Abstract
Primary biliary cirrhosis is an autoimmune disease of the liver in which T helper 1 cytokines predominate over those of T helper 2 in the pathogenesis. Interleukin-18 (IL-18), for which the gene was recently cloned, is a novel T helper 1 cytokine, which augments interferon-gamma production. We designed this study to clarify the role of IL-18 in primary biliary cirrhosis and to examine whether serum IL-18 level can be a prognostic indicator for the disease. Serum IL-18 levels were measured using an enzyme linked immuno sorbent assay with mouse monoclonal antibodies. Twenty-two healthy volunteers, 31 patients with primary biliary cirrhosis (Scheuer's stage I, 13; II, 10; and IV, 8), 20 patients with autoimmune hepatitis, 11 patients with virus-related liver cirrhosis and six patients with obstructive jaundice were enrolled. Significant differences of serum IL-18 levels were observed between patients with Scheuer's stage IV and those with stage I, or II, virus-related liver cirrhosis and obstructive jaundice (P < 0·05). The IL-18 levels in primary biliary cirrhosis increased according to the disease progression, and fell promptly after living-related liver transplantation. Moreover, serum IL-18 levels in primary biliary cirrhosis were correlated with serum bilirubin concentrations and the Risk scores of the Mayo Clinic prognostic model for the disease. The IL-18 levels observed in patients with autoimmune hepatitis were also elevated, and correlated with the activity of the disease. These results indicate that serum interleukin-18 levels reflect the severity of primary biliary cirrhosis, the activity of autoimmune hepatitis, and may be an additive prognostic indicator in primary biliary cirrhosis.
Keywords: interferon-gamma-inducing factor, IL-18, primary biliary cirrhosis, autoimmune hepatitis
Introduction
Primary biliary cirrhosis (PBC) is a disease characteristic of the progressive inflammation in the intralobular bile epithelia which occurs predominantly in middle-aged women [1]. Immunological disorders in the intralobular bile ducts are involved in the pathogensis of PBC, and the constitutive appearance of serum antimitochondrial antibody is the most characteristic feature of the humoral immune response and the most useful diagnostic indicator [2,3]. Several lines of evidence have recently demonstrated that the destruction of the bile duct epithelia is due to the activated cellular immunity against the E2 component of the mitochondrial enzyme pyruvate dehydrogenase complex [4,5]. Subtyping of the lymphocytes infiltrating in the liver has shown a predominance of CD4+ T cells over CD8+ T cells [6,7]. Analysis of the cytokine gene expression in the liver and cytokine levels in the culture supernatants of lymphocytes from both the liver and the peripheral blood has revealed that T helper 1 (Th1)-like cells are predominantly activated in the progression of PBC [8,9]. Th1 cytokines such as interleukin (IL)-2 and interferon (IFN)-gamma are expressed more strongly than the Th2 cytokines such as IL-4, IL-5, IL-6 and IL-10 in the affected lymphocytes [10].
The gene for interferon-gamma-inducing factor was cloned in 1995 [11] and the protein was subsequently designated as IL-18 [12]. The fundamental function of IL-18 is an enhancement of Th1 cytokine production by CD4+ T cells [13,14]. Through IFN-gamma production, IL-18 augments perforin-dependent cytotoxicity of liver natural killer (NK)-T cells [15–17] and Fas ligand-mediated cytotoxicity of Th1 cells [18]. In these functions, macrophages and Kupffer cells are responsible for IL-18 production in response to antigen-stimulation [19], and synergy of IL-12 and IL-18 has been demonstrated [20].
Based on this large body of evidence, there is increased awareness of IL-18 participation in various human diseases. However, to date, there have been only a few reports of IL-18 participation in human diseases [21–23]. Since Th1 cytokines are dominant in PBC, there was a possibility that serum IL-18 levels would be elevated in the patients. On the other hand, several Th1 cytokines, especially IFN-gamma, are upregulated in transplant rejection [24,25], suggesting a possibility that serum IL-18 levels might increase after liver transplantation. In order to address this, we have developed an enzyme linked immuno sorbent assay (ELISA) system specific for measuring human IL-18 concentration in serum [26]. In a preliminary study, we examined the serum levels before and after living-related liver transplantation (LRLT) in three patients with end-stage PBC. As a result, we found that serum IL-18 levels were markedly elevated before LRLT, but decreased rapidly after the operation. Therefore, we examined serum IL-18 levels in patients with various stages of PBC, and report here that IL-18 in serum increases according to the disease progression, and the level is a useful prognostic indicator in patients with PBC.
Materials and methods
The subjects in this study were 31 patients with PBC; 20 with autoimmune hepatitis (AIH) (10 cases each before and during corticosteroid treatment), and 11 with virus-related advanced liver cirrhosis (LC, HBV, two cases; HCV, nine cases). Additionally, six patients with obstructive jaundice who were admitted to our hospital were included. Twenty-two healthy volunteers of hospital staff (eight males and 14 females, average age 45 years) were also enrolled. The current medical examination in the healthy volunteers showed that HBs-antigen and HCV-antibody were negative and their liver function tests were normal. The diagnosis of PBC, AIH and LC was made histologically from liver samples obtained by peritoneoscopic examination, and the staging of PBC was determined by Scheuer's classification [27]. Of the 31 patients with PBC, 13, 10 and eight patients were in stage I, II and IV, respectively. Among the eight patients with stage IV disease, two died from hepatic failure and four patients received LRLT.
Most samples were obtained after fasting and before treatment was given except for half of the cases of AIH. In three patients with PBC, serum IL-18 levels were examined serially during the course of disease progression. Serum IL-18 levels were measured by a specific human IL-18 ELISA using two neutralizing monoclonal antibodies as previously reported [26]. The minimum level detectable and the upper limit with this assay are 10 and 1000 pg/ml, respectively, and the levels in all samples were measured in duplicate. Serum IL-12 and IFN-gamma were also measured in healthy volunteers and patients with PBC using enzyme immunoassay kits (Immunotech, Marseille, France). In all measurements, each standard cytokine preparation was used as an internal control. Laboratory data were also examined on the day when the samples for IL-18 measurement were taken. Risk scores (R) in the formula of the Mayo prognostic model for PBC [28] were calculated for individual patients. Statistical analyses of the differences between the groups were performed with the Tukey–Kramer's test using the StatView-J5·0 program (Abacus Concepts Inc., Berkeley, CA). P < 0·05 were considered significant. The study was approved by our institutional review board, and informed consent for the use of the samples for research was obtained from all patients.
Results
In the laboratory examination, serum bilirubin levels in patients with obstructive jaundice or Scheuer's stage IV PBC were significantly higher than those of other groups. Serum alkaline phosphatase levels in patients with stage IV PBC or obstructive jaundice were higher than those in patients with stage I PBC (Table 1).
Table 1.
Clinical characteristics of patients
| Age (years) | ALT (IU/l) | ALP (IU/l) | PLT (× 104/µl) | Total bilirubin (mg/dl) | Total cholesterol (mg/dl) | |
|---|---|---|---|---|---|---|
| Scheuer I (n = 13) | 49 ± 12 | 64 ± 56 | 209 ± 119 | 22 ± 8 | 0·6 ± 0·3 | 246 ± 61 |
| Scheuer II (n = 10) | 53 ± 6 | 33 ± 20 | 237 ± 162 | 23 ± 9 | 0·8 ± 0·5 | 196 ± 47 |
| Scheuer IV (n = 8) | 49 ± 10 | 65 ± 32 | 521 ± 498 | 19 ± 12 | 7·8 ± 7·8 | 236 ± 117 |
| AIH (n = 20) | 52 ± 14 | 168 ± 235 | 160 ± 76 | 18 ± 8 | 3·0 ± 4·7 | 188 ± 33 |
| LC (viral) (n = 11) | 58 ± 7 | 56 ± 34 | 161 ± 91 | 8 ± 3 | 1·0 ± 0·6 | 153 ± 29 |
| Obstructive jaundice (n = 6) | 66 ± 3 | 114 ± 51 | 567 ± 235 | 22 ± 8 | 9·1 ± 4·2 | 195 ± 56 |
| Control (n = 22) | 45 ± 10 | 25 ± 6 | 99 ± 21 | ND | 0·7 ± 0·2 | ND |
Values are expressed as means ± SD. ND, not determined; AIH, autoimmune hepatitis; LC, liver cirrhosis; ALT, alanine aminotransferase; ALP, alkaline phosphatase; PLT, platelet count.
Serum IL-18 levels (mean ± SD) of healthy volunteers, LC and obstructive jaundice were 140·7 ± 62·4, 183·7 ± 110·3, 236·0 ± 48·9 pg/ml, respectively. In patients with PBC, the IL-18 levels were 158·1 ± 65·9, 289·9 ± 236·4 and 508·8 ± 200·5 pg/ml in Scheuer's stage I, II and IV, respectively, as shown in Fig. 1. Significant differences of IL-18 levels were also observed between Scheuer's stage IV patients and patients with stage I or II disease, LC, obstructive jaundice and healthy volunteers (P < 0·05). These results suggest that serum IL-18 levels increased according to the disease progression (Fig. 1).
Fig. 1.
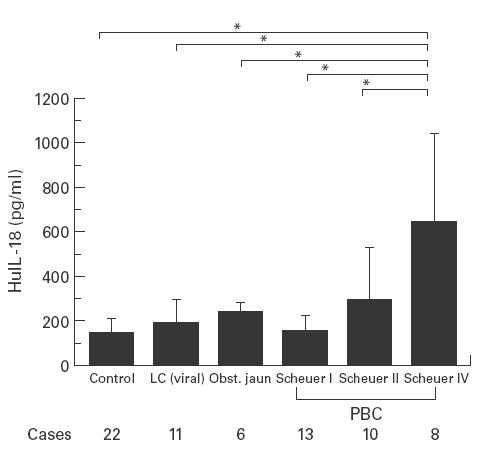
Serum IL-18 levels in healthy volunteers and patients with virus-related liver cirrhosis, obstructive jaundice and primary biliary cirrhosis (mean ± SD). Significant differences of the levels were observed between PBC patients with Scheuer's stage IV and those with I or II disease, healthy volunteers and virus-related liver cirrhosis (*P < 0·05).
The changes in serum IL-18 levels in three PBC patients in whom serum IL-18 levels were measured serially are shown in Fig. 2. Of these three patients, two have received LRLT and one is being followed-up at an out patient clinic. Serum IL-18 levels were elevated concomitantly with or prior to the increase of serum bilirubin and continued to be elevated up to the time of LRLT (Fig. 2).
Fig. 2.
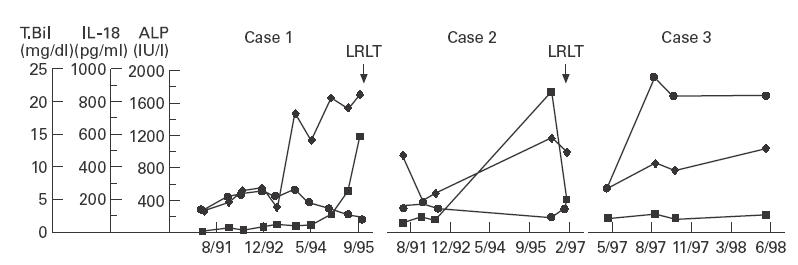
Changes in serum IL-18 levels in three patients with primary biliary cirrhosis. Serum IL-18 levels elevated with the increase of serum bilirubin in two out of three cases. However, the level in case 1 elevated approximately one and half years prior to the increase of serum bilirubin. LRLT, Living-related liver transplantation (✦, IL-18; ▪, total bilirubin; •, ALP).
We have analysed the correlation between serum IL-18 levels and various clinical parameters in all PBC patients. Serum IL-18 levels were not correlated with age, serum ALT, AST, ALP, cholesterol level or blood platelet counts. The levels were correlated significantly with serum bilirubin levels and the R scores calculated by the Mayo Clinic prognostic model, indicating that serum IL-18 levels reflected the severity of PBC (Table 2 and Fig. 3).
Table 2.
Correlation coefficient and P-value between serum IL-18 level and various parameters of patients with primary biliary cirrhosis
| Correlation coefficient | P-value | |
|---|---|---|
| Age | 0·067 | 0·719 |
| ALT | 0·095 | 0·612 |
| ALP | 0·308 | 0·243 |
| PLT | 0·115 | 0·538 |
| Total bilirubin | 0·621 | 0·0002* |
| Total cholesterol | 0·080 | 0·668 |
| R (risk score) | 0·617 | 0·001* |
Significant.
ALT, alanine aminotransferase; ALP, alkaline phosphatase; PLT, platelet count.
Fig. 3.
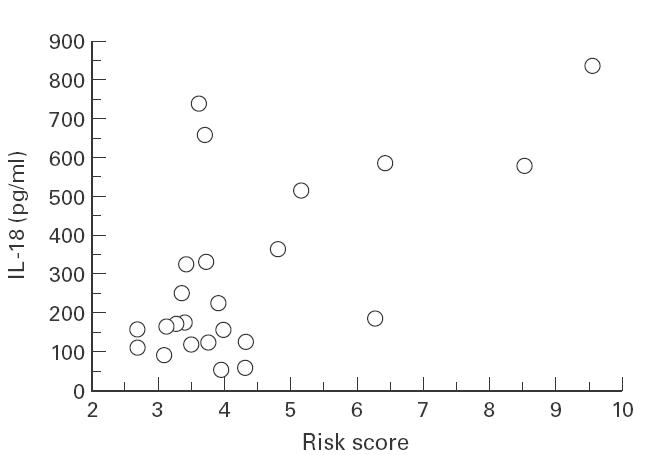
Scatter plots of serum IL-18 levels and the R scores calculated by the Mayo prognostic model in 24 PBC patients. The correlation coefficient was 0·617 (P < 0·001).
We also measured the levels of serum IL-12 and IFN-gamma in patients with PBC. Serum IFN-gamma levels in most cases of PBC were below the detection limit, and no significant differences in levels of either cytokines was observed among the groups with Scheuer's stage IV, I or II disease and healthy volunteers (Fig. 4). Furthermore, serum IL-18 levels were correlated with neither the serum IFN-gamma nor IL-12 levels (data not shown).
Fig. 4.
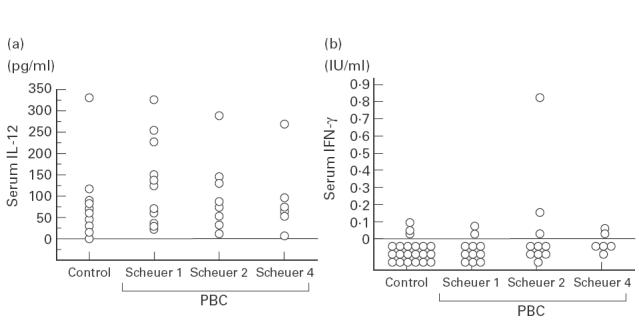
Serum IL-12 (a) and IFN-gamma (b) levels in healthy volunteers and in PBC patients at various histological stages of the Scheuer's classification. Serum IFN-gamma levels were under the detection limit in the almost all patients with PBC and healthy volunteers. Regarding serum IL-12 levels, no significant differences were found between healthy volunteers and PBC patients, regardless of the stage of PBC.
Serum IL-18 levels in patients with AIH before and during treatment with corticosteroid were 617·2 ± 534·0 and 625·9 ± 486·3 pg/ml, respectively. The IL-18 levels in AIH were significantly higher than those in all groups except for stage IV PBC patients (P < 0·05). Concerning the correlation with parameters of liver function, the IL-18 levels were significantly correlated with serum ALT and bilirubin levels, indicating that serum IL-18 also reflected the activity of AIH (Table 3).
Table 3.
Correlation coefficient and P-value between serum IL-18 level and various parameters in patients with autoimmune hepatitis
Significant.
ALT, alanine aminotransferase; ALP, alkaline phosphatase.
Discussion
Our data demonstrated that serum IL-18 levels increased significantly in PBC patients with Scheuer's stage IV, showing the significant differences between PBC patients with stage IV and healthy volunteers, LC, obstructive jaundice and those with stage I or II PBC. The levels also correlated significantly with serum bilirubin levels and the R scores in the Mayo Clinic prognostic model. These results indicate that serum IL-18 levels reflect the severity of PBC, and that the increase is not simply caused by the deteriorated liver function observed in advanced LC or cholestasis.
In Scheuer's histological staging of PBC, the inflammation of bile duct epithelia is more pronounced in stage II than in stage IV. Therefore, if IL-18 is produced as a proinflammatory cytokine, the levels should be higher in patients with stage II than in those with stage IV. However, our results were not consistent with this hypothesis. One possibility is that active inflammation may still remain in the stage IV disease since PBC is heterogeneous in histology [29]. Another is that early PBC conditions are characterized by eosinophilic reactions which may represent inflammation due to a Th2 response initially that is then shifted to a Th1 response later in Stage IV disease [30]. This may explain why inflammation is seen early on but not reflected in a Th1 cytokine profile until later. Furthermore, there is a possibility that the elevation in stage IV PBC may be due to the disturbed excretion of this cytokine into the biliary tract by severe cholestasis. Different mechanisms might be responsible for the elevation of the serum IL-18 level in PBC, but this remains speculative.
Interestingly, serum IL-18 levels in AIH patients are also elevated, and correlate significantly with serum ALT in contrast to stage IV PBC and bilirubin levels similar to PBC. Since serum bilirubin level is a most important variable for the prediction of prognosis in PBC patients [31–33], and ALT is also an important marker for decision of liver transplantation in AIH patients [34], serum IL-18 levels might be an useful indicator for the severity of both diseases.
Complement mediated cytolysis or antibody-dependent cytotoxic reactions are proposed to be main pathogenetic mechanisms in AIH [35]. However, T cell mediated cytotoxicity against hepatocytes has been indicated in animal models of AIH [36,37]. Although a significant elevation of serum IL-18 suggests a possibility that Th1 cells are involved in the pathogenesis of AIH, further investigation is necessary in the future.
Serum IL-18 levels have previously been shown to correlate with serum IFN-gamma and IL-12 levels [20]. However, in this study, neither the IFN-gamma nor the IL-12 levels were correlated with the serum IL-18 level or the severity of PBC. Particularly, IFN-gamma levels in the peripheral blood were very low. Since the number of IFN-gamma mRNA-positive cells is correlated with the inflammatory activity in the affected portal areas in PBC [8], the low level of IFN-gamma in the peripheral blood may be due to the localization of this cytokine in the liver. Examination of these cytokine levels in the tissue may elucidate a role of IL-18 and the relationships between IL-18 and IFN-gamma or IL-12 in PBC more precisely.
PBC is one of the most favourable candidates for orthotopic liver transplantation. Compared to the expected survival predicted by the formula of the Mayo Clinic far superior survival following transplantation has been mentioned [38]. As we have shown, serum IL-18 levels increased simultaneously with or prior to the elevation of serum bilirubin and fell promptly after LRLT in patients with stage IV PBC. Furthermore, the level was significantly correlated with the prognostic index outlined by the Mayo Clinic. These results suggest that the serum IL-18 level may be an additive prognostic indicator before and after liver transplantation in PBC. In conclusion, we first found that serum IL-18 levels increased in patients with stage IV PBC and those with AIH, and reflected the severity or activity of the diseases. Further studies will be required to identify IL-18-producing cells and the degradation pathway of IL-18 in these autoimmune diseases.
Acknowledgments
This work was supported by grants from Intractable Hepatitis Committee, the Japanese Ministry of Health and Welfare and from Japanese Ministry of Education (No. 10670474).
References
- 1.Ahrens JEH, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis. Medicine (Baltimore) 1950;29:299–364. doi: 10.1097/00005792-195012000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Munzo LE, Thomas HC, Scheuer PJ, Doniach D, Sherlock S. Is mitochondrial antibody diagnostic of primary biliary cirrhosis? Gut. 1981;22:136–40. doi: 10.1136/gut.22.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker JG, Doniach D, Roitt I, Sherlock S. Serological test in diagnosis of primary biliary cirrhosis. Lancet. 1965;1:827–31. doi: 10.1016/s0140-6736(65)91372-3. [DOI] [PubMed] [Google Scholar]
- 4.Van de Water J, Ansari AA, Surh CD, et al. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J Immunol. 1991;146:89–94. [PubMed] [Google Scholar]
- 5.Van de Water J, Ansari A, Prindiville T, et al. Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J Exp Med. 1995;181:723–33. doi: 10.1084/jem.181.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van de Water J, Shimoda S, Niho Y, Coppel R, Ansari A, Gershwin ME. The role of T cells in primary biliary cirrhosis. Semin Liver Dis. 1997;17:105–13. doi: 10.1055/s-2007-1007188. [DOI] [PubMed] [Google Scholar]
- 7.Colucci G, Schaffner F, Paronetto F. In situ characterization of the cell-surface antigens of the mononuclear cell infiltrate and bile duct epithelium in primary biliary cirrhosis. Clin Immunol Immunopatholol. 1986;41:35–42. doi: 10.1016/0090-1229(86)90049-8. [DOI] [PubMed] [Google Scholar]
- 8.Harada K, Van de Water J, Leung PS, L CR, Ansari A, Nakanuma Y, Gershwin ME. In situ nucleic acid hybridaization of cytokines in primary biliary cirrhosis: predominance of the Th1 subset. Hepatology. 1997;25:791–6. doi: 10.1002/hep.510250402. [DOI] [PubMed] [Google Scholar]
- 9.Berg PA, Klein R, Rocken M. Cytokines in primary biliary cirrhosis. Semin Liver Dis. 1997;17:115–23. doi: 10.1055/s-2007-1007189. [DOI] [PubMed] [Google Scholar]
- 10.Martinez OM, Villanueva JC, Gershwin ME, Krams SM. Cytokines pattern and cytotoxic mediators in primary biliary cirrhosis. Hepatology. 1995;21:113–9. [PubMed] [Google Scholar]
- 11.Okamura H, Tsutsi H, Komastsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 12.Ushio S, Namba M, Okura T, et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherchia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–9. [PubMed] [Google Scholar]
- 13.Tomura M, Ahou XY, Maruo S, et al. A critical role for IL-18 in the proliferation and activation of NK1.1+ CD3- cells. J Immunol. 1998;160:4738–46. [PubMed] [Google Scholar]
- 14.Tomura M, Maruo S, Mu J, et al. Differential capacities of CD4+, CD8+ and CD4–CD8- T cell subsets to express IL-18 receptor and produce IFN-gamma in response to IL-18. J Immunol. 1998;160:3759–65. [PubMed] [Google Scholar]
- 15.Zhang T, Kawakami K, Qureshi MH, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–9. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Micallef MJ, Yoshida K, Kawai S, et al. In vivo antitumor effects of murine interferon-gamma-inducing factor/interleukin-18 in mice bearing syngeneic Meth A sarcoma malignant ascites. Cancer Immunol Immunother. 1997;43:361–7. doi: 10.1007/s002620050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dao T, Mehal WZ, Cripe IN. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J Immunol. 1998;161:2217–22. [PubMed] [Google Scholar]
- 18.Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. IFN-gamma-iniducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996;157:3967–73. [PubMed] [Google Scholar]
- 19.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secreete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–8. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micallef MJ, Ohtsuki T, Kohno K, et al. Intereron-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human Y cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–51. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 21.Monteleone G, Trapasso F, Parrello T, et al. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163:143–7. [PubMed] [Google Scholar]
- 22.Pizarro TT, Michie MH, Bentz M, et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–35. [PubMed] [Google Scholar]
- 23.McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260–9. doi: 10.1136/gut.46.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thai NL, Fu F, Quian S. Cytokine mRNA profiles in mouse orthotopic liver transolantation. Transplantation. 1995;59:274–81. [PubMed] [Google Scholar]
- 25.Kupiec-Weglinski JW, Wasowska JW, Papp ICD. CD4 mAb therapy modultaes alloantibody production and intracardiac graft deposition in association with selective inhibition of Th1 lymphokines. J Immunol. 1993;151:5053–61. [PubMed] [Google Scholar]
- 26.Taniguchi M, Nagaoka K, Kunikata T, et al. Characterization of anti-human interleukin-18 (IL-18)/interferon-gamma-inducing factor (IGIF) monoclonal antibodies and their application in the measurment of human IL-18 by ELISA. J Immunol Methods. 1997;206:107–13. doi: 10.1016/s0022-1759(97)00094-x. [DOI] [PubMed] [Google Scholar]
- 27.Scheuer PJ. Primary biliary cirrhosis. Proc R Soc Med. 1967;60:1257–60. [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson ER, Grambsch PM, Fleming TR, Fischer LD, Langworthy A. Prognosis in primary biliary cirrhosis' Model for dicision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 29.Sherlock S, Dooley J. Diseases of the liver and biliary system. 9. London: Blackwell, Scientific Publications; 1993. Primary biliary cirrhosis; pp. 236–48. [Google Scholar]
- 30.Shindo M, Mullin GE, Braun-Elwert L, Bergasa NV, Jones EA, James SP. Cytokine mRNA expression in the liver of patients with primary biliary cirrhosis (PBC) and chronic hepatitis B (CHB) Clin Exp Immunol. 1996;105:254–9. doi: 10.1046/j.1365-2249.1996.d01-759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benhamou JP. Indications for liver transplantation in primary biliary cirrhosis. Hepatology. 1994;20:11S–13S. doi: 10.1016/0270-9139(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 32.Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 33.Goudie BM, Burt AD, Macfarlane GJ, Boyle P, Gillis CR, MacSween RN, Watkinson G. Risk factors and prognosis in primary biliary cirrhosis. Am J Gastroenterol. 1989;84:713–6. [PubMed] [Google Scholar]
- 34.Sanchez-Urdazpal L, Czaja AJ, van Hoek B, Krom RA, Wiesner RH. Prognostic features and role of liver transplantation in severe corticosteroid-treated autoimmune chronic active hepatitis. Hepatology. 1992;15:215–21. doi: 10.1002/hep.1840150208. [DOI] [PubMed] [Google Scholar]
- 35.McFarlane IG. Pathogenesis of autoimmune hepatitis. Biomed Pharmacother. 1999;53:255–63. doi: 10.1016/S0753-3322(99)80096-1. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa M, Mori Y, Mori T, et al. Adoptive transfer of experimental autoimmune hepatitis in mice − cellular interaction between donor and recipient mice. Clin Exp Immunol. 1988;73:276–82. [PMC free article] [PubMed] [Google Scholar]
- 37.Kohda H, Sekiya C, Kanai M, Yoshida Y, Uede T, Kikuchi K, Namiki M. Flow cytometric and functional analysis of mononuclear cells infiltrating the liver in experimental autoimmune hepatitis. Clin Exp Immunol. 1990;82:473–8. doi: 10.1111/j.1365-2249.1990.tb05474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiesner RH. Liver transplantation for primary biliary cirrhosis and primary biliary cholangitis: predicting outcomes with natural history model. Mayo Clin Proc. 1998;73:575–88. doi: 10.4065/73.6.575. [DOI] [PubMed] [Google Scholar]


