Abstract
The role of interleukin (IL)-8 as mediator in the recruitment of leucocytes into the CSF was investigated during experimental pneumococcal meningitis. Rabbits were inoculated intracisternally with approximately 106 CFU Streptococcus pneumoniae, and treated (i) intravenously with 5 mg of a monoclonal antibody to IL-8 (n = 7) or 5 mg of an isotype control antibody (n = 6); (ii) intracisternally with anti-IL-8, 100 µg (n = 5), 10 µg (n = 4), 1 µg (n = 4), 0·1 µg (n = 2). Ten rabbits served as untreated control group. Intravenous treatment with anti-IL-8 attenuated the pleocytosis significantly compared to untreated rabbits (P < 0·04) or rabbits treated with an isotype control antibody (P < 0·02). In contrast, intracisternal treatment with anti-IL-8 failed to attenuate the pleocytosis (P > 0·05). These results show, that IL-8 plays an important role in the recruitment of leucocytes during experimental pneumococcal meningitis, and that the functional activity of IL-8 in this process appears to be on the bloodstream side of the microvascular endothelium of the brain.
Keywords: IL-8, meningitis, Streptococcus pneumoniae
Introduction
The mortality rate of pneumococcal meningitis has not changed significantly since the introduction of penicillin in the 1940s, and it can still reach a high of approximately 25%, with neurological sequelae occurring in half of the survivors [1,2]. Host inflammatory reaction to invading pathogens, characterized by a rapid accumulation of neutrophils in the meninges and in the CSF, appears to be largely responsible for the development of brain damage in bacterial meningitis [3]. Cytokines are important mediators in this process [4], although several studies lately have questioned previous findings [5] and now indicate that the proinflammatory cytokines, tumour necrosis factor-α (TNFα) and IL-1β, are a result rather than the cause of pleocytosis [6–8]. Therefore, studies of newer candidates (e.g. chemokines) are required for a comprehensive understanding of the mechanisms behind the recruitment of neutrophils during bacterial meningitis.
Although IL-8, a member of the CXC-chemokine family, is one of the most potent inducers of neutrophil chemotaxis and activation in vitro and in vivo[9], surprisingly little is known about its role in bacterial meningitis. We have previously shown, that patients with bacterial meningitis have highly elevated levels of IL-8 in the CSF, and that IL-8, to some degree, is useful in the differential diagnosis between neutrophilic and lymphophilic meningitis [10]. The in vitro chemotactic activity for neutrophils in infected CSF has been found to correlate to the corresponding CSF IL-8 levels. Furthermore, neutralizing IL-8 activity by adding an antibody to IL-8 to infected CSF resulted in a reduction of the in vitro chemotactic activity towards neutrophils [11]. In an experimental meningitis model, further support of a role of IL-8 in the recruitment of neutrophils has been generated: (i) low CSF levels of IL-8 in rabbits pretreated with granulocyte-colony-stimulating-factor were associated with a decreased pleocytosis during pneumococcal meningitis [12]; (ii) IL-8 levels starts to raise in CSF before the pleocytosis starts to emerge, and (iii) blockage of leucocyte entry into the brain, did not attenuate the CSF IL-8 levels, indicating that IL-8 is produced by cells within the brain during pneumococcal meningitis [6].
To more directly address a possible role of IL-8 in regulating the pleocytosis during pneumococcal meningitis, we report the effect of administration (either systemically or intracisternally) of specific monoclonal antibodies directed against IL-8.
Materials and methods
Meningitis model
A rabbit meningitis model was used, as previously described [6,12]. In brief, rabbits were inoculated intracisternally with approximately 1 × 106 CFU Streptococcus pneumoniae, type 3 strain [68034, Statens Serum Institut (SSI), Copenhagen, Denmark], as confirmed by quantitative cultures. CSF and blood samples were taken every second hour during a 16-h study period. CSF and blood samples were tested for bacterial concentration and WBC count.
Study design
Inhibition of the pleocytosis with anti-IL-8
(i) Infected rabbits were treated intravenously with 5 mg of WS-4 (IgG1), a mouse monoclonal antibody, that can neutralize rabbit IL-8, prepared as previously described [13,14], dissolved in pyrogen free PBS (SSI), and administered over 1 h immediately after the bacterial inoculation (n = 7). This dose of WS-4 has previously been shown to inhibit local recruitment of leucocytes in another rabbit model [15]. Infected rabbits treated intravenously with 5 mg of TpM-1 (IgG1), a mouse monoclonal antibody towards Toxoplasma membrane antigen [15], also dissolved in pyrogen free PBS, served as control group (n = 6).
(ii) Infected rabbits were treated with an intracisternal injection of WS-4, 100 µg (n = 5) immediately after the bacterial inoculation. This dose was chosen to achieve a CSF concentration of WS-4 at approximately the same level as the concentration of WS-4 obtained in serum during intravenous therapy with 5 mg of WS-4. In addition, experiments with intracisternal injection of WS-4 in lower doses than 100 µg were performed: 10 µg (n = 4), 1 µg (n = 4), 0·1 µg (n = 2). Infected untreated rabbits served as control group (n = 10). One uninfected rabbit was injected intracisternally with 100 µg of WS-4.
Stimulation of the pleocytosis with IL-8
(i) Uninfected rabbits were given an intracisternal injection of recombinant human IL-8 (endothelial cell-derived, Genzyme, Cambridge, MA, USA), dissolved in pyrogen free PBS in doses of 1 ng, 10 ng, 100 ng, and 200 ng (n = 4), and in doses of 100 ng, 100 ng, and 10 ng together with rhTNFα (Genzyme), dissolved in pyrogen free PBS in doses of 105 U, 104 U, and 104 U, respectively (n = 3). Stock dilutions of the cytokines were freshly prepared on the day of experiments, and the activity of the preparations were subsequently tested by an ELISA (IL-8) and by a bioassay (TNFα) as previously described [12]. The doses of IL-8 were chosen to cover the range of IL-8 levels found in the CSF of patients with bacterial meningitis or during experimental pneumococcal meningitis (0·2–40 ng/ml) [10,12]. Human and rabbit IL-8 have a very high degree of homology [16], and we found that the rhIL-8, used in this study, had chemotactic activity (number of migrated cells per field) for rabbit neutrophils [IL-8, 100 ng, 285 (260–304); 10 ng, 63 (57–160); 1 ng, 0 (0); 0 ng, 0 (0); 1:200 dilution of zymosan-activated serum, 247 (192–318)]. An 8-h study period was chosen according to previous studies, where peak levels of WBC were observed within 6 h after local injections of IL-8 and/or TNFα[17]. (ii) Infected rabbits were treated with an intracisternally injection of rhIL-8 in doses of 0·01 ng, 0·1 ng, 1 ng, 10 ng, and 100 ng (n = 5). The study period was 16 h.
CSF analysis
WBC and differential counts were determined on an automatic cell counter (Swelab, Årsta, Sweden). The lowest detectable WBC was 100 cells/µl.
Statistical analysis
All results are provided as medians and ranges (min − max). Comparison between groups was performed by the nonparametric Mann–Whitney test. When appropriate, correction with the Bonferoni's coefficient was performed to compensate for multiple comparisons. P < 0·05 was considered statistically significant.
Results
Effect of treatment with a monoclonal antibody to IL-8
In pneumococcal meningitis, intravenous treatment with WS-4 (n = 7) resulted in a significant attenuation of the pleocytosis compared to rabbits treated intravenously with a control antibody (n = 6) between 10 and 16 h (P < 0·02, Fig. 1). A significant difference was also observed between WS-4-treated and rabbits not receiving any antibodies (n = 10, Table 1) between 10 and 16 h (P < 0·04), whereas there was no significant difference between rabbits treated with the control antibody and untreated rabbits at any time-point during the 16 h study period (P > 0·05).
Fig. 1.
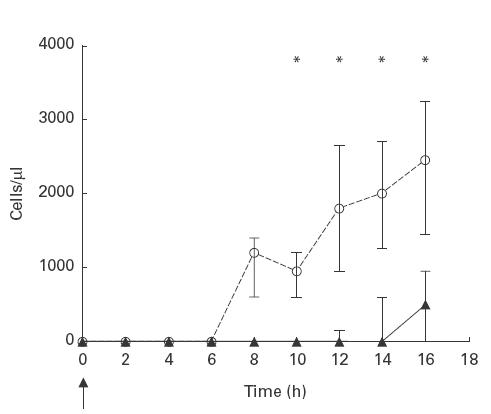
The effect of intravenous treatment with a monoclonal antibody to IL-8 on CSF WBC levels during experimental pneumococcal meningitis. WS-4/Tmp-1 (5 mg) was given as an intravenous bolus infusion over 1 h immediately after the intracisternal inoculation of approximately 106 CFU of Streptococcus pneumoniae, type 3 at 0 h (arrow). CSF WBC levels were significant attenuated in WS-4 treated rabbits (medians, closed symbols, solid line, n = 7) compared to rabbits treated with an isotype control antibody, Tmp-1 (medians, open symbols, dashed line, n = 6) between 10 and 16 h. *P < 0·05 (Mann–Whitney test). Bars indicate 25th and 75th percentiles.
Table 1.
The effect of intracisternal treatment with a monoclonal antibody to IL-8 (WS-4) on CSF WBC levels during experimental pneumococcal meningitis
| Hours | Untreated control (n = 10) cells/µl | WS-4 treated 100 µg (n = 5) cells/µl | WS-4 treated 10 µg (n = 4) cells/µl | WS-4 treated 1 µg (n = 4) cells/µl | WS-4 treated 0·1 µg (n = 2) cells/µl |
|---|---|---|---|---|---|
| 0 | < 100 (< 100) | < 100 (< 100) | < 100 (< 100) | < 100 (< 100) | < 100 (< 100) |
| 2 | < 100 (< 100) | < 100 (< 100) | < 100 (< 100) | < 100 (< 100) | < 100 (< 100) |
| 4 | < 100 (< 100) | 500 (< 100–700) | < 100 (< 100) | < 100 (< 100) | < 100 (< 100) |
| 6 | < 100 (< 100–1100) | 400 (< 100–700) | 741 (< 100–1300) | < 100 (< 100) | 200 (< 100–400) |
| 8 | 250 (< 100–1342) | 390 (< 100–1200) | 500 (400–2188) | 650 (< 100–1200) | 600 (400–800) |
| 10 | 600 (< 100–1200) | 500 (300–600) | 1700 (500–2900) | 650 (< 100–1700) | 800 (800) |
| 12 | 1200 (< 100–2100) | 700 (300–2000) | 1801 (600–3900) | 700 (< 100–2000) | 2100 (1300–2900) |
| 14 | 1020 (300–8200) | 1200 (300–4000) | 2500 (600–4900) | 2050 (300–2800) | 2565 (2200–2931) |
| 16 | 2550 (700–10900) | 2550 (800–10000) | 3800 (600–8700) | 2150 (1100–3000) | 3033 (2200–3867) |
*All data are shown as medians and ranges (min − max). WS-4 was injected intracisternally immediately after the intracisternal inoculation of approximately 1 × 106 CFU Streptococcus pneumoniae, type 3 (at 0 h). No significant difference was observed between WS-4 treated and untreated control rabbits at any time-point (P > 0·05 (Mann–Whitney test)).
In contrast to intravenous treatment, intracisternal injection of WS-4 in a dose of 100 µg (n = 5) to infected rabbits caused no attenuation in the pleocytosis compared to infected rabbits not receiving any antibodies (n = 10) during a 16-h study period (P > 0·05, Table 1). There was a minor elevation in CSF WBC [500 (0–700) cells/µl], that peaked approximately 4 h after the intracisternal injection of 100 µg of WS-4 compared to untreated rabbits. This may be due to a nonspecific stimulation of the pleocytosis by the antibody, because intracisternal injection of WS-4, 100 µg × 1 to an uninfected control rabbit, also caused a similar elevation in CSF WBC (data not shown). This nonspecific stimulation of the pleocytosis approximately 4 h after the intracisternal injection was not observed, when infected rabbits were treated intracisternally with lower doses of WS-4 [10 µg × 1 (n = 4), 1 µg × 1 (n = 4), 0·1 µg × 1 (n = 2)] (Table 1). Again, no attenuation of the pleocytosis was observed in WS-4 treated rabbits as compared to untreated control rabbits and no dose/response pattern for the WS-4 treatment was demonstrated (P > 0·05).
Effect of intracisternal injection of IL-8
Intracisternal injection of recombinant human IL-8 in doses between 1 and 200 ng in uninfected rabbits resulted in no pleocytosis in any of the rabbits studied during an 8-h study period (n = 4, data not shown). This was not due to an inactivation of IL-8 when present in the CSF, since we could detect high CSF IL-8 levels (approximately 40 ng/ml) within 1 h after the intracisternal injection of 100 ng (data not shown). The CSF elimination for human recombinant IL-8 was biphasic during an 8-h study period (data not shown). Intracisternal injections of IL-8 together with TNFα in uninfected rabbits did not result in any pleocytosis during an 8-h study period (n = 3, data not shown).
In pneumococcal meningitis, intracisternal injections of various doses of IL-8 immediately after the bacterial inoculation (n = 5) caused no apparent alteration in the kinetics of the pleocytosis compared to untreated rabbits during the 16-h study period (data not shown).
Discussion
In the present study, we found that intravenously treatment with a monoclonal antibody to IL-8 attenuated the neutrophil pleocytosis during experimental pneumococcal meningitis, indicating that this inflammatory mediator is critical important in the development of pleocytosis. To our knowledge, this is the first study to directly demonstrate this. However, a study by Diab et al. [18] using a rat model of H. influenzae meningitis has previously found, that intravenous treatment with a polyclonal antibodies to rat-macrophage inflammatory protein (MIP)-2, which is proposed to be a substitute for IL-8 as a major neutrophil chemoattractant in rats was able to attenuate the pleocytosis. Furthermore, intravenous treatment with anti-IL-8 has previously been shown to inhibit neutrophil recruitment across the blood/brain barrier in experimental reperfusion injury following transient ischaemia [19], as well as local neutrophil accumulation in other body compartments, e.g. skin [13,15], lung [20] and kidney [21].
Interestingly, intracisternal treatment with anti-IL-8, in contrast to intravenous treatment, failed to attenuate the pleocytosis during experimental pneumococcal meningitis. Intracisternal treatment with anti-IL-8, 100 µg × 1 caused a minor elevation in CSF WBC approximately 4 h after start of therapy, where after CSF WBC began to decrease for both uninfected as well as infected rabbits. However, there is no evidence that this minor elevation in CSF WBC early during the course of pneumococcal meningitis affected the subsequent pleocytosis, since the development and the kinetics of the pleocytosis was similar to untreated rabbits or rabbits treated intracisternally with lower doses of WS-4 (10, 1 and 0·1 µg), where no early elevation in CSF WBC was observed. We are fairly certain that intracisternal treatment with anti-IL-8 in doses between 0·1 and 10 µg (resulting in a CSF concentrations of the antibody between approximately 20–2000 ng/ml) should be high enough to inhibit the chemotactic activity of IL-8 within the CSF during experimental pneumococcal meningitis (the maximum CSF IL-8 concentrations detected in rabbits with pneumococcal meningitis during the 16 h study period was <2 ng/ml), since WS-4 in equal or higher concentrations than the cytokine in vitro inhibits the chemotactic activity of rabbit IL-8 against rabbit neutrophils [13]. Saukonnen et al. have previously shown, in support of our results, that intracisternal treatment with antibodies to other chemokines (MIP-1 and 2) caused no attenuation of the pleocytosis in experimental pneumococcal meningitis [5].
In addition to this, we observed no pleocytosis after intracisternal injection of IL-8 alone or together with TNFα. Moreover, intracisternal injection of IL-8 caused no enhancement of the pleocytosis in experimental pneumococcal meningitis. Thus, the presence of IL-8 in the CSF seems to have no influence on the recruitment of neutrophils during experimental meningitis. Although, using an ELISA, we were able to detect IL-8 at high levels in the CSF after the intracisternal injection, we cannot fully exclude a lack of chemotactic activity of human IL-8 when present in rabbit CSF. However, we believe that it is an unlikely explanation, since (i) human IL-8 was chemotactic for rabbit neutrophils in vitro, and (ii) the in vitro chemotactic activity of infected CSF for neutrophils, indeed, is related to the presence of IL-8 [11]. In conflict with our findings, injection of a very high dose of human IL‐8 (1 μg) into the hippocampus of rats resulted in an accumulation of neutrophils in the meningesus well as a minor accumulation of neutrophils along the injection track, but, surprisingly, not in brain parenchyma [22]. The significance of human IL-8 in the rat model may, however, be limited as mentioned above, since no direct homology to IL-8 has been identified in rats or mice [16]. This is in contrast to the high degree of homology between rabbit and human IL-8, in which activity indeed can be blocked by the same antibodies [13]. Moreover, the difference between the study by Andersson et al. [22] and ours may as well be due to difference in the injection site (cisterna magna versus hippocampus) and could indicate tissue specific activity of IL-8 within the brain. Further studies are required to investigate the functional activity of IL-8 in the various brain tissues using animal models where IL-8 is naturally occurring (e.g. rabbit model).
Various local cells within the brain are able to produce IL-8 including astrocytes, microglia, and endothelial cells [4]. Our results in this study suggest, that the endothelial cells most likely are a key producer of IL-8, since the site of activity of IL-8, seems to be on the bloodstream side of the microvascular endothelium during experimental pneumococcal meningitis. Indeed, immune-staining also showed that IL-8 was primarily detected in the cerebral microvascular endothelium during experimental cerebral reperfusion injury in rabbits [19]. However, endothelial cells of postcapillary venules and small veins can also transport IL-8 that was injected into the skin, from the apical site to the luminal surface by trancytosis [23]. But it remains to be defined whether endothelial cells of the blood–brain barrier also transport IL-8, locally produced in the perivascular tissue, to the luminal surface by trancytosis.
We also found, that intracisternal injection of recombinant human TNFα in doses up to 105 U resulted in no detectable CSF levels of IL-8 nor no pleocytosis during an 8-h study period (unpublished observations). Our results confirm previous observations by Ramilo et al. [24], but are in conflict with the findings by Saukkonen et al. who were able to induce pleocytosis after intracisternal injection with 104 U rhTNFα[5]. The discrepancy between the results of Saukkonen and our results may be due to differences in preparation and storage of the rhTNFα. Further studies are required to define whether intracisternal inoculation of rabbit TNFα, previously found to induce pleocytosis in the rabbit model [24], results in subsequent IL-8 release into the CSF.
In conclusion, we found that IL-8 plays an important role in the recruitment of neutrophils during experimental pneumococcal meningitis, and that the functional activity of IL-8 in this process appears to be on the bloodstream side of the microvascular endothelium of the brain. Further studies are required to determine the cellular source of the sequential IL-8 production during experimental pneumococcal meningitis.
Acknowledgments
This work was supported in part from the læge Sofus Carl Emil Friis', Ebbe Celinder's, Ydes' and købmand Svend Hansen's foundation. We thank Anna Asanovski, Bo Sønder Hansen and Jacob Vang for skillful technical assistance.
References
- 1.Durand ML, Calderwood SB, Weber DJ. Acute bacterial meningitis in adults: a review of 493 episodes. N Engl J Med. 1993;328:21–8. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 2.Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. N Engl J Med. 1997;337:970–6. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 3.Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–72. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 4.Tauber MG, Moser B. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin Infect Dis. 1999;28:1–12. doi: 10.1086/515079. [DOI] [PubMed] [Google Scholar]
- 5.Saukkonen K, Sande S, Cioffe C, et al. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990;171:439–48. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Østergaard C, Yieng-Kow RV, Benfield T, Frimodt-Møller N, Espersen F, Lundgren JD. Inhibition of leukocyte entry into the brain by the selectin-blocker, fucoidin decreases interleukin (IL)-1 levels, but increases IL-8 levels in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect Immun. 2000;68:3153–7. doi: 10.1128/iai.68.6.3153-3157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitsch A, Trostdorf F, Bruck W, Schmidt H, Fischer FR, Nau R. Central nervous system TNF alpha-mRNA expression during rabbit experimental pneumococcal meningitis. Neurosci Lett. 1997;237:105–8. doi: 10.1016/s0304-3940(97)00830-6. [DOI] [PubMed] [Google Scholar]
- 8.Zysk G, Brück W, Huitinga I, et al. Elimination of blood-derived macrophages inhibits the release of interleukin-1 and the entry of leukocytes into the cerebrospinal fluid in experimental pneumococcal meningitis. J Neuroimmunol. 1997;73:77–80. doi: 10.1016/s0165-5728(96)00173-7. [DOI] [PubMed] [Google Scholar]
- 9.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–64. [PubMed] [Google Scholar]
- 10.Østergaard C, Benfield TL, Sellebjerg F, Kronborg G, Lohse N, Lundgren JD. Interleukin-8 in cerebrospinal fluid from patients with septic and aseptic meningitis. Eur J Clin Microbiol Infect Dis. 1996;15:166–9. doi: 10.1007/BF01591492. [DOI] [PubMed] [Google Scholar]
- 11.Spanaus KS, Nadal D, Pfister HW, et al. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J Immunol. 1997;158:1956–64. [PubMed] [Google Scholar]
- 12.Østergaard C, Benfield T, Gesser B, et al. Pretreatment with granulocyte colony-stimulating factor attenuates the inflammatory response but not the bacterial load in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect Immun. 1999;67:3430–6. doi: 10.1128/iai.67.7.3430-3436.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada A, Sekido N, Kuno K, et al. Expression of recombinant rabbit IL-8 in Escherichia coli and establishment of the essential involvement of IL-8 in recruiting neutrophils into lipopolysaccharide-induced inflammatory site of rabbit skin. Int Immunol. 1993;5:681–90. doi: 10.1093/intimm/5.6.681. [DOI] [PubMed] [Google Scholar]
- 14.Ko Y, Mukaida N, Panyutich A, et al. A sensitive enzyme-linked immunosorbent assay for human interleukin-8. J Immunol Methods. 1992;149:227–35. doi: 10.1016/0022-1759(92)90254-q. [DOI] [PubMed] [Google Scholar]
- 15.Larsen CG, Thomsen MK, Gesser B, et al. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J Immunol. 1995;155:2151–7. [PubMed] [Google Scholar]
- 16.Mukaida N, Matsumoto T, Yokoi K, Harada A, Matsushima K. Inhibition of neutrophil-mediated acute inflammatory injury by an antibody against interleukin-8 (IL-8) Inflamm Res. 1998;47:S151–S157. doi: 10.1007/s000110050308. [DOI] [PubMed] [Google Scholar]
- 17.Matsukawa A, Yoshinaga M. Sequential generation of cytokines during the initiative phase of inflammation, with reference to neutrophils. Inflamm Research. 1998;47:S137–S144. doi: 10.1007/s000110050304. [DOI] [PubMed] [Google Scholar]
- 18.Diab A, Abdalla H, Li HL, et al. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1 alpha attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect Immun. 1999;67:2590–601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto T, Ikeda K, Mukaida N, et al. Prevention of cerebral edema and infarct in cerebral reperfusion injury by an antibody to interleukin-8. Lab Invest. 1997;77:119–25. [PubMed] [Google Scholar]
- 20.Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993;365:654–7. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]
- 21.Wada T, Tomosugi N, Naito T, et al. Prevention of proteinuria by the administration of anti-interleukin 8 antibody in experimental acute immune complex-induced glomerulonephritis. J Exp Med. 1994;180:1135–40. doi: 10.1084/jem.180.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson PB, Perry VH, Gordon S. Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the central nervous system. J Exp Med. 1992;176:255–9. doi: 10.1084/jem.176.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middleton J, Neil S, Wintle J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–95. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 24.Ramilo O, Saez Llorens X, Mertsola J, et al. Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]


