Abstract
The pathogenesis of pulmonary sarcoidosis has been related to an increased production of Th1-like cytokines. However, cytokine expression in sarcoidosis has not been systematically studied at a single-cell level. We therefore investigated the expression of IL-2, IL-4, IL-13, tumour necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) intracellularly in bronchoalveolar lavage (BAL) and peripheral blood CD3+ T lymphocytes from patients with pulmonary sarcoidosis (radiologic stage II–III, n = 8) and normal controls (n = 9) by flow cytometry. In contrast to IL-4 and IL-13, the percentage of T lymphocytes expressing intracellular IL-2 (49·3 ± 21·3% versus 14·5 ± 15·6%), IFN-γ (75·5 ± 14·9% versus 32·6 ± 18·7%) and TNF-α (68·3 ± 18·7% versus 36·8 ± 20·8%) was significantly higher in patients with sarcoidosis than in normal controls (each P < 0·005). In contrast to BAL lymphocytes, expression of these cytokines in peripheral blood lymphocytes did not differ between patients with sarcoidosis and normal controls. Close correlations were observed between the percentages of BAL lymphocytes expressing intracellular IL-2, IFN-γ and TNF-α, but not for IL-4 or IL-13. Analysis of the expression of these cytokines in T lymphocyte subsets revealed IL-2, IFN-γ, and TNF-α in CD4+ as well as CD8+ T lymphocytes, suggesting a contribution of TC1 cells to the production of proinflammatory cytokines in sarcoidosis. We conclude that a Th1-like cytokine pattern can be observed in CD4+ as well as in CD8+ BAL T lymphocytes in patients with pulmonary sarcoidosis.
Keywords: sarcoidosis, Th1, bronchoalveolar lavage, intracellular cytokine measurement
Introduction
Sarcoidosis is a chronic multisystem, granulomatous disease of unknown aetiology. The lung is the most commonly involved organ and progressive pulmonary inflammation which can result in fibrosis can lead to respiratory impairment and death, if untreated. In sarcoidosis the pulmonary interstitium is infiltrated predominantly by activated, proliferating CD4+ T lymphocytes which can be recovered in bronchoalveolar lavage (BAL) [1,2]. These CD4+ T cells express surface antigens associated with cell activation [1,3] and analysis of T cell receptor usage has suggested that lesional CD4+ T cells are oligoclonal [4]. Sarcoidosis has been termed a ‘compartmentalized inflammation’ [5], since activated lymphocytes are only present in involved organs while cytokine release and expression of antigens associated with cell activation from peripheral blood lymphocytes do not differ from those of healthy volunteers [2,4,6].
IL-2 production by T lymphocytes residing in the lung of patients with sarcoidosis which in situ leads to proliferation of T cells [7,8] and can enhance lymphocyte migration [9] has been correlated with the number of lung T cells in sarcoidosis [7,8]. In addition, increased expression of mRNA for interferon-gamma (IFN-γ) in lymph nodes [10] as well as an increase in the spontaneous release of IFN-γ from mononuclear cells [11] has been reported from patients with sarcoidosis. In a recent study [12] which compared different techniques of intracellular cytokine staining an increase in the expression of IFN-γ was observed in patients with sarcoidosis. Since the expression of IFN-γ in the absence of IL-4 has been attributed to a particular subset of T helper cells termed Th1-like T cells [13], it has been postulated that Th1-like lymphocytes are involved in the pathogenesis of sarcoidosis [10,14–16]. Accordingly, IL-4 concentrations were not elevated in BAL fluid of patients with sarcoidosis [16], but IL-4 gene expression has recently been detected by reverse transcriptase-polymerase chain reaction (RT-PCR) in T cell clones from patients with pulmonary sarcoidosis [17]. In addition, elevated tumour necrosis factor-alpha (TNF-α) gene expression has been shown in patients with sarcoidosis [10].
Although it has been hypothesized that the Th1-type cytokines IL-2 and IFN-γ are produced by activated Th1-like lymphocytes, the concomitant expression of IL-2, IFN-γ, IL-4 and IL-13 has not yet been studied systematically in sarcoidosis at a single-cell level. In order to test the hypothesis that pulmonary sarcoidosis is associated with an infiltration of the lung with T cells expressing Th1 cytokines, we analysed the expression of IL-2, IFN-γ, TNF-α, IL-4 and IL-13 in BAL T lymphocytes from patients with sarcoidosis and healthy controls. We used a recently described method to quantify intracellular cytokine expression by flow cytometry [18,20]. This approach allows the simultaneous detection of intracellular cytokine production and lymphocyte surface antigen expression on single cells and can thus provide insights into the frequency of T cells capable of producing different cytokines, as well as into the relative amount of cytokine produced by each individual cell.
Materials and methods
Materials
Antibodies specific for CD3, CD4, CD8, CD25, CD45, HLA-DR, and isotype-matched control antibodies labelled with FITC, PE and PE/CY5 were obtained from Dako (Hamburg, Germany). Antibodies specific for IL-2 (rat IgG2a, MQ1-17H12), IL-4 (mouse IgG1, MP4-25D2), IL-13 (rat IgG2a, JES10-5A2), IFN-γ (mouse IgG1, 4S.B3), and TNF-α (mouse IgG1, clone MAb11) were all purchased from PharMingen (Hamburg, Germany). The isotype-matched, directly conjugated (FITC, PE) control antibodies were also obtained from PharMingen. Saponin, brefeldine A, phorbolester (PMA) and Ca-Ionophore A23187 were purchased from Sigma (Deisenhofen, Germany) and Ficoll–Paque from Seromed (Berlin, Germany). Dulbecco's PBS was obtained from Life Technologies Ltd (Paisley, UK).
Subjects
A total of eight patients (four females and four males, mean age 39·3 ± 9·7 years) who underwent bronchoscopy for routine diagnostic evaluation of suspected pulmonary sarcoidosis were included in the study. All patients had pulmonary involvement, as demonstrated by a conventional chest x-ray corresponding to a radiological classification of stage II or III sarcoidosis. The diagnosis of pulmonary sarcoidosis was established in all patients based on clinical criteria with confirmation of non-caseating epithelioid granulomas on tissue biopsies. None of the patients had evidence of an alternative diagnosis or exposure to any inorganic material known to cause granulomatous disease, and there was no evidence of such an exposure in histological sections. Tuberculosis was excluded by a negative Mantoux skin test and absence of auramine–rhodamine-positive rods in histological sections of pulmonary biopsies. None of the patients had received anti-inflammatory treatment with corticosteroids or other immunosuppressive therapy prior to inclusion into the study.
Nine normal healthy volunteers (four females and five males, mean age 31·1 ± 8·6 years) with normal pulmonary function, who did not receive any current medication, served as controls.
All subjects were non-smokers and gave their consent after being informed about the nature and purpose of the study. Local ethics committee approval was obtained. Further patient characteristics are listed in Table 1.
Table 1.
Lung function parameters of patients with sarcoidosis and healthy volunteers
| Lung function parameters | TLC (l) | TLC% (predicted) | IVC (l) | IVC% (predicted) | FEV1 (l) | FEV1 % (predicted) | CO-Diff. capacity (mmol/min/kPa/l) | CO-Diff. % (predicted) |
|---|---|---|---|---|---|---|---|---|
| Controls | 6·8 ± 1·3 | 103·1 ± 7·8 | 5·0 ± 1·1 | 95·0 ± 14·5 | 3·7 ± 0·8 | 100·9 ± 12·2 | 1·84 ± 0·3 | 90·1 ± 12·7 |
| Patients with sarcoidosis | 5·9 ± 1·2 | 84·6 ± 7·9 | 4·2 ± 1·5 | 78·6 ± 18·4 | 3·2 ± 1·0 | 83·4 ± 17·6 | 1·64 ± 0·2 | 80·4 ± 10·9 |
TLC, Total lung capacity; TLC%, in % of predicted total lung capacity; IVC, inspiratory vital capacity; IVC%, in % of predicted inspiratory vital capacity; FEV1, forced exspiratory volume of 1 s; FEV1 %, in % of predicted forced exspiratory volume of 1 s; CO-Diff. %, CO diffusion capacity in % of predicted.
Bronchoalveolar lavage
Bronchoscopy was performed according to standard guidelines. Thirty minutes prior to the procedure patients received 0·5 mg of atropine and 12·5 mg codeine intramuscularly. Local anaesthesia of the oropharynx was achieved by Novesine spray (Wander, Switzerland) until gag reflexes subsided. Bronchoscopy was performed using a Pentax EB-1830T2 videobronchoscope through which 120 ml of normal prewarmed saline in aliquots of 20 ml were instilled into a subsegment of the right middle lobe. BAL fluid was then immediately aspirated by gentle hand suction into plastic tubes and kept at 4°C on ice.
Processing of BAL cells
BAL samples were filtered through a two-layer sterile gauze into sterile plastic vials (Falcon, Oxnard, CA), centrifuged at 4°C and 500 g for 10 min. The supernatant was removed and cells were washed twice in PBS. The total cell number was counted using a Neubauer haemocytometer (Brand, Wertheim, Germany). Differential cell counts were performed after Giemsa staining (Merck, Darmstadt, Germany) of cell smears with 1000 cells per slide counted.
Isolation of peripheral blood mononuclear cells
Venous blood was drawn into sterile plastic containers containing 0·2 ml EDTA (Sarstedt, Nümbrecht, Germany) prior to the bronchoscopy and was separated on a gradient of Ficoll with a density of 1·077 g/l for 20 min at 1330 g. The band of peripheral blood mononuclear cells (PBMC) at the interface was collected and washed twice.
Analysis of T lymphocyte subsets in BAL
PBMC and BAL cells were incubated in the presence of saturating concentrations of fluorescein-conjugated MoAb against the surface markers CD3, CD4, CD8, CD25, CD45, and HLA-DR for 20 min at room temperature in the dark. Non-specific fluorescence was detected by incubating mouse IgG of the same isotype, but with irrelevant antigen specificity. After two washes with PBS the cells were analysed by flow cytometry (FACScan; Becton Dickinson, Heidelberg, Germany).
Analysis of intracellular cytokine expression in T lymphocytes in PBMC from peripheral blood and BAL cells
Intracellular cytokine measurement was performed as recently described [18,20]. Briefly, PBMC and BAL cells were stimulated with 10−8 m PMA and 10−6 m Ca-Ionophore A23187 for 4 h and cytokine release was blocked by adding brefeldine A (10 μg/ml). Subsequently cells were washed twice with PBS/1% fetal calf serum (FCS)/0·1% NaN3 and fixed in 4% paraformaldehyde in PBS pH 7·4 for 15 min on ice. Cells were then incubated for 30 min at room temperature with PE-conjugated (PE/CY5) MoAbs specific for CD3, CD4, or CD8. Following two washes in PBS/1% FCS/0·1% NaN3 the cells were permeabilized by using 0·1% saponin/PBS. Thereafter, cells were incubated with anti-IL-2,-IL-4,-IL-13,-IFN-γ,or-TNF-α for 20 min at room temperature in the dark and then washed twice with 0·1% saponin/PBS. Finally, the cells were resuspended in PBS and analysed by flow cytometry. The percentage of positive cells was also determined using isotype-matched IgG antibodies as a control. The relative fluorescence intensity (RFI) was calculated as intensity of cytokine staining/intensity of isotype matched control antibody staining according to Nakamura et al. [34].
Statistical analysis
Data are expressed as arithmetic mean ± s.d. After testing for normality data were compared with Student's t-test (paired and unpaired) and correlated with Spearman's rank correlation.
Results
BAL differential cell count and analysis of lymphocyte subpopulations
The total cell number, differential cell counts, and the percentage of CD3+, CD4+, CD8+, CD25+, and HLA-DR+ lymphocytes, as well as the CD4/CD8 ratio of both groups are listed in Table 2. The percentage of lymphocytes (40·9 ± 17·5% versus 14·7 ± 6·4%) as well as the mean total lymphocyte count was significantly elevated in the BAL fluid of patients with sarcoidosis compared with healthy volunteers (P < 0·0001). In addition, there was an increased expression of CD25 (13·4 ± 5·1% versus 7·3 ± 1·3%, P = 0·01) as well as HLA-DR (15·2 ± 3·4% versus 6·2 ± 4·5%, P = 0·03) on BAL lymphocytes of patients with sarcoidosis. Although the percentage of the CD4+ lymphocytes (64·3 ± 17·8%) in BAL of patients with sarcoidosis was higher than that of normal controls (53·4 ± 12·4%), this difference failed to reach statistical significance. Similar results were obtained for the CD4/CD8 ratio, which was 3·2 ± 2·1 in patients with sarcoidosis and 2·2 ± 0·9 in controls (P = 0·214).
Table 2.
Bronchoalveolar lavage (BAL): differential cell count, total cell count, percentage of CD4+, CD8+ and CD25+ lymphocytes (CD3+), and CD4/CD8 ratio
| BAL | Total cell count (× 106 cells) | Lymphocytes | CD4+ cells (%) | CD4/8 | CD25+ cells (%) |
|---|---|---|---|---|---|
| Controls | 6·9 ± 3·14 × 106 | 14·7 ± 6·4% | 53·4 ± 12·4% | 2·2 ± 0·9 | 7·3 ± 1·3% |
| Patients with sarcoidosis | 15·4 ± 7·85 × 106 | 40·3 ± 16·3% | 64·3 ± 17·8% | 3·1 ± 2·0 | 13·4 ± 5·1% |
Intracellular cytokine production
Analysis of the intracellular cytokine expression of CD3+ BAL lymphocytes following in vitro stimulation with PMA/A23187 revealed a significantly elevated percentage of IFN-γ-positive cells (75·5 ± 14·9%) in patients with sarcoidosis compared with normal controls (32·6 ± 18·7%, P < 0·0001). Similar results were obtained for IL-2 and TNF-α, which were expressed by 49·3 ± 21·3% and 68·3 ± 18·7% of lymphocytes from patients with sarcoidosis compared with 14·5 ± 15·6% and 36·8 ± 20·8%, respectively, in normal controls (each P < 0·005) (Fig. 1). In addition, the intensity of the intracellular cytokine expression as measured by the RFI for these three cytokines (Fig. 1) was significantly elevated in lymphocytes from patients with sarcoidosis (IFN-γ 26·9 ± 12·8 versus 4·7 ± 5·4 RFI; IL-2 5·4 ± 2·9 versus 1·2 ± 0·3 RFI; TNF-α 12·1 ± 10 versus 3·5 ± 3·2 RFI, respectively, in sarcoidosis versus normal controls; all P < 0·05). The obtained values for the RFI for IL-2 clearly separated patients with sarcoidosis from normal controls.
Fig. 1.
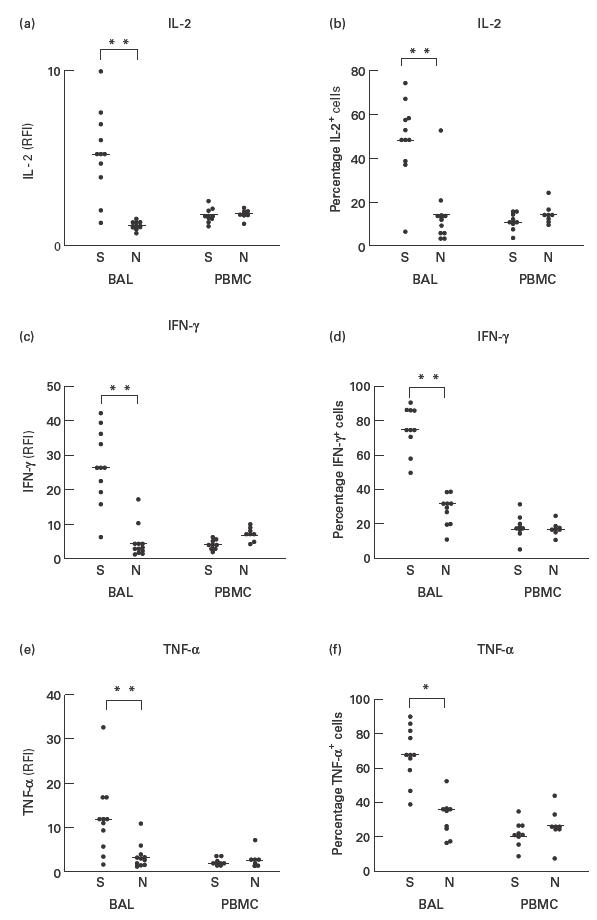
Comparison of intracellular cytokine expression in patients with sarcoidosis (S) and healthy volunteers (N). (a) Relative mean fluorescence (RFI) of IL-2 in bronchoalveolar lavage (BAL) and peripheral blood lymphocytes (CD3+). (b) Percentage of IL-2+ BAL and peripheral blood lymphocytes (CD3+). (c) RFI of IFN-γ in BAL and peripheral blood lymphocytes (CD3+). (d) Percentage of IFN-γ+ BAL and peripheral blood lymphocytes. (e) RFI of tumour necrosis factor-alpha (TNF-α) in BAL and peripheral blood lymphocytes (CD3+). (f) Percentage of TNF-α+ BAL and peripheral blood lymphocytes. **P < 0·005; *P < 0·05.
The analysis of the expression of IL-4 and IL-13 in CD3+ lymphocytes revealed no statistical difference between patients with sarcoidosis (IL-4 2·8 ± 1·8%, IL-13 4·0 ± 2·7%) and healthy volunteers (IL-4 3·3 ± 3·1%, IL-13 4·0 ± 2·7%). Using double staining we were able to show that 74·8 ± 13·2% of all T lymphocytes in patients with sarcoidosis expressed a Th1-like cytokine pattern defined as IFN+/IL-4− cells, compared with a percentage of 32·0 ± 20·3% of the T lymphocytes in healthy controls.
Furthermore, a close correlation was observed between the RFI for intracellular IL-2, IFN-γ, and TNF-α and the percentage of positive cells for the respective cytokine, suggesting that in sarcoidosis not only the expression of these cytokines in single cells but also the number of cells capable of producing the cytokines in question is increased. Moreover, there was a close correlation between the percentage of lymphocytes expressing IFN-γ and TNF-α (r = 0·94, P < 0·0001), between IFN-γ and IL-2 (r = 0·86, P < 0·0001), as well as between TNF-α and IL-2 (r = 0·86, P < 0·001) (Fig. 2), which was not observed for IL-4 or IL-13. In addition, there was a large number of cells which co-expressed IFN-γ and TNF-α (63·4 ± 19·8%).
Fig. 2.
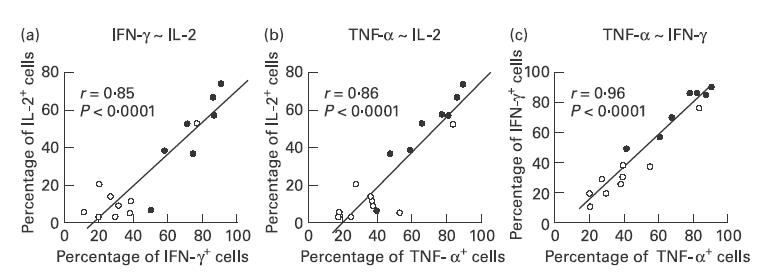
Correlations between IL-2+, IFN-γ+, and tumour necrosis factor-alpha (TNF-α)+ bronchoalveolar lavage (BAL) lymphocytes (CD3+) in patients with sarcoidosis (•) and healthy volunteers (○). (a) Correlation between IFN-γ+ and IL-2+ cells. (b) Correlation between TNF-α+ and IL-2+ cells. (c) Correlation between TNF-α+ and IFN-γ+ cells.
In contrast to these findings in BAL lymphocytes, the observed differences in intracellular cytokine expression were not detectable in peripheral blood T lymphocytes from patients with sarcoidosis and healthy volunteers (Fig. 1). In addition, we could not find any significant differences in the percentage of IL-4+ (sarcoidosis 2·9 ± 1·8% versus normal 3·4 ± 3·2%), and IL-13+ (sarcoidosis 4·0 ± 2·9% versus normal 2·0 ± 1·6%) lymphocytes when patients with sarcoidosis and healthy volunteers were compared.
Analysis of cytokine production by CD4+ and CD8+ T lymphocyte subpopulations
Analysis of T lymphocyte subsets revealed that IL-2, IFN-γ and TNF-α in sarcoidosis were expressed not only in the CD4+ lymphocyte subset but also by CD8+ cells (Fig. 3). Although in patients with sarcoidosis there was a trend towards a higher percentage of CD8+ T cells expressing IFN-γ following in vitro stimulation compared with CD4+ T lymphocytes (85·8 ± 6·4% versus 72·9 ± 4·9%), this difference failed to reach statistical significance. Likewise, there was no statistically significant difference in the percentage of CD4+ or CD8+ lymphocytes expressing IL-2 (CD4+ 54·1 ± 9·0%, CD8+ 49·6 ± 7·9%), TNF-α (CD4+ 72·3 ± 6·1%, CD8+ 69·9 ± 5·9%), IL-4 (CD4+ 2·1 ± 1·6%, CD8+ 3·0 ± 2·7%), and IL-13 (CD4+ 4·8 ± 4·0%, CD8+ 3·7 ± 2·2%). Finally, there was no statistical difference between the percentage of CD4+ (72·4 ± 10·1%) or CD8+ (85·3 ± 7·3%) cells expressing a Th1-like cytokine cluster, as defined by IFN+/IL-4− cells.
Fig. 3.
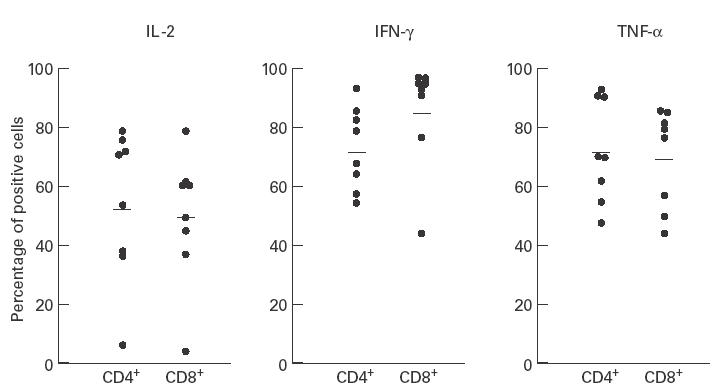
Percentage of IL-2+, IFN-γ+, tumour necrosis factor-alpha (TNF-α)+ bronchoalveolar lavage (BAL) T lymphocytes of patients with sarcoidosis separated according to CD4+ and CD8+ phenotype.
Correlation of surface markers, differential count and cytokine expression
Expression of CD25 and HLA-DR has been associated with T lymphocyte activation in sarcoidosis. In order to study whether the expression of these surface antigens is a marker for the increased production of IL-2, IFN-γ, and TNF-α following in vitro stimulation, in vivo expression of these surface markers was correlated with intracellular cytokine expression in vitro. Expression of CD25 and HLA-DR was detected on 13·4 ± 5·1% and 15·2 ± 3·4%, respectively, of all BAL T lymphocytes in patients with sarcoidosis and on 7·3 ± 1·3% and 6·2 ± 4·5% of normal controls (P < 0·05). However, there was no correlation between the expression of CD25 and any of the cytokines measured intracellularly. This was different however, for HLA-DR expression, which correlated with IL-2 production (P = 0·048, r = 0·56). Still, in patients with sarcoidosis the mean percentage of cells expressing HLA-DR (15·2%) was significantly lower compared with the mean percentage of cells expressing intracellular IL-2 (51·8%) in patients with sarcoidosis.
Furthermore, a close correlation was observed between the RFI of IL-2 in BAL lymphocytes and the degree of lymphocytic alveolitis as assessed by the total number of lymphocytes recovered from BAL fluid of these patients (r = 0·82, P = 0·042) (Fig. 4).
Fig. 4.
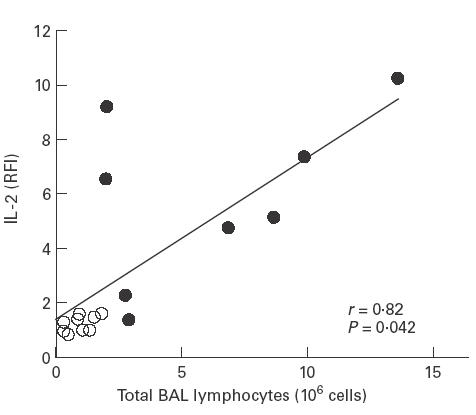
Correlation between IL-2 relative mean fluorescence (RFI) of bronchoalveolar lavage (BAL) T lymphocytes and total BAL T lymphocyte count of patients with sarcoidosis (•) and healthy volunteers (○) (r = 0·82, P = 0·042).
Correlation of cytokine expression and pulmonary function tests
There was a negative correlation between the percentage of IL-2+/CD3+ T lymphocytes (r = −0·64, P = 0·01) as well as the RFI for IL-2 and the total lung capacity (TLC percentage of predicted) (r = −0·67, P = 0·006) (Fig. 5). No other correlations were found between parameters of pulmonary function and cytokine up-regulation in patients with sarcoidosis.
Fig. 5.
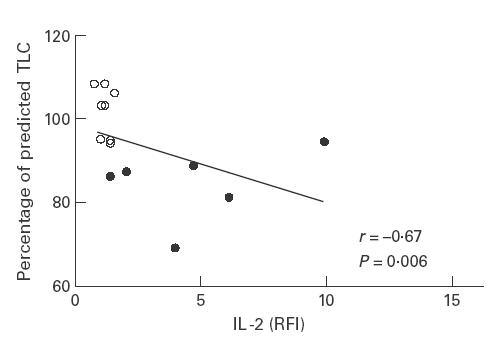
Correlation between the IL-2 relative mean fluorescence (RFI) of bronchoalveolar lavage (BAL) T lymphocytes and total lung capacity (TLC percentage of predicted) in patients with sarcoidosis (•) and healthy volunteers (○) (r = −0·67, P = 0·006).
Discussion
Previous studies using bioassays have reported a spontaneous production of IL-2 [7] and IFN-γ [11] from purified BAL T lymphocytes and in BAL supernatants by ELISA [16]. Based on these findings it has been hypothesized by several authors that sarcoidosis is a Th1-driven disease [14–16]. In a methodological study comparing different methods of intracellular cytokine staining, Krouwels et al. [12] reported an increased expression of IFN-γ in three patients with sarcoidosis as assessed by flow cytometry. In keeping with these findings we could show in this study that the number of T lymphocytes in patients with sarcoidosis which express Th1-like cytokines such as IFN-γ and IL-2 are markedly increased compared with normal controls. Up to 90% of the BAL lymphocytes of patients with sarcoidosis in fact stained positive for these so-called Th1-like cytokines. Furthermore, the intensity of staining per cell was also significantly elevated in patients with sarcoidosis, suggesting an increased cytokine production per cell. These findings contrast with the results obtained for IL-4 and IL-13, which were similarly expressed in patients with sarcoidosis and normal controls.
The pathogenesis of sarcoidosis has been closely associated with a predominance of activated T helper lymphocytes [1,2], namely in the early stage of this disease, and this is reflected by an increased CD4/CD8 ratio [21]. However, patients with longer duration of disease may also have an increase in the numbers of CD8+ cells in BAL [22–24]. This might account for our observation of an only slightly elevated mean CD4/CD8 ratio in our patient population which consisted exclusively of patients with radiologic stage II and stage III disease. In contrast to the current hypothesis of a CD4+ lymphocyte-mediated pathogenesis of sarcoidosis, we were able to show that in addition to CD4+ lymphocytes IL-2, TNF-α and IFN-γ are also produced by CD8+ lymphocytes. This finding is in contrast to the results of Saltini et al. [1], who showed that the spontaneous production of IL-2 from BAL lymphocytes was higher in the CD4+ subpopulation compared with CD8+ cells. However, this investigation was limited to three patients with sarcoidosis. Our method of intracellular cytokine detection however, is also limited by the fact that it does not measure cytokine release but the potential of cells to produce cytokines following a specific stimulus. Yet, based on the percentage of cytokine-positive CD8+ cells as well as the intracellular signal of these cytokines in individual cells, it appears that CD8+ T lymphocytes have the potential to produce proinflammatory cytokines in a similar fashion to CD4+ cells, and it can be speculated that CD8+ T lymphocytes are also a relevant source of these proinflammatory cytokines in patients with stage II and III sarcoidosis.
In addition to IL-2 and IFN-γ production we were able to show that a high percentage of T lymphocytes in sarcoidosis also produce TNF-α. Elevated concentrations of TNF-α which has been shown to be the major cytokine involved in granuloma formation [25] have previously been reported in BAL fluid of patients with sarcoidosis [16]. Macrophages have been shown to be a source of this cytokine [6,26]. Our findings imply that in sarcoidosis T lymphocytes might be another source of TNF-α. This assumption is supported by several other studies which have demonstrated TNF-α production by T lymphocytes following stimulation with two different stimuli (e.g. IL-2/anti-CD3 or PMA/A21387) [27,28]. Similar to our observations, Ludviksson et al. have recently shown an increased production of TNF-α by CD4+ lymphocytes in patients with Wegener's granulomatosis [29]. Thus, our findings are compatible with the assumption that in sarcoidosis TNF-α is also produced by T cells.
It has been suggested that activated T lymphocytes proliferate locally at the sites of sarcoid inflammation [3]. Pinkston et al. [7] demonstrated a spontaneous proliferation of lymphocytes as well as IL-2 production in situ which was not present in peripheral blood of affected patients. Our findings of a close correlation between intracellular IL-2 production in lymphocytes and the total lymphocyte count in BAL suggest that IL-2 production is related to the degree of lymphocytic alveolitis. Thus, although our results were obtained with a completely different experimental setup, they are in agreement with observations by other authors [5,7], suggesting that inflammation in sarcoidosis is localized to the involved organ.
Previous data have indicated that elevated concentrations of spontaneously released IL-2 from cultured BAL cells can indicate disease progression in sarcoidosis [30]. Interestingly, we found a negative correlation between the RFI for IL-2 as well as the percentage of IL-2+ T lymphocytes and the total lung capacity (% of predicted) of our patients, suggesting that the IL-2 up-regulation in sarcoidosis is related to the impairment in pulmonary function. Whether however, analysis of intracellular IL-2 production in BAL lymphocytes following in vitro stimulation can also yield prognostic information remains at present unclear. Comparative studies with other interstitial lung diseases are needed to investigate whether intracellular cytokine staining might be useful in the diagnosis of sarcoidosis.
Activation of T lymphocytes in sarcoidosis has been associated with the expression of surface antigens such as CD25 and HLA-DR [1,3]. Our observation however, that the percentage of lymphocytes which can produce Th1-like cytokines upon in vitro stimulation by far exceeds the number of CD25+ and HLA-DR+ cells, suggests a preactivation of these cells in vivo which however, was not present in cells in peripheral blood. Similar to previous investigations [30,31] there was no correlation between CD25 expression and intracellular IL-2 production. However, in contrast to previous findings [32], in our study we found a relationship between HLA-DR expression on BAL lymphocytes which has been reported as a marker of progression of sarcoidosis [32] and IL-2 production in vitro.
Our finding of a close correlation between IFN-γ, TNF-α and IL-2 (P < 0·000 01, r > 0·85) suggests a common mechanism of up-regulation of these cytokines. A close relationship and reciprocal activation between BAL lymphocytes and macrophages resulting in granuloma formation has been suggested [6]. Although not further investigated in our study, macrophage-derived cytokines such as IL-12 which causes a Th1 shift in lymphocytes [33] or IL-15, which similar to IL-2 can induce T cell proliferation [19], could be related to the marked overproduction of Th1-like cytokines by BAL lymphocytes observed in our study.
In conclusion, we provide evidence that a Th1-like cytokine pattern can be found in single lymphocytes of the CD4 as well as the CD8 phenotype in patients with stage II–III sarcoidosis. Intracellular cytokine staining might be useful to study cytokine expression in sarcoidosis. Although our results strongly suggest that this method might also aid in the diagnosis and monitoring of sarcoidosis, further studies are required to address those questions.
Acknowledgments
The authors wish to thank Sieglinde Bock for her expert technical assistance. This work was supported by a grant from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (FKZ 01GC9701/7).
References
- 1.Saltini C, Spurzem JR, Lee JJ, Pinkston P, Crystal RG. Spontaneous release of interleukin 2 by lung T lymphocytes in active pulmonary sarcoidosis is primarily from the Leu3+DR+ T cell subset. J Clin Invest. 1986;77:1962–70. doi: 10.1172/JCI112525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunninghake GW, Crystal RG. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981;305:429–34. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- 3.Semenzato G, Agostini C, Trentin L, et al. Evidence of cells bearing interleukin-2 receptor at sites of disease activity in sarcoid patients. Clin Exp Immunol. 1984;57:331–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Grunewald J, Olerup O, Persson U, Ohrn MB, Wigzell H, Eklund A. T-cell receptor variable region gene usage by CD4+ and CD8+ T cells in bronchoalveolar lavage fluid and peripheral blood of sarcoidosis patients. Proc Natl Acad Sci USA. 1994;91:4965–9. doi: 10.1073/pnas.91.11.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller-Quernheim J, Saltini C, Sondermeyer P, Crystal RG. Compartmentalized activation of the interleukin 2 gene by lung T lymphocytes in active pulmonary sarcoidosis. J Immunol. 1986;137:3475–83. [PubMed] [Google Scholar]
- 6.Müller-Quernheim J, Pfeifer S, Mannel D, Strausz J, Ferlinz R. Lung-restricted activation of the alveolar macrophage/monocyte system in pulmonary sarcoidosis. Am Rev Respir Dis. 1992;145:187–92. doi: 10.1164/ajrccm/145.1.187. [DOI] [PubMed] [Google Scholar]
- 7.Pinkston P, Bitterman PB, Crystal RG. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983;308:793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- 8.Hunninghake GW, Bedell GN, Zavala DC, Monick M, Brady M. Role of interleukin-2 release by lung T-cells in active pulmonary sarcoidosis. Am Rev Respir Dis. 1983;128:634–8. doi: 10.1164/arrd.1983.128.4.634. [DOI] [PubMed] [Google Scholar]
- 9.Dubinett SM, Huang M, Lichtenstein A, et al. Tumor necrosis factor-alpha plays a central role in interleukin-2-induced pulmonary vascular leak and lymphocyte accumulation. Cell Immunol. 1994;157:170–80. doi: 10.1006/cimm.1994.1214. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron A, Bonay M, Kambouchner M, et al. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J Immunol. 1997;159:3034–43. [PubMed] [Google Scholar]
- 11.Robinson BW, McLemore TL, Crystal RG. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985;75:1488–95. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krouwels FH, Nocker RET, Snoek M, et al. Immunocytochemical and flow cytofluorimetric detection of intracellular IL-4, IL-5 and IFN-gamma—applications using blood-and airway-derived cells. J Immunol Methods. 1997;203:89–101. doi: 10.1016/s0022-1759(97)00016-1. [DOI] [PubMed] [Google Scholar]
- 13.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 14.Moller DR, Forman JD, Liu MC, et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol. 1996;156:4952–60. [PubMed] [Google Scholar]
- 15.Milburn HJ, Poulter LW, Dilmec A, Cochrane GM, Kemeny DM. Corticosteroids restore the balance between locally produced Th1 and Th2 cytokines and immunoglobulin isotypes to normal in sarcoid lung. Clin Exp Immunol. 1997;108:105–13. doi: 10.1046/j.1365-2249.1997.d01-979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker C, Bauer W, Braun RK, et al. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med. 1994;150:1038–48. doi: 10.1164/ajrccm.150.4.7921434. [DOI] [PubMed] [Google Scholar]
- 17.Bäumer I, Zissel G, Schlaak M, Müller-Quernheim J. TH1/TH2 cell distribution in pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 1997;16:171–7. doi: 10.1165/ajrcmb.16.2.9032124. [DOI] [PubMed] [Google Scholar]
- 18.Jung T, Lack G, Schauer U, et al. Decreased frequency of interferon-gamma-and interleukin-2-producing cells in patients with atopic diseases measured at the single cell level. J Allergy Clin Immunol. 1995;96:515–27. doi: 10.1016/s0091-6749(95)70296-2. [DOI] [PubMed] [Google Scholar]
- 19.Agostini C, Semenzato G, James DG. Immunological, clinical and molecular aspects of sarcoidosis. Mol Aspects Med. 1997;18:91–165. doi: 10.1016/s0098-2997(97)84114-3. [DOI] [PubMed] [Google Scholar]
- 20.Krug N, Thurau AM, Lackie P, et al. A flow cytometric method for the detection of intracellular basic proteins in unseparated peripheral blood and bone marrow eosinophils. J Immunol Methods. 1996;190:245–54. doi: 10.1016/0022-1759(95)00272-3. [DOI] [PubMed] [Google Scholar]
- 21.Valeyre D, Saumon G, Georges R, et al. The relationship between disease duration and noninvasive pulmonary explorations in sarcoidosis with erythema nodosum. Am Rev Respir Dis. 1984;129:938–42. doi: 10.1164/arrd.1984.129.6.938. [DOI] [PubMed] [Google Scholar]
- 22.Verstraeten A, Demedts M, Verwilghen J, et al. Predictive value of bronchoalveolar lavage in pulmonary sarcoidosis. Chest. 1990;98:560–7. doi: 10.1378/chest.98.3.560. [DOI] [PubMed] [Google Scholar]
- 23.Greening AP, Nunn P, Dobson N, Rudolf M, Rees AD. Pulmonary sarcoidosis: alterations in bronchoalveolar lymphocytes and T cell subsets. Thorax. 1985;40:278–83. doi: 10.1136/thx.40.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward K, O'connor C, Odlum C, Fitzgerald MX. Prognostic value of bronchoalveolar lavage in sarcoidosis: the critical influence of disease presentation. Thorax. 1989;44:6–12. doi: 10.1136/thx.44.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 26.Ziegenhagen MW, Benner UK, Zissel G, Zabel P, Schlaak M, Müller-Quernheim J. Sarcoidosis: TNF-alpha release from alveolar macrophages and serum level of sIL-2R are prognostic markers. Am J Respir Crit Care Med. 1997;156:1586–92. doi: 10.1164/ajrccm.156.5.97-02050. [DOI] [PubMed] [Google Scholar]
- 27.Turner M, Londei M, Feldmann M. Human T cells from autoimmune and normal individuals can produce tumor necrosis factor. Eur J Immunol. 1987;17:1807–14. doi: 10.1002/eji.1830171220. [DOI] [PubMed] [Google Scholar]
- 28.Steffen M, Ottmann OG, Moore MA. Simultaneous production of tumor necrosis factor-alpha and lymphotoxin by normal T cells after induction with IL-2 and anti-T3. J Immunol. 1988;140:2621–4. [PubMed] [Google Scholar]
- 29.Ludviksson BR, Sneller MC, Chua KS, et al. Active Wegener's granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998;160:3602–9. [PubMed] [Google Scholar]
- 30.Müller-Quernheim J, Pfeifer S, Kienast K, Zissel G. Spontaneous interleukin 2 release of bronchoalveolar lavage cells in sarcoidosis is a codeterminator of prognosis. Lung. 1996;174:243–53. doi: 10.1007/BF00173139. [DOI] [PubMed] [Google Scholar]
- 31.Müller-Quernheim J, Kronke M, Strausz J, Schykowski M, Ferlinz R. Interleukin-2 receptor gene expression by bronchoalveolar lavage lymphocytes in pulmonary sarcoidosis. Am Rev Respir Dis. 1989;140:82–8. doi: 10.1164/ajrccm/140.1.82. [DOI] [PubMed] [Google Scholar]
- 32.Müller-Quernheim J, Pfeifer S, Strausz J, Ferlinz R. Correlation of clinical and immunologic parameters of the inflammatory activity of pulmonary sarcoidosis. Am Rev Respir Dis. 1991;144:1322–9. doi: 10.1164/ajrccm/144.6.1322. [DOI] [PubMed] [Google Scholar]
- 33.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 34.Nakamura H, Fujishima S, Soejima K, et al. Flow cytometric detection of cell-associated cytokines in alveolar macrophages. Eur Respir J. 1996;9:1181–7. doi: 10.1183/09031936.96.09061181. [DOI] [PubMed] [Google Scholar]


