Abstract
Atopic eczema (AE) is characterized by the persistence of infiltrating T lymphocytes in the dermis. To test the hypothesis that dysregulation of normal T cell apoptosis may contribute to the pathogenesis and chronicity of AE we compared patients with a normal resolving immune response (Mantoux reaction (MR)) induced in healthy volunteers by cutaneous PPD injection. Significantly less T cell apoptosis was observed in lesional skin of AE patients compared with either the peak or the resolution phase of the MR (P < 0·0001). The low incidence of T cell apoptosis in AE was associated with significantly increased levels of Bcl-2 relative to Bax (P < 0·0001) and significantly decreased CD95-L expression (P < 0·002) compared with the resolving MR. The cytokines IL-15 and interferon-beta (IFN-β), which prevent activated T cell apoptosis, were expressed maximally on day 7 and day 14 of the MR, respectively. In contrast, AE patients expressed high levels of both IL-15 and IFN-β in cutaneous lesions at the same time. This suggests that the co-expression of two anti-apoptotic cytokines, which are not found together during resolving cutaneous responses, may contribute to excessive T cell survival which leads to the persistence of inflammation in patients with AE.
Keywords: atopic eczema, apoptosis, IL-15, interferon-beta
Introduction
During the resolution phase of a ‘normal’, self-limiting inflammatory response, most of the expanded T cell population is cleared by the process of apoptosis, while a minority survive to establish T cell memory [1,2]. In chronic inflammation however, activated T cells are not cleared, and the response ‘fails to resolve’ [3–5]. This raises the question of whether chronic inflammation is generated as a result of dysregulation of T cell apoptosis.
Classical tuberculin PPD-induced DTH responses and atopic eczema (AE) are both examples of cutaneous inflammatory responses in which infiltrating T lymphocytes are thought to play a major pathogenic role [6–8]. There is increasing evidence to suggest that AE represents a DTH-type response to environmental allergens/antigens, triggered by IgE receptor-bearing cutaneous antigen-presenting cells (APC) [9]. Whereas PPD-induced DTH responses are resolving in terms of leucocyte infiltration by day 14 after challenge [10], the cutaneous inflammation in AE frequently persists well beyond this time point. The possibility that a dysregulation of normal T cell apoptosis, perhaps as a result of the intralesional cytokine microenvironment, may contribute to the pathogenesis and chronicity of AE has not previously been addressed, but is clearly of relevance to the development of successful future therapies.
Activated T cell apoptosis may occur via two distinct pathways. The first involves interaction of CD95 or related molecules with their ligands [11]. The second occurs following withdrawal of cytokines, including those which signal via the γ-chain of the IL-2 receptor (IL-2R) [2,12,13] and type I interferons [4,14,15]. These two groups of cytokines rescue T cells from death via different mechanisms [2,4,16]. The generation of chronic inflammation after antigenic stimulation may reflect an aberrant rescue of T cells from apoptosis resulting from the over-expression of such anti-apoptotic cytokines and the inappropriate survival of excess numbers of T cells [3,5]. We have recently shown that in humans, there are phases of apoptosis that occur at the peak of a DTH response on day 7, and also during the resolution phase on day 14 [10]. While the death observed on day 7 is likely to be CD95-mediated, the apoptosis on day 14 was due to cytokine withdrawal [10].
In this study we investigated the intralesional expression of apoptosis regulatory proteins and cytokines which regulate these molecules in chronic lesional AE. We compared this with a resolving T cell-mediated PPD-induced DTH response, in order to test the hypothesis that dysregulation of T cell apoptosis may contribute to the persistence of the cutaneous T cell infiltrate in AE.
PATIENTS and METHODS
Patients samples
Normal skin was obtained from surgical specimens in five all female, age range 17–39 years, median 25 years. Twenty healthy volunteers, previously immunized with bacille Calmette–Guérin (BCG) (15 male, age range 23–59 years, median 30 years) were recruited from laboratory and Hospital personnel. Subjects had no personal or family history of allergic rhinitis (hay fever), asthma or eczema. Mantoux tests were performed on the volar surface of the non-dominant forearm. Testing was first with 0·1 ml of a 1:10 000 solution of tuberculin PPD (Evans Medical Ltd, Leatherhead, UK) and then, if negative at 48–72 h, with 1:1000 strengths. All subjects showed a positive response, the details of which are published [10]. We examined initiation and resolution of the reaction by biopsy of early and late time points after PPD injection. Each volunteer had one 4-mm punch biopsy taken from the intradermal injection site at either 12 h, 72 h, 7 or 14 days after the procedure (five subjects per time point). The criteria for the diagnosis of AE were based on those of Hanifin & Rajka [17]. The diagnosis was made when, in the presence of a rash of typical morphology and distribution, at least two other basic features were present. Nine patients with AE were recruited from a specialist eczema clinic: seven male, age range 21–49 years, median 28 years. All had long-standing disease (range 11–48 years, median 27 years), involving >60% of the body surface area. None had been treated with topical steroids, systemic or UVR therapy for at least 1 month prior to biopsy. Four-millimetre punch biopsies were taken from unexcoriated and clinically uninfected lesional AE, present for at least 2 weeks. Lesions were biopsied at this time point for comparison to day 14 (resolution) of the Mantoux reaction (MR). Ethics committee approval and subjects' informed consent were obtained before performing the biopsies. We also investigated lesional versus non-lesional skin in three patients to determine if there were pre-existing cutaneous abnormalities in cytokine production. All biopsies were mounted in ‘Cryo-M-Bed’ (Bright's Instrument Co. Ltd, Huntingdon, UK) and snap-frozen in isopentane cooled in a bath of liquid nitrogen. Cryostat sections (6 μ m) were mounted on poly L-lysine-coated slides, air-dried for 2 h, and either used immediately or stored, wrapped in cling film at −20°C prior to immunohistologic staining.
Antibodies used in the study
T cells were stained with a cocktail of MoAbs which have been described in detail elsewhere [10]. Antibodies to CD45RO (UCHL-1, IgG2a) and CD3 (UCHT1, IgG2a) were kindly provided by Professor P. C. L. Beverley (University College and Middlesex School of Medicine, London, UK). Antibodies to CD4, Ki67 and Bcl-2 were purchased from Dako Ltd. (High Wycombe, UK). Anti-Bax (p21) antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). CD95-ligand was stained using Nok-1 antibody (PharMingen, San Diego, CA), and also kindly provided by Professor H. Yagita (Juntendo University School of Medicine, Tokyo, Japan). Fibroblasts were stained with an antibody (Dia110,ASO2, IgG1) purchased from Dianova (Hamburg, Germany). For cytokine staining, antibodies to IL-15, IL-7 and transforming growth factor-beta (TGF-β) were obtained from Genzyme Diagnostics (Cambridge, MA). Anti-IL-2 antibody was obtained from Serotec Ltd (Oxford, UK), anti-IL-4 was obtained from PharMingen, and anti-interferon-beta (IFN-β) from Harlan Sera-Lab Ltd (Loughborough, UK).
Histological staining
An indirect immunoperoxidase technique was used to detect T cells, and ASO2+ cell numbers and distribution as previously described [10]. Three control preparations were employed. Sections of normal human tonsil, in which the distribution and pattern of staining could be tested against tissue architecture, were used as positive controls in each experiment. In addition, control incubations to detect background staining were performed on sections of each skin sample, omitting the primary antibody. Third, isotype specificity was confirmed by comparison with staining with irrelevant MoAbs of the same isotype as the MoAbs used on tonsil sections. CD4:CD8 ratios and proportions of T cell subsets expressing CD45RO, Ki67, Bcl-2 and Bax were determined by immunofluorescence as previously described [10]. Sections were incubated with appropriate combinations of primary MoAbs followed by immunoglobulin isotype-specific FITC- or TRITC-conjugated affinity-purified goat anti-mouse or goat anti-rabbit (Southern Biotechnology Associates, Birmingham, AL) second layer antibodies. The distribution of IL-2, IL-15, IL-7, IL-4, IFN-β and CD95-L in skin sections was determined by biotin/streptavidin staining as previously described [10]. Controls were performed on skin sections as above using the biotin/streptavidin second and third layers alone. Isotype specificity was confirmed by comparison with staining with an irrelevant IgG1 or IgG2 MoAb on skin sections.
Identification of apoptotic T cells
The presence of apoptotic T cells within perivascular infiltrates was confirmed using a combination of indirect immunofluorescence and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling (TUNEL) methodologies [10]. In previous studies we standardized different methods for detecting apoptotic cells to ensure that the results obtained using the TUNEL method were comparable to that obtained by Annexin 5, propidium iodide and morphological analysis [3]. Sections were stained with anti-T cell antibodies, fixed in 4% paraformaldehyde, washed, and permeabilized by incubating with 0·1% Triton X-100 (Rohm & Haas, Philadelphia, PA), 0·1% sodium citrate for 2 min on ice. Sections were then incubated with TUNEL reaction mixture (In situ cell death detection kit, fluorescein; Cat. no. 1684795; Boehringer Mannheim). In each experiment, sections of normal human tonsil, in which apoptotic cells were present predominantly in germinal centres, were used as positive controls. Negative controls were performed as per the manufacturer's instructions.
Quantification of immunohistology
For immunoperoxidase studies, the number and distribution of positive cells were quantified in each section using an image analysis system (Seescan Imaging Ltd, Cambridge, UK; mag. × 320) as previously described [10]. When assessing the proportion of perivascular cells expressing cytokines or CD95-L expression, the number of cells with either surface or cytoplasmic staining was divided by the total number of cells counted per frame area. For quantification of interstitial ASO2+ and IFN-β-expressing cells the frame was centred on interstitial areas, in between perivascular infiltrates, and counted in five different areas per section (frame area 4·5 × 104 μ m2). For immunofluorescence and TUNEL studies, the distribution and percentages of T cells were estimated in each section using a Zeiss fluorescence microscope (× 400 magnification) in the five largest dermal perivascular inflammatory cell infiltrates present in the sections.
Statistical analysis
Measurements were taken from five areas (using the image analysis system or fluorescence microscopy as described above) in each subject and mean values and s.d. were calculated for each individual. AE patients and different time points of the DTH response were considered as separate groups. A minimum of three subjects was investigated at each time point/group. Using the anova method, the results obtained in AE were compared with those in normal skin (time 0) and the height (day 7) and resolution phases (day 14) of the DTH response and were tested for significance including time point/group and subject as factors in the analysis. For the purpose of visually displaying the data, the mean values for the different subjects were used to calculate an overall mean value and s.d. for each time point/group.
Results
Characteristics of infiltrating T cells
We investigated the nature of the T cell infiltrate in AE and compared it with the infiltrating T cells during the induction and resolution phases of the MR. In chronic lesional AE, infiltrating T cells were concentrated in perivascular areas, predominantly in the papillary and upper reticular dermis. As shown in Fig. 1a, the mean perivascular T cell number in AE was significantly greater than in normal skin before PPD injection (P < 0·0001), lower than at the height of the MR on day 7 (P < 0·0001), but not significantly different from that found during the resolution phase of the MR on day 14 (P < 0·68). Numbers of CD4+ cells exceeded CD8+ cells in all subjects. The mean perivascular CD4:CD8 ratio in AE (4·24 ± 2·1) was not significantly different from that on day 7 (3·25 ± 0·79) and day 14 (4·6 ± 0·56) of the MR. Dual immunofluorescence studies revealed that 86 ± 3·1% (mean ±s.d.) of perivascular CD4 cells in AE were CD45RO+. This high proportion of CD4+CD45RO+ cells resembled that seen 14 days after intradermal PPD (86·9 ± 4·2%), and was significantly greater than in normal skin (45·3 ± 25·5%, P < 0·0001) and 7 day MR (80·7 ± 14·3%, P < 0·003). Thus, the distribution, phenotypic characteristics and numbers of infiltrating T cells in 2-week-old AE lesions were similar to those observed during the resolution phase (day 14) of PPD-induced DTH responses.
Fig. 1.
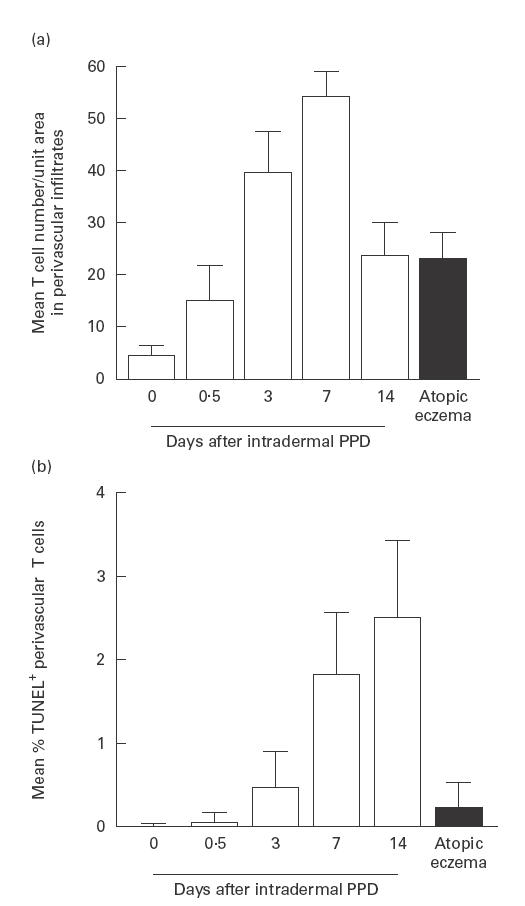
Perivascular T cell numbers in normal skin, Mantoux reactions (MR) and chronic atopic eczema (AE). (a) T cells were stained by an indirect immunoperoxidase method and quantified in each section using an image analysis system as described in PATIENTS and METHODS (normal skin n = 5; MR, days 0·5, 3 and 14, n = 5, day 7 n = 4; AE, n = 9). Error bars indicate s.d. (b) Apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling (TUNEL) staining in normal skin, MR and chronic AE. Dual immunofluorescence (IMF) was used to count proportions of perivascular T cells expressing TUNEL reactivity (normal skin, days 7 and 14 MR, n = 4, day 0·5 and 3 MR, n = 5; AE n = 9). Cells were quantified using a Zeiss fluorescence microscope as described in PATIENTS and METHODS. Error bars indicate s.d.
T cell proliferation and apoptosis
Dual immunofluorescence studies revealed that in AE a mean of 6·9 ± 2·1% (range 3·6–9·6%) of perivascular T cells expressed Ki67, a marker for proliferating cells (n = 9). This was significantly lower than at the height (day 7) of the DTH response (18·8 ± 3·7%; n = 4), but greater than in normal skin (0%, P < 0·0001 in both cases; n = 3). Although slightly higher, it resembled that observed on day 14 (resolution) of the MR (4·2 ± 3·4%; n = 5).
In the MR, increased T cell apoptosis was observed at day 7, coinciding with maximal levels of T cell proliferation, and also during the resolution phase on day 14, when proliferative activity was considerably reduced (Fig. 1b). The percentage of apoptotic T cells identified in AE subjects (mean 0·23 ± 0·3%, Fig. 1b, Fig. 2B) was significantly lower than those obtained on days 7 and 14 or the MR (Fig. 1b, Fig. 2A; P = 0·0001 in both cases). The relative kinetics of Ki67 and TUNEL expression make it unlikely that the TUNEL staining detected proliferating rather than apoptotic cells. For example, maximal numbers of Ki67+ cells were found on day 7 of the MR and this substantially decreased by day 14 (see above). In contrast, TUNEL+ cells were at maximal levels at day 14. In addition, we have optimized our TUNEL staining in parallel with that for other apoptotic markers to rule out non-specific reactivity by any of the methods used [3]. Previous studies showed that the ratio of the anti-apoptotic molecule Bcl-2 to the pro-apoptotic molecule Bax determines the susceptibility of T cells to apoptosis [11,12]. We therefore investigated the expression of these molecules in lesional T cells in patients with AE. We also investigated the expression of CD95-L in perivascular infiltrates in AE, to see if the low rates of T cell apoptosis observed might be due to altered levels of this molecule compared with the resolving MR. As shown in Fig. 3, dual immunofluorescence studies revealed that a mean of 74·5 ± 8·2% of perivascular T cells in AE expressed Bcl-2. In addition, > 98% of these cells expressed Bax (data not shown). This level of Bcl-2 expression was significantly greater than in normal skin (38·7 ± 16%) and than during the resolution phase (day 14) of the DTH response (23·5 ± 3%; P < 0·0001). Bax expression by T cells was > 98% throughout the time course of the MR. The level of T cell Bcl-2 expression in AE was similar to that at the height (day 7) of the MR (70·9 ± 6%).
Fig. 2.
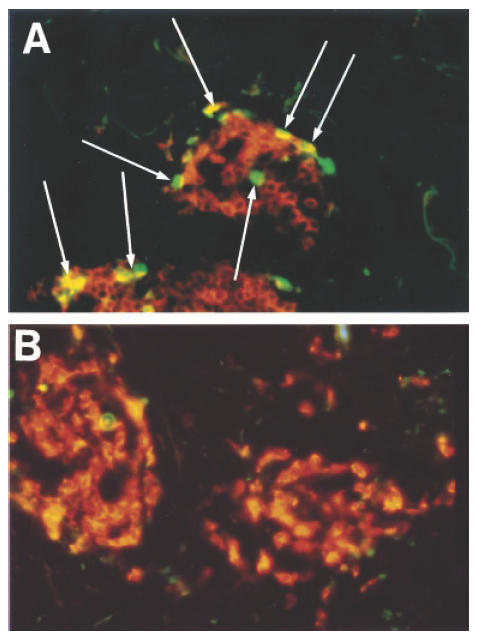
The incidence of T cell apoptosis within perivascular infiltrates during the resolution phase (day 14) of the Mantoux reaction (MR) ((A) see arrows) and in a patient with atopic eczema (AE) (B) was determined by two-colour immunofluorescence staining (see legend to Fig. 1). T cells (orange) and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling (TUNEL) (green) staining were quantified using a Zeiss fluorescence microscope as described in Patients and Methods.
Fig. 3.
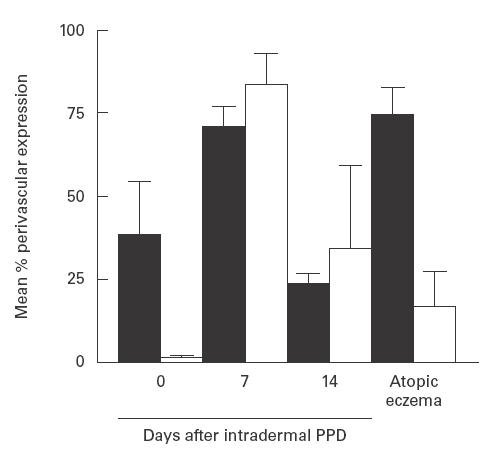
T cell Bcl-2 (▪) and CD95 ligand expression (□) in perivascular infiltrates in normal skin, Mantoux reactions (MR) and chronic atopic eczema (AE). Dual immunofluorescence studies were performed and the proportion of T cells which also expressed Bcl-2 was quantified using a Zeiss fluorescence microscope as described in PATIENTS and METHODS. A biotin/streptavidin method and an image analysis system were used to determine the proportion of perivascular cells expressing CD95 ligand. Cells with cytoplasmic or membrane positivity were counted (normal skin and day 7 MR, n = 4; day 14 MR, n = 5; AE, n = 8). Error bars indicate s.d.
In the DTH response maximal CD95-L expression occurred on day 7, coinciding with maximal levels of T cell proliferation (Fig. 3). Although substantially reduced, there was still greater CD95-L expression on day 14 of the MR compared with normal skin, suggesting that CD95-mediated death may have a minor role during the resolution phase of the MR. The histological distribution of CD95-L during DTH responses has been shown elsewhere [10]. In AE patients, a mean of 16·9 ± 10·6% of cells expressed low levels of CD95-L in perivascular areas (Fig. 3). The percentage of positive perivascular cells was significantly lower than 7 and 14 days after intradermal PPD (P < 0·0001). This suggests that the decreased T cell apoptosis in AE may be due to both the increased expression of Bcl-2 and decreased expression of CD95-L compared with resolving cutaneous inflammation in normal individuals. The majority of T cells were positive for CD95 expression on days 7 and 14 of the DTH response and in AE lesions (not shown).
Expression of anti-apoptotic cytokines
Previous studies have shown that the IL-2R common γ-chain family of cytokines [12,13] and type-1 interferons [4,14,15] can prevent activated T cell death. We first investigated if the expression of the IL-2R γ-chain signalling cytokines in these lesions was associated with the inhibition of apoptosis and contributed to the persistence of chronic inflammation in AE. IL-2 expression was markedly down-regulated in chronic lesional AE compared with the height of the MR. Although a minority of perivascular cells expressed weak membranous IL-2 (Table 1), no strongly positive cells were identified. Reduced levels of IL-7 expression were present in the AE specimens examined (n = 5) when compared with 7 or 14 day DTH (Table 1). In addition, the intensity of staining and proportion of IL-4-expressing cells in all the AE patients were reduced compared with day 7 and day 14 MR (n = 8, Table 1). IL-2, IL-4 and IL-7 were therefore unlikely to play a significant role in up-regulating Bcl-2 in AE patients. In 7/9 AE subjects, the distribution and intensity of staining obtained with IL-15 resembled that in 3- and 7-day MR, where peak expression of this cytokine occurred (Fig. 4a–c, Table 1). Epidermal keratinocytes (KC) showed moderate–strong cytoplasmic staining in 7/9 subjects. In the papillary and upper reticular dermis a majority of perivascular cells strongly expressed IL-15. These cells comprised large oval and dendritic cells with cytoplasmic staining and small lymphocytes with surface staining.
Table 1.
Cytokine expression in perivascular cells in after intradermal PPD injection (Mantoux reaction (MR)) and atopic eczema (AE)
| Days after intradermal PPD | |||||
|---|---|---|---|---|---|
| Cytokine | 0 | 7 | 14 | AE | |
| IL-15 | % perivascular expression* | 1·9 | 84·6 | 7·9 | 54·3 |
| Range (%) | 0–5 | 75–90 | 5–10 | 20–90 | |
| Intensity of staining† | ++ | ++ | + | ++/+++ | |
| IL-2 | Percent perivascular expression | 0 | 65·7 | 3·5 | 14·8 |
| Range (%) | 60–75 | 0–5 | 0–35 | ||
| Intensity of staining | – | +++ | ++ | + – | |
| IL-7 | Percent perivascular expression | 7·1 | 56·3 | 36·4 | 8 |
| Range (%) | 5–10 | 50–65 | 30–40 | 5–15 | |
| Intensity of staining | + | +++ | ++ | + | |
| IL-4 | Percent perivascular expression | 37·6 | 75·2 | 85·4 | 52·7 |
| Range (%) | 25–50 | 60–80 | 80–95 | 25–80 | |
| Intensity of staining | ++ | +++ | +++/++ | ++/+++ | |
| IFN-β | Positive interstitial cell number/unit area in upper dermis‡ | 0·8 | 5·9 | 12·2 | 15·8 |
| s.d. | 1·2 | 1·8 | 2·1 | 2·3 | |
| Intensity of staining | + | ++ | +++ | +++ | |
Cells with both membranous and cytoplasmic staining were taken into account when calculating the mean and range percentages of positive perivascular cells. A minimum of three subjects were investigated at each time point in MR, for numbers of AE subjects see text.
Intensity of staining was graded as follows: –, none; +/−, very weak; +, weak; ++, moderate; +++, strong.
IFN-β-positive cells were counted per rectangular frame area centred in between perivascular infiltrates in the upper dermis.
Fig. 4.
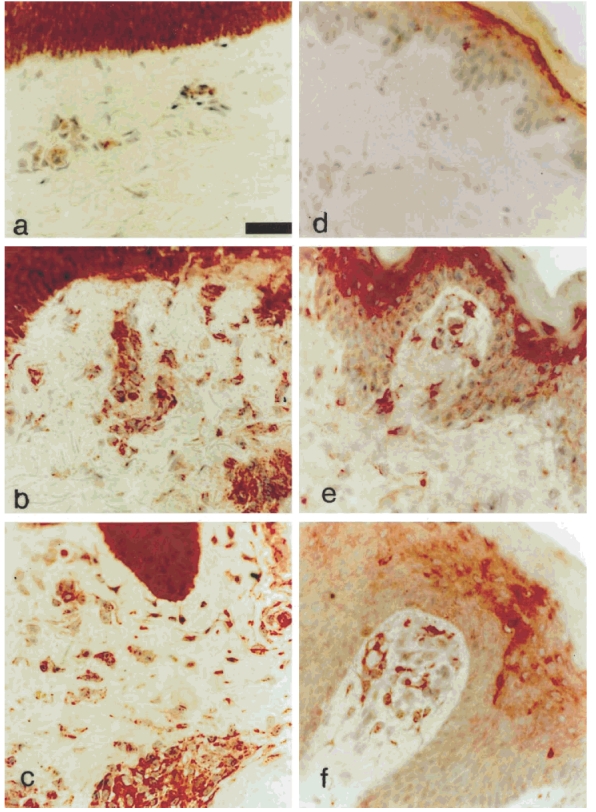
Photomicrographs stained by means of biotin/streptavidin technique with MoAb in skin sections. Staining with anti-IL-15 MoAb (a–c) shows strongly positive keratinocytes, but little dermal staining in normal skin (a), strong positivity in keratinocytes, perivascular areas and interstitial cells on day 14 of the Mantoux reaction (MR) (b). Similar strong positivity is observed in keratinocytes, interstitial (predominantly dendritic) cells and perivascular areas in atopic eczema (AE) (c). Staining with anti-IFN-β antibody (d–f) shows weak suprabasal keratinocyte positivity in normal skin (d), and increased positivity in keratinocytes and positive dendritic cells in the papillary dermis on day 3 of the MR (e). In AE weak–moderate keratinocyte positivity, and numerous strongly positive dendritic and perivascular cells in the papillary dermis are present. (Original mag. × 400, calibration bar 24 μ m.)
IFN-β has recently been implicated both in the generation of T cell memory [18], and the persistence of T cell-mediated inflammation by its ability to prevent both cytokine deprivation [4,15,16] and also CD95-mediated activation-induced cell death [16]. We therefore investigated the expression of this cytokine in chronic lesional AE and during the MR. Little IFN-β was found in the dermis in normal skin (Fig. 4d). As shown in Table 1, after PPD injection, IFN-β increased to a peak at day 14 (see also Fig. 4d,e). This cytokine therefore showed maximal expression late in the MR compared with the IL-2R γ-chain family. In AE patients, this cytokine was strongly expressed in KC throughout the epidermis in all subjects (n = 9). Endothelial cells showed moderate–strong cytoplasmic expression and a majority of cells in perivascular infiltrates were positive (Fig. 4f). Numerous IFN-β+ interstitial dendritic and spindle-shaped cells were observed. An overall mean of 15·8 ± 2 IFN-β+ interstitial cells/unit area (UA) (range 12·4–18·4 IFN-β+ cells/UA) were present in AE patients. This number was significantly greater than that in normal skin (0·8 ± 1), on day 7 (5·9 ± 1·8; P = 0·0001 in both cases) and also day 14 of the MR (12·2 ± 2; P = 0·002, see Table 1). Preliminary observations suggest that the majority of IFN-β-secreting cells in both the MR and in AE are fibroblasts, but a subset of macrophages and also dendritic cells are a source of this cytokine (data not shown).
Discussion
In this study, we addressed the hypothesis that the persistence of cutaneous inflammation in AE is due to the lack of apoptosis of infiltrating cells. A central observation of this investigation was that there was significantly less T cell apoptosis in lesional skin from AE patients compared with either the peak or resolution of the MR. This suggested that although the distribution and phenotypic characteristics of infiltrating T cells in chronic AE resembled those observed in the MR, certain microenvironmental factors in AE lesions were preventing the apoptosis of these cells. It was therefore important to investigate the expression of apoptosis regulatory molecules in AE lesions to determine why there was a lack of T cell death.
An important observation was that the anti-apoptotic cytokines IL-15, which is expressed maximally between days 3 and 7, and IFN-β, which is expressed maximally at day 14 during the MR, were both elevated simultaneously in lesions from AE patients. The co-expression of these cytokines, which prevent apoptosis by different mechanisms [4], may be responsible in part for the prevention of T cell apoptosis in AE. The apoptosis at the peak of the MR on day 7 is unlikely to be due to cytokine deprivation, since high levels of IL-2 and IL-15 are present [10]. Instead, it is likely that the death is associated with high levels of CD95-L expressed at this time. Our data would also suggest that the elevated Bcl-2 expression in T cells from AE patients compared with those found during the resolution of the MR on day 14 was due to IL-15, which prevents apoptosis, in part, by up-regulation of this molecule [12,13]. The reduced lesional CD95-L expression observed may also contribute to the persistence of the T cell infiltrate in chronic AE lesions. It is clearly of importance to determine why the expression of IL-15 and IFN-β are elevated in AE.
The possibility that IL-15 up-regulation in AE represents a primary genetic abnormality is unlikely, since preliminary investigation in three patients where paired biopsies of lesional and non-lesional skin were investigated showed no significant up-regulation of IL-15 or IFN-β in non-lesional skin (data not shown). The high level of IL-15 expression observed in epidermal and dermal dendritic cells might occur as a result of their ongoing activation by percutaneously absorbed antigens via IgE/IgE-receptor mediated triggering [9]. Recent data in monocyte-derived dendritic cells from AE patients have shown that they are capable of up-regulating synthesis of tumour necrosis factor-alpha (TNF-α) and IL-8 after cross-linking of Fcε RI on their surface (Katoh N, Bieber T. FCεRI mediates production of proinflammatory cytokines by human monocytes and monocyte-derived dendritic cells. J Invest Dermatol 1998; 110 (Abstr.):647). It would now be important to determine if these interactions also lead to up-regulation of IL-15 and if dysregulated T cell–fibroblast or T cell–dendritic cell interactions at sites of inflammation provide the trigger for the generation of chronicity. It would be important to compare the MR and AE with chronic DTH-type lesions such as contact dermatitis to determine if the initiating mechanism, i.e. DTH versus IgE, may itself affect the chronicity of the response [7,9,17]. A recent study which showed that there is reduced B cell apoptosis associated with high Bcl-2 expression in patients supports the possibility that IL-2R common γ-chain cytokine secretion, which up-regulates Bcl-2 expression, is dysregulated in AE (Takayama K, Satoh T, Yokozeki H, Katayama I, Nishioka K. Expression of Bcl-2 on B lymphocytes contributes to overexpression of IgE in atopic dermatitis. J Invest Dermatol 1998; 110 (Abstr.):634). Another point for future investigation would be whether down-regulation of cutaneous IL-15 or IFN-β expression occur in AE patients after successful therapy. Further studies will be needed to determine accurately the interactions between different cell types which may trigger the over-production of IL-15 and IFN-β in AE.
Acknowledgments
This work was supported by a grant from the Sir Jules Thorn Charitable Trust (number 95/04A).
References
- 1.Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin KM. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994;6:279–89. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 2.Akbar AN, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T cell death. Immunol Today. 1997;18:72–76. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 3.Salmon M, Scheel-Toellner D, Huissoon AP, et al. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997;99:439–46. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilling D, Akbar AN, Girdlestone J, et al. Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–50. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Salmon M, Pilling D, Borthwick NJ, Akbar AN. Inhibition of T cell apoptosis: a mechanism for the persistence of chronic inflammation. Immunologist. 1997;5:87–92. [Google Scholar]
- 6.Gibbs J, Ferguson J, Brown R, et al. Histometric study of the localisation of lymphocyte subsets and accessory cells in human Mantoux reactions. J Clin Pathol. 1984;37:1227–34. doi: 10.1136/jcp.37.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapsenberg M, Wirenga E, Bos J, Jansen H. Functional subsets of allergen-reactive human CD4+ T cells. Immunol Today. 1991;12:392–5. doi: 10.1016/0167-5699(91)90137-I. [DOI] [PubMed] [Google Scholar]
- 8.Poulter LW, Seymour GJ, Duke O, Janossy G, Panayi G. Immunohistological analysis of delayed-type hypersensitivity in man. Cell Immunol. 1982;74:358–69. doi: 10.1016/0008-8749(82)90036-3. [DOI] [PubMed] [Google Scholar]
- 9.Bieber T. FcER1-expressing antigen-presenting cells: new players in the atopic game. Immunol Today. 1997;18:311–3. doi: 10.1016/s0167-5699(97)01046-3. [DOI] [PubMed] [Google Scholar]
- 10.Orteu CH, Poulter LW, Rustin MA, Sabin CA, Salmon M, Akbar AN. The role of apoptosis in the resolution of T cell-mediated cutaneous inflammation. J Immunol. 1998;161:1619–29. [PubMed] [Google Scholar]
- 11.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akbar AN, Borthwick NJ, Wickremasinghe RG, et al. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-x (L) but not pro-apoptotic (bax, bcl-x (S) gene expression. Eur J Immunol. 1996;26:294–9. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 13.Vella T, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–5. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko S, Suzuki N, Koizumi H, Yamamoto S, Sakane T. Rescue by cytokines of apoptotic death induced by IL-2 deprivation of human antigen-specific T cell clones. Clin Exp Immunol. 1997;109:185–93. doi: 10.1046/j.1365-2249.1997.4191324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–9. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheel-Toellner D, Pilling D, Akbar AN, et al. Inhibition of T cell apoptosis by interferon-beta rapidly reverses nuclear translocation of protein kinase C-delta. Eur J Immunol. 1999;29:2603–12. doi: 10.1002/(SICI)1521-4141(199908)29:08<2603::AID-IMMU2603>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Hanifin J, Rajka J. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- 18.Tough DF, Sun S, Zhang X, Sprent J. Stimulation of naive and memory T cells by cytokines. Immunol Rev. 1999;170:39–47. doi: 10.1111/j.1600-065x.1999.tb01327.x. [DOI] [PubMed] [Google Scholar]


