Abstract
The acidic ribosomal proteins of the protozoan parasite Leishmania infantum have been described as prominent antigens during both human and canine visceral leishmaniasis. In this study we present data showing that the intraperitoneal administration in BALB/c mice of the Leishmania LiP2a protein, in the absence of any added adjuvants, elicited a strong humoral response as an indication that the protein is a potent immunogen. Despite the evolutionary conservation of the acidic ribosomal proteins, the antibody response was found to be specific for the Leishmania protein. Another remarkable finding was the observation that the LiP2a protein stimulates the in vitro proliferation of splenocytes from either LiP2a-immunized or naive BALB/c mice. Since similar proliferative indices were observed in T cell-enriched cultures, it is likely that the LiP2a stimulating activity is due mainly to T lymphocyte expansion. Also, the stimulatory effect was demonstrated to be antigen-specific, since the proliferation was abrogated by the presence of anti-LiP2a antibodies. Interestingly, the LiP2a protein stimulated the production of substantial amounts of IFN-γ in cultured splenocytes from LiP2a-immunized mice. Our data indicate therefore that the immunostimulatory properties shown by this antigen should be taken into account when developing therapeutic and prophylactic vaccines against leishmaniasis.
Keywords: Leishmania, acidic ribosomal protein, humoral response, interferon-gamma, lymphoproliferation
Introduction
Leishmania species are protozoan parasites of vertebrates capable of causing a wide spectrum of human and veterinary diseases known as leishmaniasis. The spectrum of diseases ranges from small cutaneous ulcers, which heal spontaneously, to disseminated lethal infections. The clinical manifestations of leishmaniasis depend on complex interactions between the virulence characteristics of the infecting Leishmania species and the status of host immune system [1].
Experimental infection of inbred strains of mice with L. major constitutes an excellent model for the study of immunoregulation of the host response to this intracellular pathogen. The central finding that has evolved from this model is the observation that protective immunity to L. major-infection correlates with a robust Th1 cell response that ensures the production of macrophage-activating cytokines, particularly IFN-γ, that are required for the control of the replication of the parasite. In contrast, susceptibility has been associated with the development of a Th2 cell response that is unable to mediate macrophage activation and that actively abrogates the action of Th1-derived cytokines (reviewed in [2]). Th1 and Th2 cells are also differentially activated in various clinical presentations of human leishmanial infections [3]. Thus, the common paradigm is that self-limited infections result from a strong Th1 response against leishmanial antigens, whereas the severe forms of the disease correlate with the activation of Th2 cells.
The factors that determine whether Th1 or Th2 CD4+ cells dominate during episodes of leishmaniasis have not yet been fully defined. The possibility that the antigen itself, depending on its proper nature, may play a role in the polarization of the response has been suggested [4]. In the search for Leishmania antigens we identified acidic ribosomal proteins as prominent antigens during both human and canine leishmaniasis [5,6]. Acidic ribosomal proteins, also named P proteins, are constituents of the large subunit of all ribosomes and are required for the functional activity of the ribosome [7,8]. The P protein family, highly conserved among eukaryotes, comprises three members (namely P0, P1 and P2) forming a protruding stalk, consisting of two P1/P2 homodimers attached to the ribosome through the P0 protein [9]. Remarkably, these proteins have been reported as antigens capable of inducing IgG antibody responses in systemic autoimmune diseases [10] and in the Chagas' disease [11], caused by infection by Trypanosoma cruzi. In those diseases, the antibodies are directed against the C-terminal domain, a consensus sequence shared by all three P proteins [11]. Recently, the P2 proteins have also been described as major allergens in several fungal allergies [12–14]. Because of the prominent antigenicity of the acidic ribosomal proteins observed in the sera from animals naturally infected with L. infantum, we decided to analyse the immunogenic properties of the L. infantum LiP2a ribosomal protein in the mouse model.
Materials and methods
Plasmid construction and protein purification
In order to obtain the Leishmania LiP2a protein as a non-fusion recombinant protein, the insert of a cDNA clone coding for the entire protein, derived from pMal-cRI-LiP2a [6], was obtained by digestion with SacI and HindIII restriction endonucleases and cloned in the corresponding restriction sites of the pQE31 prokaryotic expression plasmid (Qiagen, Hilden, Germany). Expression and purification of the recombinant LiP2a was performed under denaturing conditions (8 m urea, 0·1 m NaH2PO4, 0·01 m Tris–HCl) on a Ni-NTA agarose column, following the methodology provided by the supplier (Qiagen). Briefly, 100-ml cultures of Escherichia coli transformed with the recombinant plasmid were induced for 4 h by 2 mm IPTG. The pelleted cells were lysed by sonication in the purification buffer at pH 8·0. After centrifugation the supernatant was passed through to nitrilotriacetic acid-Ni2+ columns (Qiagen). After elution, fractions containing the recombinant protein were pooled and dialysed against PBS. To eliminate endotoxins, the recombinant protein preparations were further passed through a polymyxin-agarose column (Sigma, St Louis, MO). Recombinant Leishmania MBP-LiP2a, human P2 and T. cruzi P2 acidic ribosomal proteins were expressed and purified as reported elsewhere [6].
Mice and immunizations
Female 8-week-old BALB/c mice were purchased from Harlan Interfauna Iberica S.A. (Barcelona, Spain). The mice were immunized intraperitoneally on days 0 and 21 with 5 μg of the recombinant protein in 200 μl of PBS. The mice were periodically bled from the retro-orbital plexus.
Determination of antibody titres and isotypes
Standard ELISA plates were coated overnight at room temperature with 100 μl of rLiP2a (1 μg/ml in PBS). A serial dilution of the sera was carried out in order to determine the titre, which was defined as the inverse of the highest serum dilution factor giving an absorbance > 0·2. The isotype-specific analysis was done using one of the following horseradish peroxidase-conjugated anti-mouse immunoglobulins (Nordic Immunological Laboratories, Tilburg, The Netherlands): anti-IgG (1:1000 and 1:2000), anti-IgM (1:1000 and 1:2000), anti-IgG1 (1:1000 and 1:2000) or anti-IgG2a (1:500 and 1:1000). As peroxidase substrate, the orthophenylene diamine (Dako, A/S, Glostrup, Denmark) was used. The absorbance was read at 450 nm after 20 min of reaction.
Immunoblot analysis
SDS–PAGE gels were performed using standard conditions [15]. For Western blot analysis, after electrophoretic separation, the proteins were transferred to nitrocellulose membranes (Amersham, Aylesbury, UK). The transfers were blocked with 5% non-fat dried milk powder in PBS and 0·5% Tween 20. The filters were sequentially probed with primary and secondary antibodies in blocking solution. A goat anti-mouse IgG peroxidase immuno-conjugate (Nordic Immunology) was used as secondary antibody (1:2000) and the specific binding was revealed with the Western blotting detection ECL system (Amersham).
Lymphoproliferation assays
Spleens from BALB/c mice were removed aseptically. The single-cell suspension was prepared in complete RPMI medium (RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine, and 10 μm 2-mercaptoethanol). Lysis of erythrocytes was achieved by placing the cells in lysis buffer (150 mm NH4Cl, 10 mm CO3HK, 1 mm EDTA, pH 7·4) at 37°C for 2 min. Afterwards, the splenocytes were washed with complete medium and counted. Two hundred microlitres of cells (2·5 × 106 cells/ml) were plated per well in a 96-well flat-bottomed microtitre plate in the presence of various concentrations of the rLiP2a recombinant protein or 1 μg/ml of concanavalin A (Con A; Sigma). Splenocyte cultures were incubated at 37°C for the indicated times in a humidified chamber containing 5% CO2. The cultures were pulsed with 1 μCi of 3H-thymidine (5 Ci/mmol; Amersham) for the final 16 h of culture. At the end of this period the cells were harvested on filters. The incorporation of 3H-thymidine into the DNA was measured by liquid scintillation counting.
For the proliferation inhibition assays using antibodies, a specific anti-LiP2a serum was added to the splenocyte cultures at the indicated dilutions. The anti-LiP2a serum was obtained by immunization of a rabbit with the L. infantum LiP2a recombinant protein. Briefly, a New Zealand white rabbit was immunized with 0·5 mg of rLiP2a protein in Freund's complete adjuvant (FCA; Difco, Detroit, MI) followed by two boosters at 2-week intervals with 0·2 mg of the same protein in Freund's incomplete adjuvant (FIA). The serum was obtained 2 weeks after the last boost. The titre of this serum was > 50 000 as determined by ELISA using the rLiP2a as the coating antigen.
T lymphocytes were purified from splenocytes by nylon fibre columns as previously described [16]. Briefly, adherent cells were removed by incubation of splenocytes for 2 h onto plastic dishes. After centrifugation, the non-adherent population (2·5 × 107 cells) was resuspended in 0·5 ml of RPMI medium supplemented with 1% FCS and added to a 2-ml column containing 0·2 g of nylon fibre (Dupont); the nylon fibre column was washed with 10 ml of RPMI medium (containing 1% FCS) and pretreated with 4 ml of RPMI medium supplemented with 20% FCS for 1 h. The cells were added to the column and allowed to interact with the nylon fibre for 2 h at 37°C. Finally, after adding 6 ml of RPMI medium supplemented with 1% FCS, the non-bound cells (T lymphocytes) were collected, centrifuged, resuspended in complete RPMI medium and used for proliferation assays as indicated above. Purity was determined by flow cytometry before and after passage. Percentages of CD3+ cells were 30–35% before nylon wool passage and 80–85% after the purification.
Measurement of cytokines in supernatants
For cytokine determinations, splenocytes (5 × 106 cells/well) from either control or immunized mice were seeded in 24-well plates and cultured during 72 h at 37°C in the presence of 10 μg/ml of LiP2a or 1 μg/ml of Con A. Commercial ELISA kits were used to assess the release of IFN-γ, IL-4, and IL-12 into the supernatants (Endogen, Woburn, MA).
Results
Humoral response elicited by immunization of BALB/c mice with rLiP2a protein
We have previously reported that the L. infantum acidic ribosomal proteins behave as prominent antigens specifically recognized by the sera from dogs having the viscerocutaneous form of leishmaniasis and from the sera of humans affected by either mucosal or visceral leishmaniasis [5,6]. In those studies, the recombinant proteins were obtained fused to the maltose binding protein (MBP). To free the recombinant rLiP2a protein from MBP moiety, the coding region of the LiP2a gene was subcloned into the E. coli expression vector pQE31. Thus, the resulting expressed LiP2a protein contained only the 6 his-tag from the vector as exogenous sequence. The recombinant protein was expressed at high levels upon induction with IPTG and purified by affinity chromatography.
In order to analyse the immunogenic properties of the Leishmania LiP2a acidic ribosomal protein, a group of 12 BALB/c mice were immunized intraperitoneally on days 0 and 21 with 5 μg each of the rLiP2a protein in PBS. We used this immunization schedule because it has been shown to be effective with other Leishmania antigens [17]. In fact, high titres (above 10 000) of anti-LiP2a IgG were found on day 28. As is shown in Fig. 1, the humoral response showed features of a conventional secondary response since there was a progressive switch from IgM to IgG antibodies after the protein boosting as an indication that the immunization with the rLiP2a elicited a T cell-dependent immune response. At that time, a switch of antibody classes from the IgG2a to the IgG1 isotype was also observed. On day 7 after rLiP2a administration the anti-LiP2a IgG2a isotype (mean titre of 1900) was found to predominate over the IgG1 isotype (titres < 200). However, after the second administration of rLiP2a the increase in anti-LiP2a IgG titres was due primarily to the increase in the level of the IgG1 response, even though the anti-LiP2a IgG2a titres also increased. As indicated by Coffman et al. [18], a hallmark for a Th1 to Th2 cytokine shift in vivo is the shift in the antibody isotype from IgG2a to IgG1. Thus, we may conclude that i.p. administration of the rLiP2a protein primarily elicits a Th1-like response when first encountered by the cells from the immune system, which is then shifted to a predominant Th2-like response upon further stimulation with the antigen.
Fig. 1.
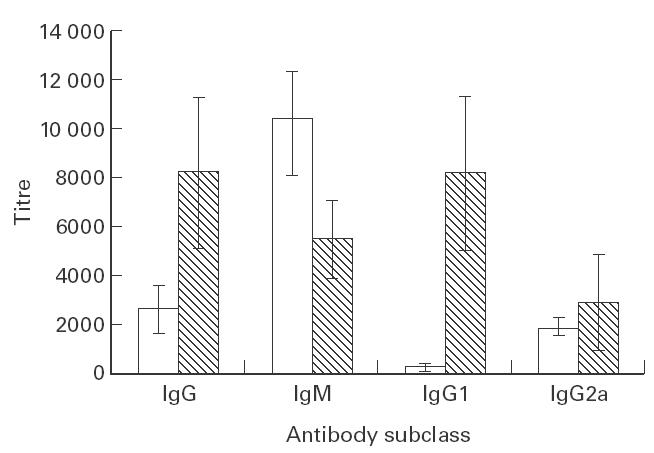
Humoral immune response induced by the immunization of BALB/c mice with the rLiP2a protein. Sera from 12 immunized mice were individually assayed by ELISA 7 days after the first immunization (□) or 7 days after the second immunization (hatched bars). Titres were determined for IgG, IgM, IgG1, and IgG2a antibody subclasses. None of the preimmune sera showed reactivity against the rLiP2a protein.
Specificity of the antibody response
Taking into account the structural features of the LiP2a antigen, with an evolutionarily conserved carboxyl-terminal domain that is the target of autoantibodies elicited during several diseases, we considered it of interest to characterize the specificity of the anti-LiP2a antibodies induced in the BALB/c mice. First, we tested the reactivity by FAST-ELISA of the 12 serum samples (obtained on day 28) against a series of seven synthetic peptides (20mer, overlapping in five residues) covering the entire Leishmania LiP2a acidic ribosomal protein (Fig. 2a). The reactivity was observed against peptides A4, A5 and A6. Remarkably however, no reactivity was observed against peptide A7 which contains the conserved carboxyl-terminal sequence. The high s.d. values obtained for peptide A5 reflect the immune response diversity between animals against that peptide, since it was recognized only by the sera from four out of the 12 immunized mice, whereas peptides A4 and A6 were recognized by the sera from all the animals (12/12). It must be noted that peptide A6 contains the main antigenic determinant of the LiP2a protein that is recognized by the sera from humans and dogs affected by leishmaniasis [5,6].
Fig. 2.
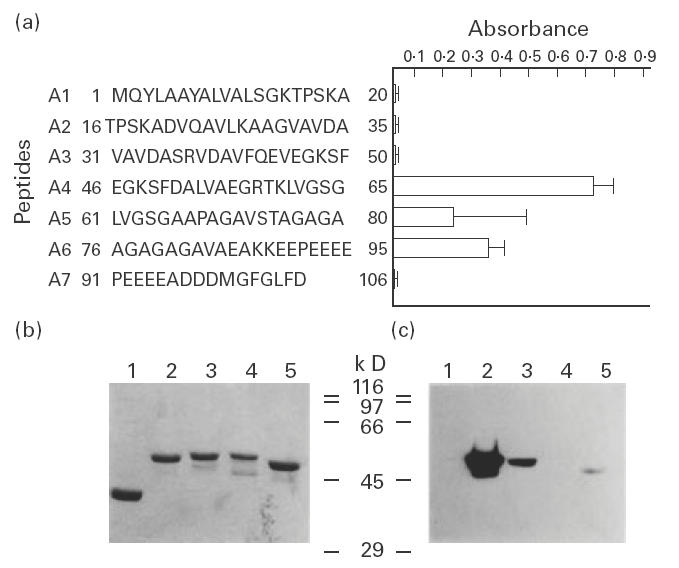
Reactivity of sera from LiP2a-immunized mice against the LiP2a synthetic peptides and analysis of the specificity of anti-LiP2a antibodies. (a) The reactivity of sera from 12 LiP2a-immunized mice against the 20mer peptides (overlapping by five amino acids), covering the whole Leishmania LiP2a protein, was determined by FAST-ELISA. The mean reactivity values (± s.d.) of sera, assayed at 1:200 dilution, against each peptide are shown. (b) Coomassie blue-stained 10% SDS–PAGE gel containing 2 μg of purified maltose binding protein (MBP; lane 1), MBP-LiP2a (lane 2), MBP-LiP2b (lane 3), MBP-human P2 (lane 4), and MBP-Trypanosoma cruzi P2 (lane 5). Molecular mass markers are shown in kD. (c) An equivalent gel to that of (b) was blotted and probed with a pool of 12 immune sera at a final dilution of 1:200.
The specificity of the humoral response elicited by the immunization with rLiP2a was also assayed by Western blotting (Fig. 2b,c). The sera from the immunized mice were incubated with a blot containing the P2 acidic ribosomal proteins from Leishmania, Homo sapiens and T. cruzi, expressed as MBP-fused recombinant proteins. As is shown in (Fig. 2c), the sera strongly reacted with the MBP-LiP2a protein. Also, a slight reactivity was observed with the Leishmania rLiP2b and the T. cruzi P2, in agreement with the significant sequence conservation shared by the P2 proteins from these two kinetoplastid protozoa [11,19]. Remarkably, no signal was obtained in the lane containing the human P2 protein, demonstrating that anti-P autoantibodies were not elicited by immunization with the rLiP2a protein.
Lymphoproliferative responses to rLiP2a in BALB/c mice
In order to analyse the cellular immune response elicited by immunization with the rLiP2a protein, spleens from immunized mice were removed 2 weeks after the last immunization. The splenic cells were stimulated in vitro with different concentrations of rLiP2a. The data presented in Fig. 3a demonstrate that the splenocytes from the immunized mice proliferated in response to the antigen in a dose-dependent manner. However, it was surprising that splenocytes from naive BALB/c mice also proliferated in the presence of rLiP2a in a similar way and dose-dependent manner as the splenocytes from the LiP2a-immunized mice (Fig. 3a).
Fig. 3.
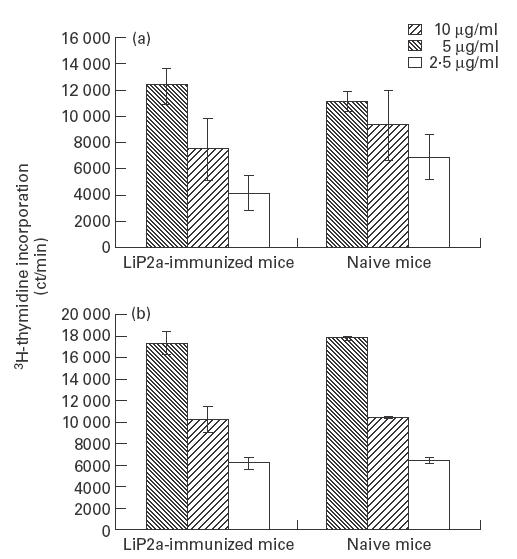
Proliferation of splenocytes and T cell-enriched populations from LiP2a-immunized and naive BALB/c mice in response to the rLiP2a protein. Splenocytes (a) or T cell-enriched populations (b), 5 × 105 cells per well, were incubated for 72 h in the presence of the indicated concentrations of rLiP2a and pulsed with 1 μCi of 3H-thymidine.
We next examined the proliferation induced by rLiP2a on T cell-enriched cultures. Thus, T cell-enriched fractions, purified by nylon fibre columns from spleens of either LiP2a-immunized or naive BALB/c mice, were incubated with different concentrations of rLiP2a. A similar type of dose-dependent proliferation was, again, observed in the T cells coming from the immunized and naive animals (Fig. 3b). Although the rLiP2a preparation used in the present study can be considered as endotoxin-free (see Materials and methods), control experiments were performed in which soluble polymyxin B (0·5 μg/ml) was additionally added to the T cell cultures. As expected, the addition of soluble polymyxin B did not reduce the 3H-thymidine incorporation of rLiP2a-stimulated cultures (data not shown), thus excluding that the observed stimulation could be due to contaminating lipopolysaccharide (LPS). To further support that the proliferation of the splenocytes was induced by rLiP2a, we performed inhibition assays using specific anti-LiP2a antibodies (Fig. 4). The results indicated that the presence of anti-LiP2a antibodies in the assay had a marked inhibitory effect on the proliferation rates of splenocytes from either rLiP2a-immunized (Fig. 4a) or naive BALB/c mice (Fig. 4b). As control, the effect of anti-LiP2a antibodies on Con A stimulation was analysed, demonstrating that the inhibitory effect of the antibody was antigen-specific.
Fig. 4.

Inhibition of the LiP2a-induced proliferation of splenocytes by the presence of a specific antibody. Splenic cells (5 × 105 cells/well) from LiP2a-immunized (a) and naive (b) mice were stimulated with either concanavalin A (Con A; 1 μg/ml) or rLiP2a (10 μg/ml) in the presence of different dilutions of an anti-LiP2a antibody. Proliferation was measured by 3H-thymidine incorporation. The incorporation of thymidine (proliferation) in either Con A- or rLiP2a-stimulated splenocytes in the absence of antibodies (No Ab) was set at 100%. Data represent the mean ± s.d. from three separate experiments.
The time course of the response of murine splenocytes to rLiP2a was also assessed (Fig. 5). Splenocytes from immunized and naive BALB/c were stimulated with either Con A or rLiP2a, and the 3H-thymidine incorporation was measured at days 1, 2, 3 and 4. In splenocytes from naive BALB/c mice, the maximum stimulation of rLiP2a was observed on days 3 and 4 (Fig. 5a). On the other hand, in splenocytes from the rLiP2a-immunized mice the maximum values of stimulation were observed on day 2. This suggests that the onset of proliferation was faster in the rLiP2a-primed cells than in the rLiP2a-umprimed cells. However, as was indicated above (Fig. 3a,b), the maximum stimulation values induced by rLiP2a were similar for both groups of mice. In the positive controls, stimulated with Con A, the peak of 3H-thymidine incorporation was observed on day 3 for the splenocytes from both immunized and naive mice (Fig. 5a,b). This fact indicates that there were no differences in the proliferative capacity between both splenocyte populations.
Fig. 5.

Time course of in vitro proliferation induced by rLiP2a on splenocytes from LiP2a-immunized (a) and naive (b) BALB/c mice. Cells (5 × 105 per well) were incubated for 24 h, 48 h, 72 h and 96 h in the presence of either 10 μg/ml of rLiP2a (▪) or 1 μg/ml of concanavalin A (•). Cultures were pulsed with 1 μCi of 3H-thymidine for the last 16 h.
Analysis of lymphokine production associated with the splenocyte proliferation induced by rLiP2a
Given the ability of the rLiP2a protein to stimulate the in vitro proliferation of splenic cells, we next examined whether lymphokine secretion was specifically induced in the LiP2a-stimulated splenocytes (Table 1). Interestingly, after stimulation with rLiP2a, the supernatants from the splenocyte cultures derived from the LiP2a-immunized mice contained significant amounts of IFN-γ (8·66 ng/ml). Also, IFN-γ secretion was detected in the rLiP2a-stimulated splenocytes derived from naive mice (1·2 ng/ml). Control experiments in which Con A was used as stimulus demonstrated that both splenocyte populations (those derived from either naive or LiP2a-immunized mice) had intrinsically the same capacity for secreting IFN-γ in response to an appropriate signal. Although Table 1 only shows the levels of IFN-γ produced after 72 h of stimulation, similar values were observed in assays performed at either 24 h or 48 h. Comparable amounts of IFN-γ were detected in the supernatants of T cell-enriched cultures derived from either naive or LiP2a-immunized mice when they were in vitro stimulated with rLiP2a (data not shown). These data demonstrate that the rLiP2a protein has the ability to stimulate the production of IFN-γ in unprimed splenocytes, although the IFN-γ production is higher when LiP2a-primed cells are present in the splenocyte culture.
Table 1.
Release of IFN-γ and IL-4 by splenocytes exposed to concanavalin A (Con A) or rLiP2a
| Concentration (mean ± s.d.)† | ||||
|---|---|---|---|---|
| Naive mice | rLiP2a-immunized mice | |||
| Stimulus* | IFN-γ (ng/ml) | IL-4 (pg/ml) | IFN-γ (ng/ml) | IL-4 (pg/ml) |
| Con A | 141·97 ± 25 | 100·04 ± 19·5 | 160 ± 38 | 69·07 ± 11·9 |
| rLiP2a | 1·2 ± 0·41 | 47·15 ± 5·22 | 8·66 ± 0·32 | 43·19 ± 23·8 |
| Medium | 0·31 ± 0·15 | 22·11 ± 5·56 | 0·22 ± 0·07 | 27·84 ± 14·7 |
The final concentration of Con A was 1 μg/ml, and the final concentration of the rLiP2a protein was 10 μg/ml. Cytokine concentrations were determined in supernatants from splenocyte cultures (5 × 106 cells/ml) after incubation with the stimulus for 72 h.
Mean ± s.d. of 12 determinations, each performed in triplicate.
A slight production of IL-4, two-fold above the value of that of unstimulated cells (Table 1), was also observed after in vitro rLiP2a stimulation of splenocytes from either LiP2a-immunized or naive mice. We also checked for the presence of IL-12 in the supernatants of splenocytes derived from both groups of mice after proliferation induced by the rLiP2a protein. In our conditions, in which the detection limit was 12 pg/ml, we could not observe the presence of this cytokine. Thus, it appears that the LiP2a is unable to elicit in vitro the production of appreciable levels of IL-12.
Discussion
An important observation deriving from this work is that the L. infantum LiP2a acidic ribosomal protein possesses the remarkable ability to generate a strong humoral response when immunized in BALB/c mice in the absence of adjuvant. This result can provide a direct explanation of previous findings in which the Leishmania acidic proteins have been described as prominent antigens during both human and canine visceral leishmaniasis [5,6]. The results presented have further shown that the LiP2a protein exhibits an unusual immunostimulatory property, since it is able to stimulate the in vitro proliferation of splenocytes from naive BALB/c mice as occurs in splenocytes from LiP2a-immunized animals. This response is also observed with T cell-enriched populations. A glance at the scientific literature on the immunological properties associated with the acidic ribosomal proteins allows us to suggest that the features described here for the L. infantum LiP2a may be a consequence of a pre-existing immune response against conserved epitopes of the acidic ribosomal proteins. Thus, antibodies against ribosomal P proteins have been found in sera from patients with infectious diseases other than leishmaniasis (for example, Chagas' disease [11]), patients with fungal allergies [12–14], and patients having autoimmune processes such as systemic lupus erythematosus (SLE [10]). The autoantibodies present in the sera of SLE patients are directed against the evolutionarily conserved carboxyl-terminal end of the P-proteins [20]. In recent works, the presence has been reported of masked anti-ribosomal P autoantibodies in both healthy children and adults [21–23]. Moreover, the existence has been suggested of an idiotypic/anti-idiotypic network of anti-P antibodies in normal individuals, and that the appearance of overt anti-P antibodies during pathogenic processes is associated with the loss of anti-idiotypic regulation [23]. Thus, we hypothesize that the immunostimulatory properties of the L. infantum LiP2a can be explained in part by the existence of this immune network against the P proteins. The present data indicate however, that the regulatory capacity of this network was not broken in the LiP2a-immunized mice since the anti-LiP2a antibodies elicited by immunization are directed against epitopes other than the carboxyl-terminal end. Thus, after LiP2a immunization, the humoral response has been demonstrated to be specific against the Leishmania protein.
In a previous work, Kemp et al. [24] have shown that crude antigen preparations of Leishmania promastigote are able to induce in vitro proliferation and IFN-γ production in peripheral blood mononuclear cells (PBMC) from individuals without known exposure to the parasite. From the present results, it can be postulated that the mitogenic properties of crude preparations of Leishmania proteins may be due to the immunostimulatory effect of the acidic ribosomal proteins on proliferation of unprimed T cells. In fact, the acidic P proteins, as multicopy ribosome components, are among the most abundant proteins present in the cell [11]. However, the Leishmania LiP2a protein is not the first molecule derived from trypanosomatids that shows immunostimulatory properties. Vaidya et al. [25] have characterized a protein expressed by T. brucei that triggers CD8+ T lymphocytes to proliferate and to secrete IFN-γ. Accordingly, this protein was called T lymphocyte triggering factor (TLTF). Also, the rLeIF, the L. braziliensis homologue to the eukaryotic ribosomal protein eIF4A protein, can be considered as an immunomodulating molecule, since it has the ability to stimulate the production of IL-12 in cultured PBMC from both patients and uninfected individuals [26]. Also, it has been reported that the Leishmania phosphoglycans selectively inhibit the synthesis of IL-12 by activated macrophages [27].
Another relevant feature associated with the immunostimulatory properties of the LiP2a protein is its ability to elicit the production of significant amounts of IFN-γ from splenocyte cells of LiP2a-immunized BALB/c mice (Table 1). In agreement with a Th1-type cytokine profile, the amount of IL-4 in supernatants of LiP2a-stimulated cultures was found to be low. On the other hand, although the subtype analysis of the repertoire of anti-LiP2a antibodies present in LiP2a-immunized BALB/c indicated that the IgG2a subtype predominates over the IgG1 subtype, this subtype increases significantly over the IgG2a subtype after an immunization boosting. The reason for that switching is at present unknown. Given that Th1 cytokines preferentially elicit an IgG2a antibody response whereas Th2 cytokines stimulate IgG1 antibodies [18], both results are somewhat controversial. It has to be taken in consideration however, that while we are analysing the in vivo humoral response, the cytokine secretion of splenocytes was stimulated in vitro. A conciliatory result could be found in the observation that a single immunization with LiP2a elicited anti-LiP2a IgG antibodies exclusively of the IgG2a isotype, and that a second immunization with the protein seems to override the Th1-like response. In order to address this apparent paradox, we are currently investigating the mechanisms by which LiP2a induces IFN-γ production. These studies are of further value since this cytokine is clearly associated with protection in experimental leishmaniasis [2,28] and has been successfully used as immunotherapeutic treatment for kala-azar patients [29]. Therefore, it is justified to carry out additional research on the immunostimulatory properties of the LiP2a protein in order to assess its use as prophylactic and therapeutic vaccine for leishmanial infection. In this regard, there are precedents indicating that ribosomal vaccines may protect against infection of intracellular bacteria, and that the ribosomal protein responsible of those immunological properties is the L7/12 protein, the prokaryotic homologue to the acidic ribosomal proteins from eukaryotes. It has been demonstrated that the recombinant ribosomal L7/12 protein from Brucella abortus induces a proliferative response of PBMC from B. abortus-vaccinated cattle [30]. Also, Bachrach et al. [31] demonstrated that the brucella ribosomal protein L7/L12 induces a DTH reaction, a response associated with protection against brucellosis in humans and animals. Remarkably, it has been recently reported that immunization of mice with the recombinant L7/L12 ribosomal protein confers protection against B. abortus infection [32].
Acknowledgments
This research was supported by grants 1FD97-0630-C02-0 (Plan Nacional de I + D), BIO99-1133 (Plan Nacional de I + D), and 08.2/0022.1/99 (Comunidad Autónoma de Madrid). An Institutional grant from Fundación Ramón Areces is also acknowledged.
References
- 1.Pearson RD, Sousa AQ. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1996;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 3.Kemp M, Theander TG, Kharazmi A. The contrasting roles of CD4+ T cells in intracellular infections in humans: leishmaniasis as an example. Immunol Today. 1996;17:13–16. doi: 10.1016/0167-5699(96)80562-7. [DOI] [PubMed] [Google Scholar]
- 4.Shearer GM, Clerici M. Vaccine strategies: selective elicitation of cellular or humoral immunity? Trends Biotechnol. 1997;15:106–9. doi: 10.1016/S0167-7799(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 5.Soto M, Requena JM, Quijada L, Alonso C. Specific serodiagnosis of human leishmaniasis with recombinant Leishmania P2 acidic ribosomal proteins. Clin Diagn Lab Immunol. 1996;3:387–91. doi: 10.1128/cdli.3.4.387-391.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soto M, Requena JM, Quijada L, Angel SO, Gomez LC, Guzman F, Patarroyo ME, Alonso C. During active viscerocutaneous leishmaniasis the anti-P2 humoral response is specifically triggered by the parasite P proteins. Clin Exp Immunol. 1995;100:246–52. doi: 10.1111/j.1365-2249.1995.tb03661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Möller W, Schrier PI, Maassen JA, Zantema A, Schop E, Reinalda H. Ribosomal proteins L7/L12 of Escherichia coli. J Mol Biol. 1983;163:553–73. doi: 10.1016/0022-2836(83)90112-2. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Madrid F, Reyes R, Conde P, Ballesta JPG. Acidic ribosomal proteins from eukaryotic cells: effect on ribosomal functions. Eur J Biochem. 1979;98:409–16. doi: 10.1111/j.1432-1033.1979.tb13200.x. [DOI] [PubMed] [Google Scholar]
- 9.Uchiumi T, Wahha AJ, Traut RR. Topography and stoichiometry of acidic proteins in large ribosomal subunits from Artemia salina as determined by crosslinking. Proc Natl Acad Sci USA. 1987;85:5580–4. doi: 10.1073/pnas.84.16.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkon K, Skelly S, Parnassa A, Moller W, Danho W, Weissbach H, Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1986;83:7419–23. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin MJ, Vazquez M, Kaplan D, Schijman AG. The Trypanosoma cruzi ribosomal P protein family: classification and antigenicity. Parasitol Today. 1993;9:381–4. doi: 10.1016/0169-4758(93)90088-w. [DOI] [PubMed] [Google Scholar]
- 12.Achatz G, Oberkofler H, Lechenauer E, et al. Molecular cloning of major and minor allergens of Aternaria alternata and Cladosporium herbarum. Mol Immunol. 1995;32:213–27. doi: 10.1016/0161-5890(94)00108-d. [DOI] [PubMed] [Google Scholar]
- 13.Mayer C, Appenzeller U, Seelbach H, Achatz G, Oberkofler H, Breitenbach M, Blaser K, Crameri R. Humoral and cell-mediated autoimmune reactions to human acidic ribosomal P2 protein in individuals sensitized to Aspergillus fumigatus P2 protein. J Exp Med. 1999;189:1507–12. doi: 10.1084/jem.189.9.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Muradia G, Curran IHA, Rode H, Vijay HM. A cDNA clone coding for a novel allergen, Cla h III, of Cladosporium herbarum identified as a ribosomal P2 protein. J Immunol. 1995;154:710–7. [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structured proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Werner C, Klouda PT, Correa MC, Vassalli P, Jeannet M. Isolation of B and T lymphocytes by nylon fiber columns. Tissue Antigens. 1977;9:227–9. doi: 10.1111/j.1399-0039.1977.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 17.Rico AI, del Real G, Soto M, Quijada L, Martínez AC, Alonso C, Requena JM. Characterization of the immunostimulatory properties of Leishmania infantum HSP70 by fusion to the Escherichia coli maltose-binding protein in normal and nu/nu BALB/c mice. Infect Immun. 1998;66:347–52. doi: 10.1128/iai.66.1.347-352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffman RL, Seymour BWP, Lebman DA, et al. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 19.Soto M, Requena JM, García M, Gómez LC, Navarrete I, Alonso C. Genomic organization and expression of two independent gene arrays coding for two antigenic acidic ribosomal proteins of Leishmania. J Biol Chem. 1993;268:21835–43. [PubMed] [Google Scholar]
- 20.Elkon K, Bonfa E, Llovet R, Danho W, Weissbach H, Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci USA. 1988;85:5186–9. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson CJ, Neas BR, Pan Z, Taylor-Albert E, Reichlin M, Stafford HA. The presence of masked antiribosomal P autoantibodies in healthy children. Arthritis Rheum. 1998;41:33–40. doi: 10.1002/1529-0131(199801)41:1<33::AID-ART5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Pan Z-J, Stafford HA. Recombinant ribosomal P2 protein can unmask anti-ribosomal P autoantibodies from healthy adults. J Lab Clin Med. 1996;127:333–9. doi: 10.1016/s0022-2143(96)90180-8. [DOI] [PubMed] [Google Scholar]
- 23.Pan Z-J, Anderson CJ, Stafford HA. Anti-idiotypic antibodies prevent the serologic detection of antiribosomal P autoantibodies in healthy adults. J Clin Invest. 1998;102:215–22. doi: 10.1172/JCI1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp M, Hansen MB, Theander TG. Recognition of Leishmania antigens by T lymphocytes from nonexposed individuals. Infect Immun. 1992;60:2246–51. doi: 10.1128/iai.60.6.2246-2251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaidya T, Bakhier M, Hill KL, Olsson T, Kristensson K, Donelson JE. The gene for a T lymphocyte triggering factor from African trypanosomes. J Exp Med. 1997;186:433–8. doi: 10.1084/jem.186.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skeiky YAW, Guderian JA, Benson DR, et al. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J Exp Med. 1995;181:1527–37. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piedrafita D, Proudfoot L, Nikolaev AV, et al. Regulation of macrophage IL-12 synthesis by Leishmania phosphoglycans. Eur J Immunol. 1999;29:235–44. doi: 10.1002/(SICI)1521-4141(199901)29:01<235::AID-IMMU235>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 28.Murray HW, Delph-Etienne S. Roles of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2000;68:288–93. doi: 10.1128/iai.68.1.288-293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundar S, Rosenkaimer F, Murray HW. Successful treatment of refractory visceral leishmaniasis in India using antimony plus interferon-gamma. J Infect Dis. 1994;170:659–62. doi: 10.1093/infdis/170.3.659. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira SC, Splitter GA. Subcloning and expression of the Brucella abortus L7/L12 ribosomal gene and T-lymphocyte recognition of the recombinant protein. Infect Immun. 1994;62:5201–4. doi: 10.1128/iai.62.11.5201-5204.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachrach G, Banai M, Bardenstein S, Hoida G, Genizi A, Bercovier H. Brucella ribosomal protein L7/L12 is a major component in the antigenicity of brucellin INRA for delayed-type hypersensitivity in brucella-sensitized guinea pigs. Infect Immun. 1994;62:5361–6. doi: 10.1128/iai.62.12.5361-5366.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira SC, Splitter GA. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine. 1996;14:950–62. doi: 10.1016/0264-410x(96)00018-7. [DOI] [PubMed] [Google Scholar]


