, Petit JM, Denis-Gay M, Julien R. Direct cardiolipin assay in yeast using the red fluorescence emission of 10-N-nonyl acridine orange. Eur J Biochem. 1995;228:113–9. doi: 10.1111/j.1432-1033.1995.tb20238.x. [DOI] [PubMed] [Google Scholar]
- 28.Petit JM, Huet O, Gallet PF, Maftah A, Ratinaud MH, Julien R. Direct analysis and significance of cardiolipin transverse distribution in mitochondrial inner membranes. Eur J Biochem. 1994;220:871–9. doi: 10.1111/j.1432-1033.1994.tb18690.x. [DOI] [PubMed] [Google Scholar]
- 29.Hoch FL. Cardiolipins and biomembrane functions. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- 30.Moreau P, Cassagne C. Phospholipid trafficking and membrane biogenesis. Biochim Biophys Acta. 1994;1197:257–90. doi: 10.1016/0304-4157(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 31.Castedo M, Macho A, Zanzami N, Hirsch T, Marchetti P, Uriel J, Kroemer G. Mitochondrial perturbations define lymphocytes undergoing apoptotic depletion in vivo. Eur J Immunol. 1995;25:3277–84. doi: 10.1002/eji.1830251212. [DOI] [PubMed] [Google Scholar]
- 32.Zanzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–44. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macho A, Castedo M, Marchetti P, et al. Mitochondrial dysfunctions in circulating T lymphocytes from human immunodeficiency virus 1 carriers. Blood. 1995;86:2481–7. [PubMed] [Google Scholar]
- 34.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 35.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide: general properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–16. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casciola-Rosen LA, Miller DK, Anhalt GJ, Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994;269:30757–60. [PubMed] [Google Scholar]
- 37.Casiano CA, Seamus JM, Green DR, Tan EM. Selective cleavage of nuclear autoantigens during CD95 (Fas/APO-1) -mediated T cell apoptosis. J Exp Med. 1996;184:765–70. doi: 10.1084/jem.184.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casciola Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in Systemic Lupus Erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mamula MJ, Jemmerson R, Hardin JA. The specificity of human anti-cytochrome c autoantibodies that arise in autoimmune disease. J Immunol. 1990;144:1835–40. [PubMed] [Google Scholar]
- 40.Lanzavecchia A. How can cryptic epitopes trigger autoimmunity? J Exp Med. 1995;181:1945–8. doi: 10.1084/jem.181.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casciola Rosen L, Rosen A, Petri M, Schlissel M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1996;93:1624–9. doi: 10.1073/pnas.93.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manfredi AA, Rovere P, Galati G. Apoptotic cell clearance in systemic lupus erythematosus. Opsonization by antiphospholipid antibodies. Arthritis Rheum. 1998;41:205–14. doi: 10.1002/1529-0131(199802)41:2<205::AID-ART4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 43.Manfredi AA, Rovere P, Heltai S, Galati G, Nebbia G, Tincani A, Balestrieri G, Sabbadini MG. Apoptotic cell clearance in systemic lupus erythematosus. Role of β2-glycoprotein I. Arthritis Rheum. 1998;41:215–23. doi: 10.1002/1529-0131(199802)41:2<215::AID-ART5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Price BE, Rauch J, Shia MA, et al. Anti-phospholipid antibodies bind to apoptotic, but not viable, thymocytes in a β2-glycoprotein I-dependent manner. J Immunol. 1996;157:2201–8. [PubMed] [Google Scholar]
- 45.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


